Take a Break for Memory Sake! Effects of Short Physical Activity Breaks on Inhibitory Control, Episodic Memory, and Event-Related Potentials in Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Fitness Assessment
2.3. Cognitive Tasks
2.3.1. Flanker Task
2.3.2. Word Recognition Task
2.4. EEG
2.4.1. Recording
2.4.2. Processing
2.4.3. ERPs
2.5. Procedure
2.6. Statistical Analysis
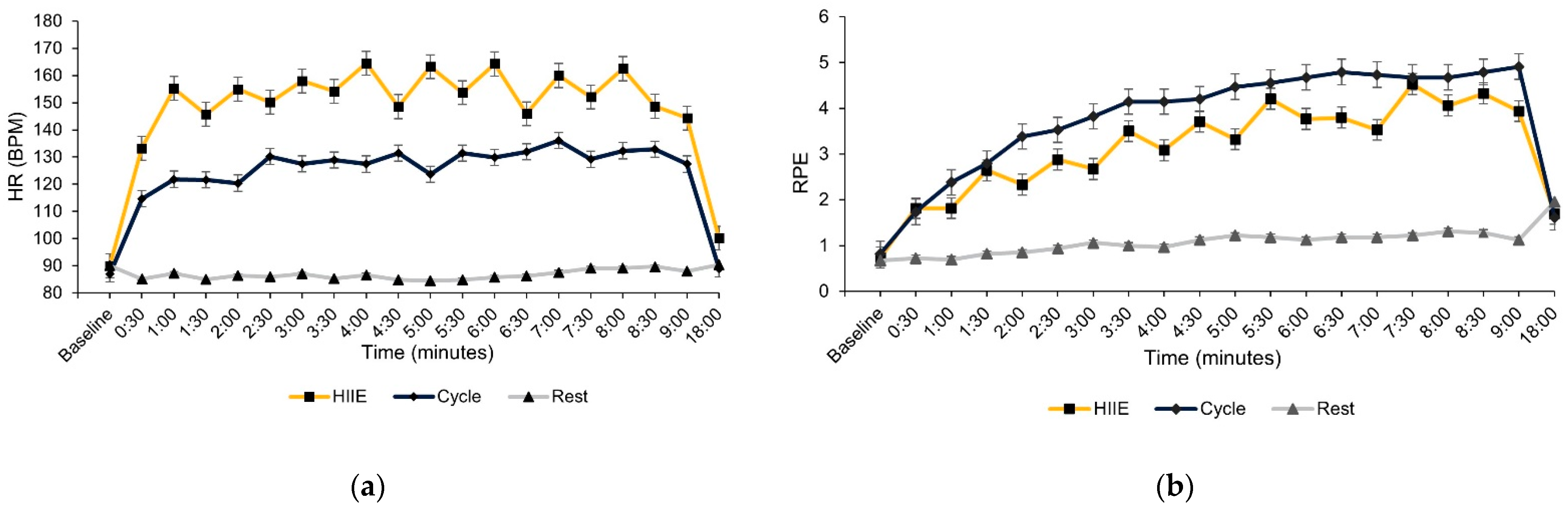
3. Results
3.1. Cognitive Task Performance
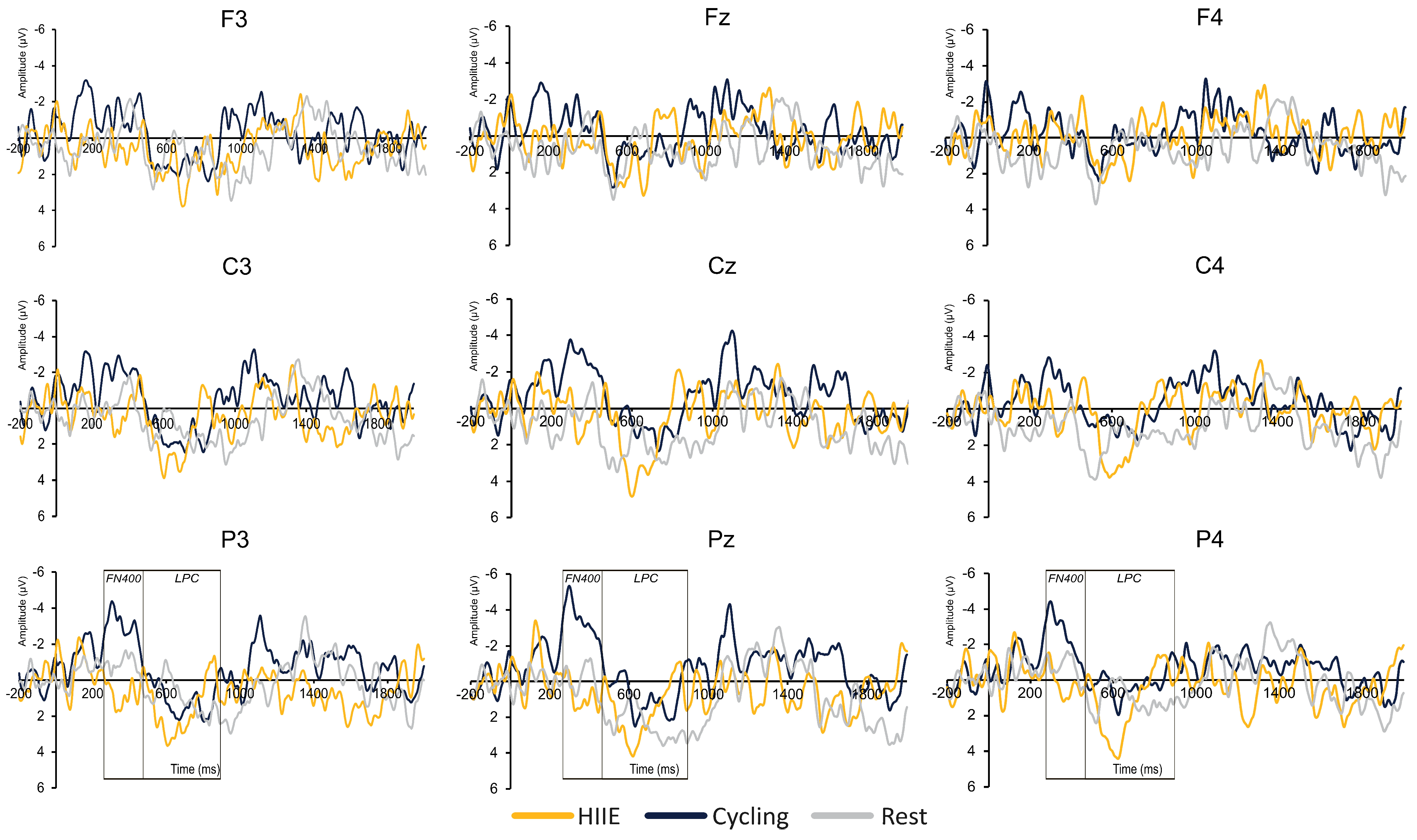
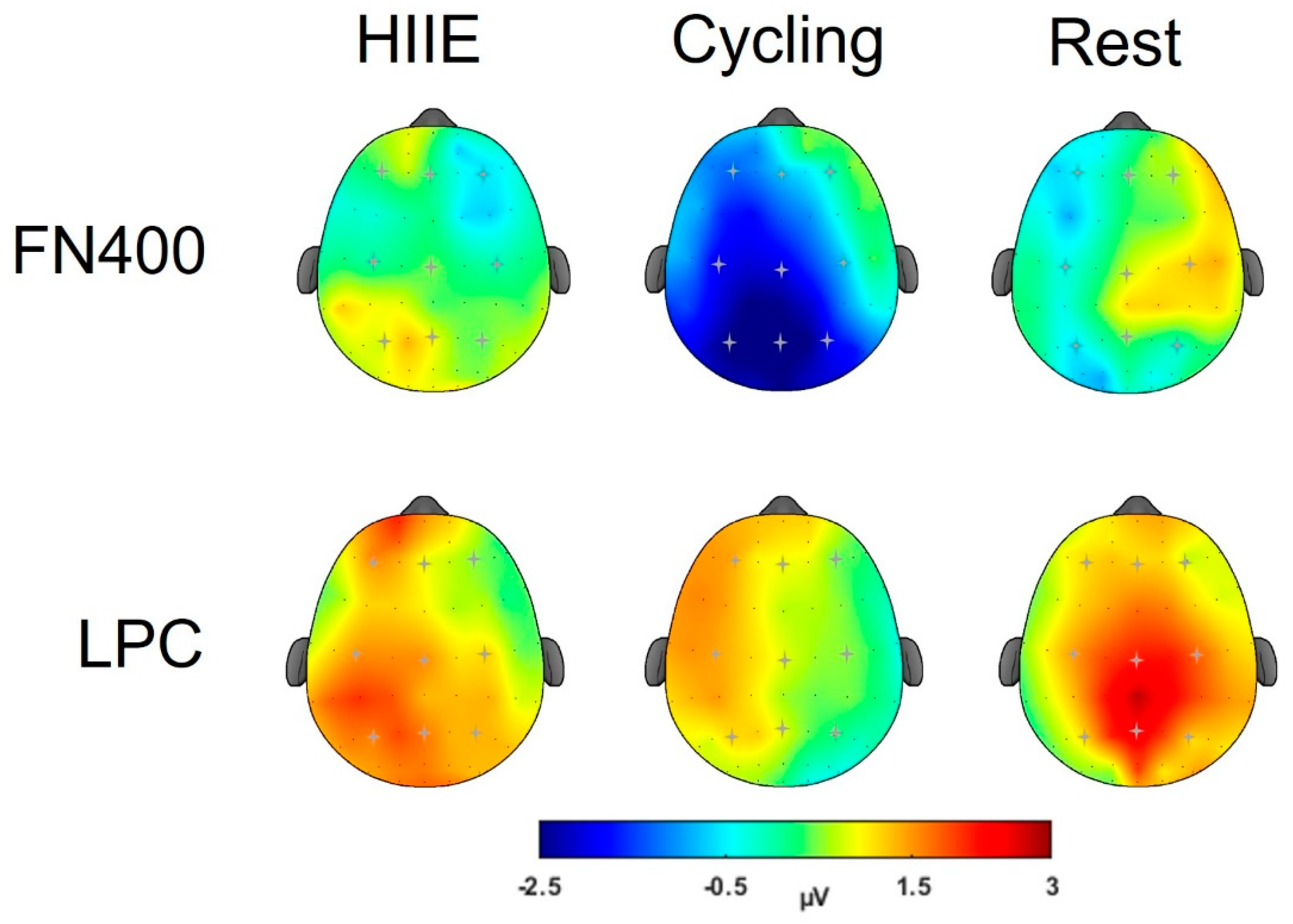
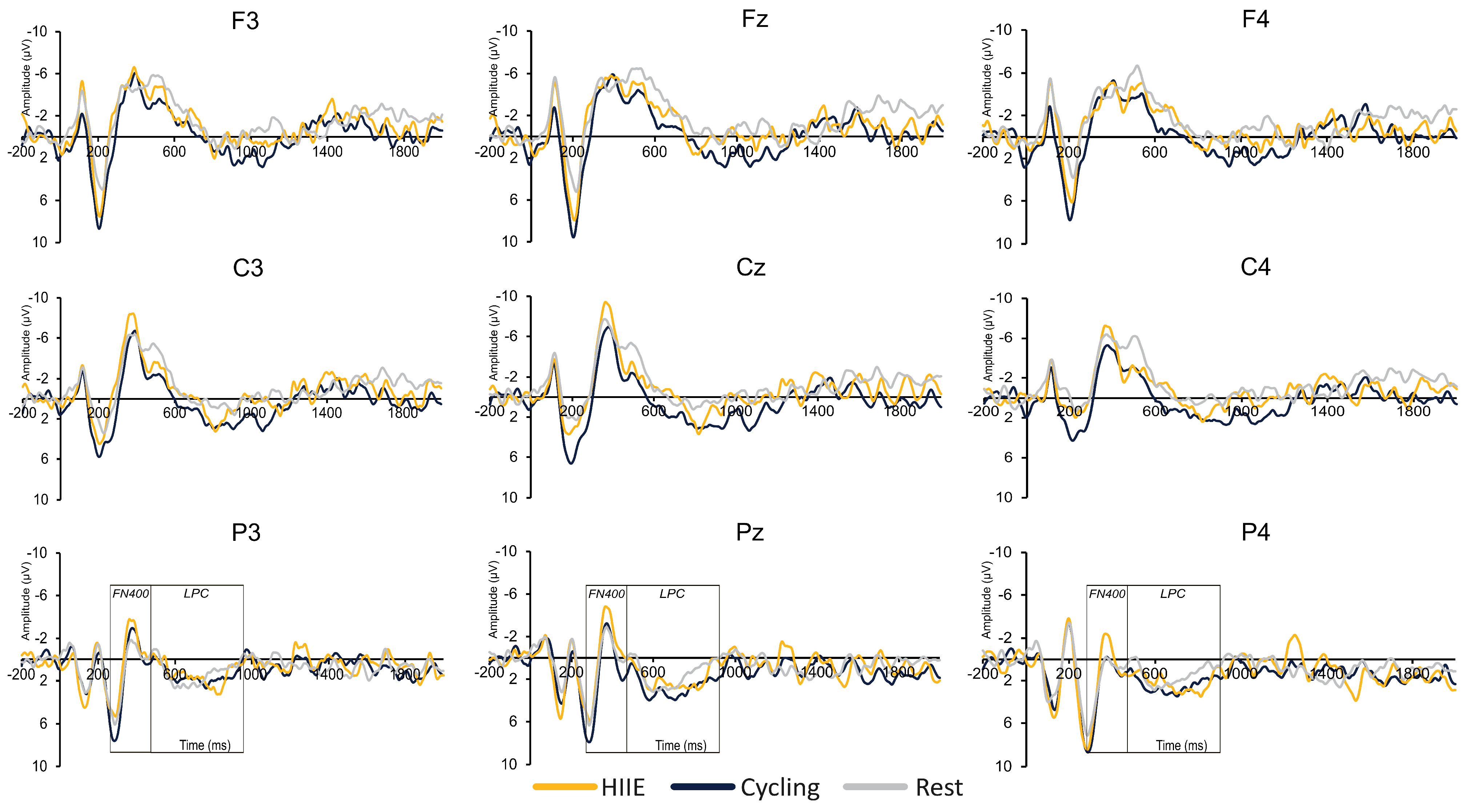
3.2. ERPs
3.2.1. P3
3.2.2. FN400
3.2.3. LPC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustafsson, M. Pandemic-Related Disruptions to Schooling and Impacts on Learning Proficiency Indicators: A Focus on the Early Grades; UNESCO Institute for Statistics: Montreal, QB, Canada, 2021; Volume 14. [Google Scholar]
- McArthur, B.A.; Racine, N.; Browne, D.; McDonald, S.; Tough, S.; Madigan, S. Recreational screen time before and during COVID-19 in school-aged children. Acta Paediatr. 2021, 110, 2805–2807. [Google Scholar] [CrossRef] [PubMed]
- Neville, R.D.; Lakes, K.D.; Hopkins, W.G.; Tarantino, G.; Draper, C.E.; Beck, R.; Madigan, S. Global Changes in Child and Adolescent Physical Activity During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022, 176, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Action Plan on Physical Activity 2018–2030. 2018. Available online: https://apps.who.int/iris/handle/10665/353808 (accessed on 11 June 2022).
- Hillman, C.H.; Logan, N.; Shigeta, T. A Review of Acute Physical Activity Effects on Brain and Cognition in Children. Transl. J. Am. Coll. Sports Med. 2019, 4, 132–136. [Google Scholar] [CrossRef]
- Booth, J.N.; Chesham, R.A.; Brooks, N.E.; Gorely, T.; Moran, C.N. A citizen science study of short physical activity breaks at school: Improvements in cognition and wellbeing with self-paced activity. BMC Med. 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- de Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Drollette, E.S.; Shishido, T.; Pontifex, M.B.; Hillman, C.H. Maintenance of cognitive control during and after walking in preadolescent children. Med. Sci. Sports Exerc. 2012, 44, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Drollette, E.S.; Scudder, M.R.; Raine, L.B.; Moore, R.D.; Saliba, B.J.; Pontifex, M.B.; Hillman, C.H. Acute exercise facilitates brain function and cognition in children who need it most: An ERP study of individual differences in inhibitory control capacity. Dev. Cogn. Neurosci. 2014, 7, 53–64. [Google Scholar] [CrossRef]
- Drollette, E.S.; Hillman, C.H. Walking effects on memory in children: Implications for individual differences in BMI. Ment. Health Phys. Act. 2020, 18, 100317. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis: Moderate exercise and executive function. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Tomporowski, P.D.; Davis, C.L.; Miller, P.H.; Naglieri, J.A. Exercise and Children’s Intelligence, Cognition, and Academic Achievement. Educ. Psychol. Rev. 2008, 20, 111–131. [Google Scholar] [CrossRef]
- Tomporowski, P.D.; McCullick, B.; Pendleton, D.M.; Pesce, C. Exercise and children’s cognition: The role of exercise characteristics and a place for metacognition. J. Sport Health Sci. 2015, 4, 47–55. [Google Scholar] [CrossRef]
- Diamond, A.; Barnett, W.S.; Thomas, J.; Munro, S. Preschool Program Improves Cognitive Control. Science 2007, 318, 1387–1388. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. The Cognitive Benefits of Exercise in Youth. Curr. Sports Med. Rep. 2015, 14, 320–326. [Google Scholar] [CrossRef]
- St Clair-Thompson, H.L.; Gathercole, S.E. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Q. J. Exp. Psychol. 2006, 59, 745–759. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Carter, C.S.; Braver, T.S.; Barch, D.M.; Cohen, J.D. Conflict Monitoring and Cognitive Control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.D.; Lambourne, K.; Szabo-Reed, A.N. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Pontifex, M.B.; Raine, L.B.; Castelli, D.M.; Hall, E.E.; Kramer, A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 2009, 159, 1044–1054. [Google Scholar] [CrossRef]
- Lambourne, K.; Tomporowski, P.D. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef]
- Tulving, E. What Is Episodic Memory? Curr. Dir. Psychol. Sci. 1993, 2, 67–70. [Google Scholar] [CrossRef]
- Etnier, J.L.; Labban, J.D.; Piepmeier, A.; Davis, M.E.; Henning, D.A. Effects of an Acute Bout of Exercise on Memory in 6th Grade Children. Pediatr. Exerc. Sci. 2014, 26, 250–258. [Google Scholar] [CrossRef]
- Mavilidi, M.F.; Okely, A.D.; Chandler, P.; Paas, F. Infusing Physical Activities into the Classroom: Effects on Preschool Children’s Geography Learning. Mind Brain Educ. 2016, 10, 256–263. [Google Scholar] [CrossRef]
- Pesce, C.; Crova, C.; Cereatti, L.; Casella, R.; Bellucci, M. Physical activity and mental performance in preadolescents: Effects of acute exercise on free-recall memory. Ment. Health Phys. Act. 2009, 2, 16–22. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Tessaro, V.H.; Teixeira, L.A.C.; Murakava, K.; Roschel, H.; Gualano, B.; Takito, M.Y. Influence of Acute High-Intensity Aerobic Interval Exercise Bout on Selective Attention and Short-Term Memory Tasks. Percept. Mot. Skills 2014, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.C.; Westfall, D.R.; Soneson, J.; Gurd, B.; Hillman, C.H. Comparison of the acute effects of high-intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology 2017, 54, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Lambrick, D.; Stoner, L.; Grigg, R.; Faulkner, J. Effects of continuous and intermittent exercise on executive function in children aged 8-10 years: Acute exercise and executive function. Psychophysiology 2016, 53, 1335–1342. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Suga, T.; Takenaka, S.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Hashimoto, T. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol. Behav. 2016, 155, 224–230. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Blough, J.; Crawford, L.; Ryu, S.; Zou, L.; Li, H. The Temporal Effects of Acute Exercise on Episodic Memory Function: Systematic Review with Meta-Analysis. Brain Sci. 2019, 9, 87. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Polich, J. Neuropsychology of P300. In The Oxford Handbook of Event-Related Potential Components; Oxford Library of Psychology, Oxford University Press: Oxford, UK, 2012; pp. 159–188. [Google Scholar]
- Pontifex, M.B.; Saliba, B.J.; Raine, L.B.; Picchietti, D.L.; Hillman, C.H. Exercise Improves Behavioral, Neurocognitive, and Scholastic Performance in Children with Attention-Deficit/Hyperactivity Disorder. J. Pediatr. 2013, 162, 543–551. [Google Scholar] [CrossRef]
- Rugg, M.D.; Curran, T. Event-related potentials and recognition memory. Trends Cogn. Sci. 2007, 11, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Curran, T. Brain potentials of recollection and familiarity. Mem. Cognit. 2000, 28, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Yonelinas, A.P. The Nature of Recollection and Familiarity: A Review of 30 Years of Research. J. Mem. Lang. 2002, 46, 441–517. [Google Scholar] [CrossRef]
- Leynes, P.A.; Bruett, H.; Krizan, J.; Veloso, A. What psychological process is reflected in the FN400 event-related potential component? Brain Cogn. 2017, 113, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.A.; Webster, C.A.; Beets, M.W.; Weaver, R.G.; Russ, L.; Michael, D.; Nesbitt, D.; Orendorff, K.L. Sedentary Time and Behavior during School: A Systematic Review and Meta-Analysis. Am. J. Health Educ. 2019, 50, 283–290. [Google Scholar] [CrossRef]
- Yli-Piipari, S.; Kulmala, J.S.; Jaakkola, T.; Hakonen, H.; Fish, J.C.; Tammelin, T. Objectively Measured School Day Physical Activity Among Elementary Students in the United States and Finland. J. Phys. Act. Health 2016, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M. Growth at Adolescence. Med. J. Aust. 1962, 2, 629. [Google Scholar] [CrossRef]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can. J. Sport. Sci. 1992, 17, 338–345. [Google Scholar] [PubMed]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. Experiments in behavior made easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Coltheart, M. The MRC Psycholinguistic Database. Q. J. Exp. Psychol. Sect. A. 1981, 33, 497–505. [Google Scholar] [CrossRef]
- Wilson, M. MRC psycholinguistic database: Machine usable dictionary. Behav. Res. Methods Instrum. Comput. 1988, 20, 6–10. [Google Scholar] [CrossRef]
- Chatrian, G.E.; Lettich, E.; Nelson, P.L. Ten Percent Electrode System for Topographic Studies of Spontaneous and Evoked EEG Activities. Am. J. EEG Technol. 1985, 25, 83–92. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Lopez-Calderon, J.; Luck, S.J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, S.H.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic EEG Artifact Removal. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 1242–1245. [Google Scholar] [CrossRef]
- Kothe, C.A.; Makeig, S. BCILAB: A platform for brain–computer interface development. J. Neural Eng. 2013, 10, 056014. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Miskovic, V.; Laszlo, S. Evaluating the efficacy of fully automated approaches for the selection of eyeblink ICA components. Psychophysiology 2017, 54, 780–791. [Google Scholar] [CrossRef]
- Davies, P.L.; Segalowitz, S.J.; Gavin, W.J. Development of Response-Monitoring ERPs in 7- to 25-Year-Olds. Dev. Neuropsychol. 2004, 25, 355–376. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.-C.; Chen, F.-T.; Moreau, D.; Drollette, E.S.; Amireault, S.; Chu, C.-H.; Chang, Y.K. Acute effects of exercise engagement on neurocognitive function: A systematic review and meta-analysis on P3 amplitude and latency. Int. Rev. Sport. Exerc. Psychol. 2022, 1–43. [Google Scholar] [CrossRef]
- Meijer, A.; Königs, M.; Vermeulen, G.T.; Visscher, C.; Bosker, R.J.; Hartman, E.; Oosterlaan, J. The effects of physical activity on brain structure and neurophysiological functioning in children: A systematic review and meta-analysis. Dev. Cogn. Neurosci. 2020, 45, 100828. [Google Scholar] [CrossRef] [PubMed]
- Drollette, E.S.; Johnson, M.N.; Meadows, C.C. No Change in Inhibitory Control or P3 Following Different High-Intensity Interval Exercise Modalities. Brain Sci. 2022, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.C.; Drollette, E.S.; Ritondale, J.P.; Khan, N.; Hillman, C.H. The acute effects of high-intensity interval training and moderate-intensity continuous exercise on declarative memory and inhibitory control. Psychol. Sport. Exerc. 2018, 38, 90–99. [Google Scholar] [CrossRef]
- Gur, R.C.; Jaggi, J.L.; Ragland, J.D.; Resnick, S.M.; Shtasel, D.; Muenz, L.; Gur, R.E. Effects of Memory Processing on Regional Brain Activation: Cerebral Blood Flow in Normal Subjects. Int. J. Neurosci. 1993, 72, 31–44. [Google Scholar] [CrossRef]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Krueger, K.; Fromme, A.; Korsukewitz, C.; Floel, A.; et al. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Ponce, P.; Frith, E. Hypothesized mechanisms through which acute exercise influences episodic memory. Acta Physiol. Int. 2018, 105, 285–297. [Google Scholar] [CrossRef]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef]
- Chowdhury, R.; Guitart-Masip, M.; Bunzeck, N.; Dolan, R.J.; Düzel, E. Dopamine Modulates Episodic Memory Persistence in Old Age. J. Neurosci. 2012, 32, 14193–14204. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The Role of Acetylcholine in Learning and Memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Mlinar, B.; Stocca, G.; Corradetti, R. Endogenous serotonin facilitates hippocampal long-term potentiation at CA3/CA1 synapses. J. Neural Transm. 2015, 122, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Stanton, P.K.; Sarvey, J.M. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J. Neurosci. Off. J. Soc. Neurosci. 1985, 5, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Real, C.C.; Ferreira, A.F.B.; Hernandes, M.S.; Britto, L.R.G.; Pires, R.S. Exercise-induced plasticity of AMPA-type glutamate receptor subunits in the rat brain. Brain Res. 2010, 1363, 63–71. [Google Scholar] [CrossRef]
- VanLeeuwen, J.E.; Petzinger, G.M.; Walsh, J.P.; Akopian, G.K.; Vuckovic, M.; Jakowec, M.W. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. Res. 2010, 88, 650–668. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, X.; Wang, J.; Li, Y. Effect of exercise training on long-term potentiation and NMDA receptor channels in rats with cerebral infarction. Exp. Ther. Med. 2013, 6, 1431–1436. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Loenneke, J.P.; Storm, B.C. Effects of acute aerobic and resistance exercise on episodic memory function. Q. J. Exp. Psychol. 2021, 74, 1264–1283. [Google Scholar] [CrossRef]
- Ishihara, T.; Drollette, E.S.; Ludyga, S.; Hillman, C.H.; Kamijo, K. Baseline Cognitive Performance Moderates the Effects of Physical Activity on Executive Functions in Children. J. Clin. Med. 2020, 9, 2071. [Google Scholar] [CrossRef]
- Johnson, M.N.; Maher, J.P.; Meadows, C.C.; Bittel, K.M.; Hevel, D.J.; Drollette, E.S. Positive affect moderates inhibitory control and positive affect following a single bout of self-select aerobic exercise. Psychol. Sport Exerc. 2022, 60, 102141. [Google Scholar] [CrossRef]


| Measures | Participants |
|---|---|
| N (females) | 36 (18) |
| Age (years) | 10.1 ± 1.1 |
| Puberty Timing | 1.9 ± 0.9 |
| BMI | 19.2 ± 3.6 |
| Fitness (mL/kg/min) | 37.6 ± 7.7 |
| Fitness percentile (%) | 42.2 ± 32.3 |
| IQ | 103.5 ± 11.7 |
| Maternal Education | |
| Advanced degree | 14 |
| Bachelor’s degree | 20 |
| Some college | 1 |
| Hispanic or Latino | |
| Yes | 7 |
| No | 28 |
| Race | |
| White or Caucasian | 25 |
| Black or African | 3 |
| Mixed | 4 |
| Asian | 2 |
| Asian, White, or Caucasian | 1 |
| Not reported | 1 |
| Model | F | df1/df2 | p | ηp2 |
|---|---|---|---|---|
| FN400 | ||||
| Frontal Region (F3, Fz, F4) | ||||
| Site (Old) | 7.58 | 1,35 | <0.01 | 0.18 |
| Site (New) | 5.17 | 1,35 | <0.02 | 0.13 |
| Central Region (C3, Cz, C4) | ||||
| Site (Old) | 6.43 | 1,35 | <0.01 | 0.15 |
| Parietal Region (P3, Pz, P4) | ||||
| Site (Old) | 13.15 | 1,35 | <0.01 | 0.27 |
| Site (New) | 12.10 | 1,35 | <0.01 | 0.25 |
| Mode (Difference) | 5.24 | 1,35 | <0.01 | 0.13 |
| Model | F | df1/df2 | p | ηp2 |
|---|---|---|---|---|
| LPC | ||||
| Frontal Region (F3, Fz, F4) | ||||
| Mode (Old) | 5.19 | 1,35 | <0.04 | 0.13 |
| Site (Old) | 13.94 | 1,35 | <0.01 | 0.28 |
| Central Region (C3, Cz, C4) | ||||
| Site (Old) | 7.13 | 1,35 | <0.01 | 0.17 |
| Mode (New) | 4.54 | 1,35 | <0.01 | 0.11 |
| Parietal Region (P3, Pz, P4) | ||||
| Site (Old) | 11.92 | 1,35 | <0.01 | 0.25 |
| Site (New) | 4.99 | 1,35 | <0.01 | 0.12 |
| Site (Difference) | 4.54 | 1,35 | <0.05 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drollette, E.S.; Pasupathi, P.A.; Slutsky-Ganesh, A.B.; Etnier, J.L. Take a Break for Memory Sake! Effects of Short Physical Activity Breaks on Inhibitory Control, Episodic Memory, and Event-Related Potentials in Children. Brain Sci. 2024, 14, 626. https://doi.org/10.3390/brainsci14070626
Drollette ES, Pasupathi PA, Slutsky-Ganesh AB, Etnier JL. Take a Break for Memory Sake! Effects of Short Physical Activity Breaks on Inhibitory Control, Episodic Memory, and Event-Related Potentials in Children. Brain Sciences. 2024; 14(7):626. https://doi.org/10.3390/brainsci14070626
Chicago/Turabian StyleDrollette, Eric S., Praveen A. Pasupathi, Alexis B. Slutsky-Ganesh, and Jennifer L. Etnier. 2024. "Take a Break for Memory Sake! Effects of Short Physical Activity Breaks on Inhibitory Control, Episodic Memory, and Event-Related Potentials in Children" Brain Sciences 14, no. 7: 626. https://doi.org/10.3390/brainsci14070626





