The Contribution of Cognitive Control Networks in Word Selection Processing in Parkinson’s Disease: Novel Insights from a Functional Connectivity Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Paradigm: Languange Tasks
2.3. MRI Acquisition Processing and Modeling
2.4. Statistical Analyses
3. Results
3.1. Demographic, Clinical, and Neuropsychological Characterization of the Samples
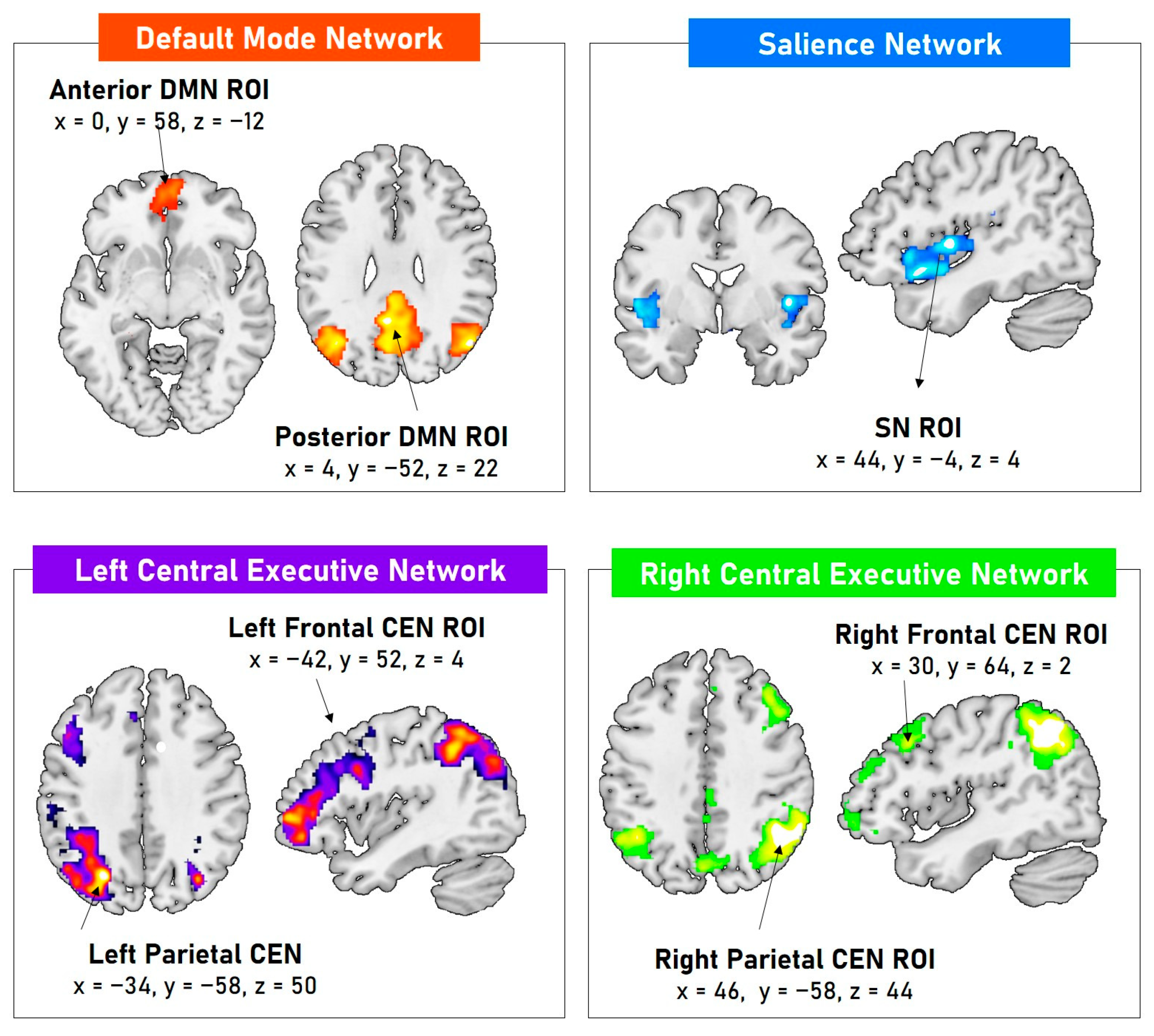
3.2. Resting-State Functional ROIs
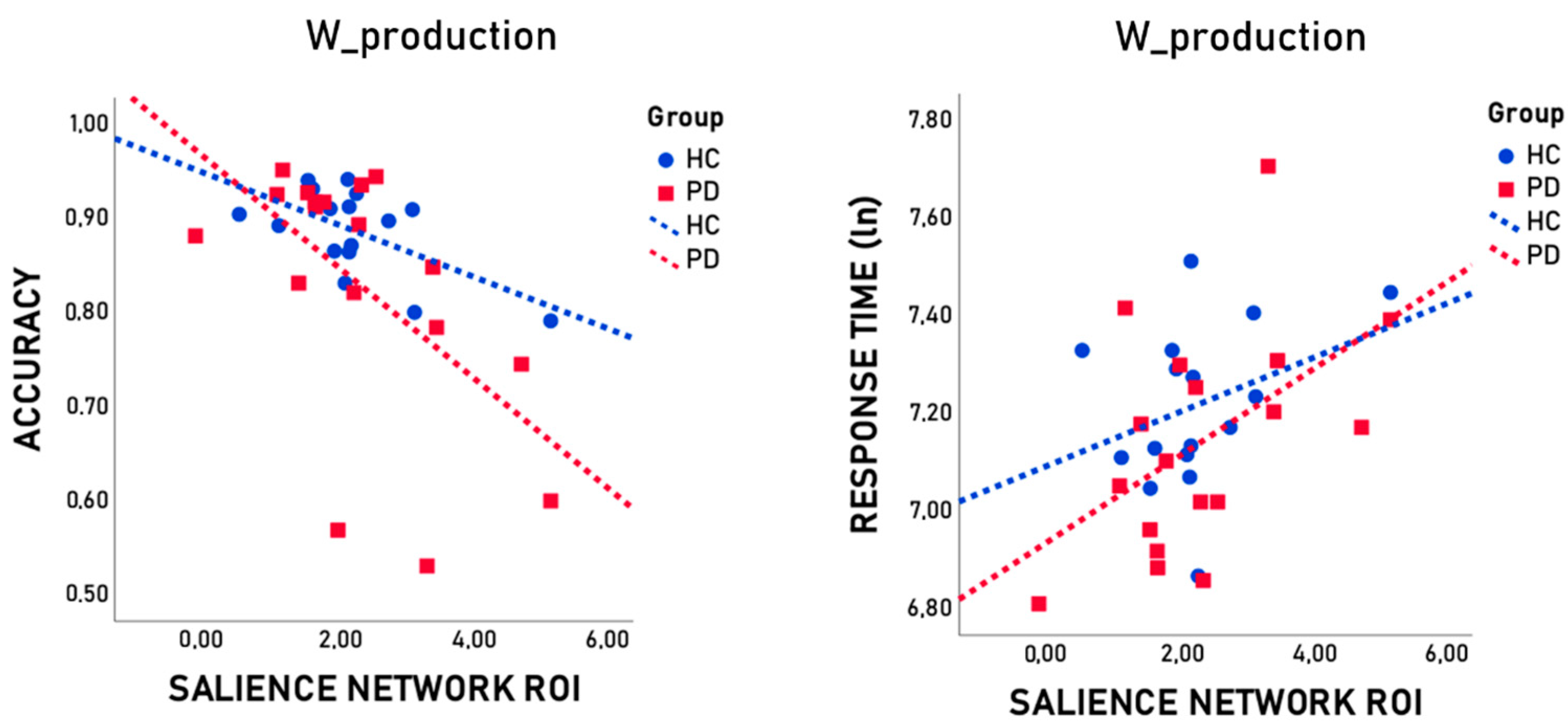
3.3. Association between Language Task Performance and Resting-State Functional ROIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anzuino, I.; Baglio, F.; Pelizzari, L.; Cabinio, M.; Biassoni, F.; Gnerre, M.; Blasi, V.; Silveri, M.C.; Di Tella, S. Production of emotions conveyed by voice in Parkinson’s disease: Association between variability of fundamental frequency and gray matter volumes of regions involved in emotional prosody. Neuropsychology 2023, 37, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Di Tella, S.; Anzuino, I.; Biassoni, F.; Ciceri, M.R.; Gnerre, M.; Nemni, R.; Cabinio, M.; Baglio, F.; Silveri, M.C. The role of the dorsal striatum in the recognition of emotions expressed by voice in Parkinson’s disease. Neurol. Sci. 2021, 42, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Piretti, L.; Di Tella, S.; Lo Monaco, M.R.; Delle Donne, V.; Rumiati, R.I.; Silveri, M.C. Impaired processing of conspecifics in Parkinson’s disease. Appl. Neuropsychol. Adult 2024, 31, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Trojano, L.; Papagno, C. Cognitive and behavioral disorders in Parkinson’s disease: An update. II: Behavioral disorders. Neurol. Sci. 2018, 39, 53–61. [Google Scholar] [CrossRef]
- Yu, R.L.; Wu, R.M. Mild cognitive impairment in patients with Parkinson’s disease: An updated mini-review and future outlook. Front. Aging Neurosci. 2022, 14, 943438. [Google Scholar] [CrossRef]
- Liu, R.; Umbach, D.M.; Tröster, A.I.; Huang, X.; Chen, H. Non-motor symptoms and striatal dopamine transporter binding in early Parkinson’s disease. Park. Relat. Disord. 2020, 72, 23–30. [Google Scholar] [CrossRef]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef]
- Cotelli, M.; Borroni, B.; Manenti, R.; Alberici, A.; Calabria, M.; Agosti, C.; Arévalo, A.; Ginex, V.; Ortelli, P.; Binetti, G.; et al. Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology 2006, 20, 558–565. [Google Scholar] [CrossRef]
- Silveri, M.C.; Ciccarelli, N.; Baldonero, E.; Piano, C.; Zinno, M.; Soleti, F.; Bentivoglio, A.R.; Albanese, A.; Daniele, A. Effects of stimulation of the subthalamic nucleus on naming and reading nouns and verbs in Parkinson’s disease. Neuropsychologia 2012, 50, 1980–1989. [Google Scholar] [CrossRef]
- Silveri, M.C.; Traficante, D.; Lo Monaco, M.R.; Iori, L.; Sarchioni, F.; Burani, C. Word selection processing in Parkinson’s disease: When nouns are more difficult than verbs. Cortex 2018, 100, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Di Tella, S.; Baglio, F.; Cabinio, M.; Nemni, R.; Traficante, D.; Silveri, M.C. Selection Processing in Noun and Verb Production in Left- and Right-Sided Parkinson’s Disease Patients. Front. Psychol. 2018, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Di Tella, S.; Baglio, F.; Pelizzari, L.; Cabinio, M.; Nemni, R.; Traficante, D.; Silveri, M.C. Uncinate fasciculus and word selection processing in Parkinson’s disease. Neuropsychologia 2020, 146, 107504. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 2007, 56, 171–184. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Nguyen, M.; Hasson, U. The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 2021, 22, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Bonnelle, V.; Ham, T.E.; Leech, R.; Kinnunen, K.M.; Mehta, M.A.; Greenwood, R.J.; Sharp, D.J. Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. USA 2012, 109, 4690–4695. [Google Scholar] [CrossRef] [PubMed]
- Ham, T.E.; de Boissezon, X.; Leff, A.; Beckmann, C.; Hughes, E.; Kinnunen, K.M.; Leech, R.; Sharp, D.J. Distinct frontal networks are involved in adapting to internally and externally signaled errors. Cereb. Cortex 2013, 23, 703–713. [Google Scholar] [CrossRef]
- Kelly, A.M.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between functional brain networks mediates behavioral variability. NeuroImage 2008, 39, 527–537. [Google Scholar] [CrossRef]
- Weissman, D.H.; Roberts, K.C.; Visscher, K.M.; Woldorff, M.G. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006, 9, 971–978. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Di Tella, S.; De Marco, M.; Baglio, F.; Silveri, M.C.; Venneri, A. Resting-state functional connectivity is modulated by cognitive reserve in early Parkinson’s disease. Front. Psychol. 2023, 14, 1207988. [Google Scholar] [CrossRef]
- Christopher, L.; Koshimori, Y.; Lang, A.E.; Criaud, M.; Strafella, A.P. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain 2014, 137, 2143–2154. [Google Scholar] [CrossRef]
- Christopher, L.; Marras, C.; Duff-Canning, S.; Koshimori, Y.; Chen, R.; Boileau, I.; Segura, B.; Monchi, O.; Lang, A.E.; Rusjan, P.; et al. Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson’s disease with mild cognitive impairment. Brain 2014, 137, 565–575. [Google Scholar] [CrossRef]
- Wolters, A.F.; van de Weijer, S.C.F.; Leentjens, A.F.G.; Duits, A.A.; Jacobs, H.I.L.; Kuijf, M.L. Resting-state fMRI in Parkinson’s disease patients with cognitive impairment: A meta-analysis. Park. Relat. Disord. 2019, 62, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Esposito, F.; Vitale, C.; Santangelo, G.; Amboni, M.; Russo, A.; Corbo, D.; Cirillo, G.; Barone, P.; Tedeschi, G. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 2012, 79, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Dove, A.; Robbins, T.W.; Barker, R.A.; Owen, A.M. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J. Neurosci. 2003, 23, 6351–6356. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, S.P.; Siri, C.; Guidi, L.; Antonini, A.; Perani, D. The neural correlates of spatial and object working memory in elderly and Parkinson’s disease subjects. Behav. Neurol. 2015, 2015, 123636. [Google Scholar] [CrossRef]
- Cascone, A.D.; Langella, S.; Sklerov, M. Frontoparietal network resilience is associated with protection against cognitive decline in Parkinson’s disease. Commun. Biol. 2021, 4, 1021. [Google Scholar] [CrossRef]
- Amboni, M.; Tessitore, A.; Esposito, F.; Santangelo, G.; Picillo, M.; Vitale, C.; Giordano, A.; Erro, R.; de Micco, R.; Corbo, D.; et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J. Neurol. 2015, 262, 425–434. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Jilka, S.R.; Scott, G.; Ham, T.; Pickering, A.; Bonnelle, V.; Braga, R.M.; Leech, R.; Sharp, D.J. Damage to the Salience Network and interactions with the Default Mode Network. J. Neurosci. 2014, 34, 10798–10807. [Google Scholar] [CrossRef]
- Yeager, B.E.; Twedt, H.P.; Bruss, J.; Schultz, J.; Narayanan, N.S. Cortical and subcortical functional connectivity and cognitive impairment in Parkinson’s disease. NeuroImage Clin. 2024, 42, 103610. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Marangolo, P.; Piras, F.; Galati, G.; Burani, C. Functional anatomy of derivational morphology. Cortex 2006, 42, 1093–1106. [Google Scholar] [CrossRef]
- Bertinetto, P.M.; Burani, C.; Laudanna, A.; Marconi, L.; Ratti, D.; Rolando, C.; Thornton, A.M. Corpus e Lessico di Frequenza dell’Italiano Scritto (CoLFIS). 2005. Available online: https://linguistica.sns.it/CoLFIS/Home.htm (accessed on 1 August 2024).
- Song, X.W.; Dong, Z.Y.; Long, X.Y.; Li, S.F.; Zuo, X.N.; Zhu, C.Z.; He, Y.; Yan, C.G.; Zang, Y.F. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 2011, 6, e25031. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Adali, T.; Pearlson, G.D.; Pekar, J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001, 14, 140–151. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Kalcher, K.; Huf, W.; Boubela, R.N.; Filzmoser, P.; Pezawas, L.; Biswal, B.; Kasper, S.; Moser, E.; Windischberger, C. Fully exploratory network independent component analysis of the 1000 functional connectomes database. Front. Hum. Neurosci. 2012, 6, 301. [Google Scholar] [CrossRef]
- Tessitore, A.; Giordano, A.; De Micco, R.; Russo, A.; Tedeschi, G. Sensorimotor connectivity in Parkinson’s disease: The role of functional neuroimaging. Front. Neurol. 2014, 5, 180. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.-L.; Valabregue, R.; Poline, J.-B. Region of interest analysis using an SPM toolbox. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, 2–6 June 2002; p. 497. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Martínez-Martín, P.; Rodríguez-Blázquez, C.; Mario, A.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Martínez-Castrillo, J.C.; Rodríguez-Violante, M.; et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Park. Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. The cognit: A network model of cortical representation. Int. J. Psychophysiol. 2006, 60, 125–132. [Google Scholar] [CrossRef]
- Bocanegra, Y.; García, A.M.; Pineda, D.; Buriticá, O.; Villegas, A.; Lopera, F.; Gómez, D.; Gómez-Arias, C.; Cardona, J.F.; Trujillo, N.; et al. Syntax, action verbs, action semantics, and object semantics in Parkinson’s disease: Dissociability, progression, and executive influences. Cortex 2015, 69, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Crescentini, C.; Mondolo, F.; Biasutti, E.; Shallice, T. Supervisory and routine processes in noun and verb generation in nondemented patients with Parkinson’s disease. Neuropsychologia 2008, 46, 434–447. [Google Scholar] [CrossRef]
- Hammers, A.; Allom, R.; Koepp, M.J.; Free, S.L.; Myers, R.; Lemieux, L.; Mitchell, T.N.; Brooks, D.J.; Duncan, J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003, 19, 224–247. [Google Scholar] [CrossRef]
- Gousias, I.S.; Rueckert, D.; Heckemann, R.A.; Dyet, L.E.; Boardman, J.P.; Edwards, A.D.; Hammers, A. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. NeuroImage 2008, 40, 672–684. [Google Scholar] [CrossRef]
- Faillenot, I.; Heckemann, R.A.; Frot, M.; Hammers, A. Macroanatomy and 3D probabilistic atlas of the human insula. NeuroImage 2017, 150, 88–98. [Google Scholar] [CrossRef]
- Kurth, F.; Zilles, K.; Fox, P.T.; Laird, A.R.; Eickhoff, S.B. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010, 214, 519–534. [Google Scholar] [CrossRef]
- Molnar-Szakacs, I.; Uddin, L.Q. Anterior insula as a gatekeeper of executive control. Neurosci. Biobehav. Rev. 2022, 139, 104736. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.T.; Price, J.L. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1996, 371, 179–207. [Google Scholar] [CrossRef]
- Robbins, T.W. Dissociating executive functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1996, 351, 1463–1470; discussion 1470–1471. [Google Scholar] [CrossRef]
- Fathy, Y.Y.; Hepp, D.H.; de Jong, F.J.; Geurts, J.J.G.; Foncke, E.M.J.; Berendse, H.W.; van de Berg, W.D.J.; Schoonheim, M.M. Anterior insular network disconnection and cognitive impairment in Parkinson’s disease. NeuroImage Clin. 2020, 28, 102364. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 2016, 42, 33–50. [Google Scholar] [CrossRef]
- Boon, L.I.; Hepp, D.H.; Douw, L.; van Geenen, N.; Broeders, T.A.A.; Geurts, J.J.G.; Berendse, H.W.; Schoonheim, M.M. Functional connectivity between resting-state networks reflects decline in executive function in Parkinson’s disease: A longitudinal fMRI study. NeuroImage Clin. 2020, 28, 102468. [Google Scholar] [CrossRef]
- Prenger, M.T.M.; Madray, R.; Van Hedger, K.; Anello, M.; MacDonald, P.A. Social Symptoms of Parkinson’s Disease. Park. Dis. 2020, 2020, 8846544. [Google Scholar] [CrossRef]
- Meloni, M.; Saibene, F.L.; Di Tella, S.; Di Cesare, M.; Borgnis, F.; Nemni, R.; Baglio, F. Functional and Cognitive Improvement After an Intensive Inpatient Multidisciplinary Rehabilitation Program in Mild to Severe Parkinson’s Disease: A Retrospective and Observational Study. Front. Neurol. 2021, 12, 626041. [Google Scholar] [CrossRef]
- Frodl, T.; Scheuerecker, J.; Schoepf, V.; Linn, J.; Koutsouleris, N.; Bokde, A.L.; Hampel, H.; Möller, H.J.; Brückmann, H.; Wiesmann, M.; et al. Different effects of mirtazapine and venlafaxine on brain activation: An open randomized controlled fMRI study. J. Clin. Psychiatry 2011, 72, 448–457. [Google Scholar] [CrossRef]
- Chen, Y.; Pressman, P.; Simuni, T.; Parrish, T.B.; Gitelman, D.R. Effects of acute levodopa challenge on resting cerebral blood flow in Parkinson’s Disease patients assessed using pseudo-continuous arterial spin labeling. PeerJ 2015, 3, e1381. [Google Scholar] [CrossRef]
- Lin, W.C.; Chen, P.C.; Huang, Y.C.; Tsai, N.W.; Chen, H.L.; Wang, H.C.; Lin, T.K.; Chou, K.H.; Chen, M.H.; Chen, Y.W.; et al. Dopaminergic Therapy Modulates Cortical Perfusion in Parkinson Disease With and Without Dementia According to Arterial Spin Labeled Perfusion Magnetic Resonance Imaging. Medicine 2016, 95, e2206. [Google Scholar] [CrossRef] [PubMed]
- Shima, A.; Inano, R.; Tabu, H.; Okada, T.; Nakamoto, Y.; Takahashi, R.; Sawamoto, N. Altered functional connectivity associated with striatal dopamine depletion in Parkinson’s disease. Cereb. Cortex Commun. 2023, 4, tgad004. [Google Scholar] [CrossRef] [PubMed]
- Boulenger, V.; Mechtouff, L.; Thobois, S.; Broussolle, E.; Jeannerod, M.; Nazir, T.A. Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia 2008, 46, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ferreiro, J.; Menéndez, M.; Ribacoba, R.; Cuetos, F. Action naming is impaired in Parkinson disease patients. Neuropsychologia 2009, 47, 3271–3274. [Google Scholar] [CrossRef]
- Cardona, J.F.; Kargieman, L.; Sinay, V.; Gershanik, O.; Gelormini, C.; Amoruso, L.; Roca, M.; Pineda, D.; Trujillo, N.; Michon, M.; et al. How embodied is action language? Neurological evidence from motor diseases. Cognition 2014, 131, 311–322. [Google Scholar] [CrossRef]
- Barsalou, L.W. Perceptual symbol systems. Behav. Brain Sci. 1999, 22, 577–609; discussion 610–660. [Google Scholar] [CrossRef]
- Price, C.J.; Moore, C.J.; Humphreys, G.W.; Wise, R.J. Segregating Semantic from Phonological Processes during Reading. J. Cogn. Neurosci. 1997, 9, 727–733. [Google Scholar] [CrossRef]
| Demographical and Clinical Data | HC [n = 16] | PD [n = 18] | Group Comparison p-Value |
|---|---|---|---|
| Age [Mean ± SD] years | 65.13 ± 7.53 | 66.83 ± 7.37 | 0.509 # |
| Education [Mean ± SD] | 13.56 ± 3.90 | 12.72 ± 4.01 | 0.541 ° |
| Sex M/F [n (%)] | 9(56.3%)/7(43.7%) | 9(50%)/9(50%) | 0.716 § |
| MoCA [Mean ± SD] | 26.20 ± 2.78 | 23.27 ± 3.36 | 0.010 # |
| UPDRS—motor part III [Mean ± SD] | 21.72 ± 9.43 | ||
| Disease duration [Mean ± SD] | 38.22 ± 29.55 | ||
| LEDD [Mean ± SD] | 274.20 ± 208.30 |
| Partial Correlations | Accuracy | lnRTs | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | V_from_N | N_from_V | W_Production | V_from_N | N_from_V | W_Production | ||
| HC | Anterior DMN ROI | r | −0.059 | 0.081 | 0.079 | 0.367 | 0.299 | 0.342 |
| p | 0.849 | 0.792 | 0.797 | 0.217 | 0.321 | 0.253 | ||
| pFDR | 0.849 | 0.849 | 0.849 | 0.642 | 0.642 | 0.642 | ||
| Posterior DMN ROI | r | −0.450 | 0.060 | −0.039 | −0.041 | −0.040 | −0.041 | |
| p | 0.123 | 0.846 | 0.900 | 0.895 | 0.897 | 0.895 | ||
| pFDR | 0.738 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | ||
| SN ROI | r | 0.117 | −0.544 | −0.410 | 0.216 | 0.195 | 0.211 | |
| p | 0.703 | 0.055 | 0.165 | 0.479 | 0.523 | 0.489 | ||
| pFDR | 0.703 | 0.330 | 0.495 | 0.628 | 0.628 | 0.628 | ||
| Left Frontal CEN ROI | r | −0.359 | 0.204 | 0.071 | 0.124 | 0.021 | 0.073 | |
| p | 0.229 | 0.505 | 0.818 | 0.686 | 0.947 | 0.812 | ||
| pFDR | 0.947 | 0.947 | 0.947 | 0.947 | 0.947 | 0.947 | ||
| Left Parietal CEN ROI | r | −0.131 | 0.270 | 0.171 | −0.068 | −0.233 | −0.158 | |
| p | 0.669 | 0.372 | 0.577 | 0.826 | 0.444 | 0.607 | ||
| pFDR | 0.802 | 0.802 | 0.802 | 0.826 | 0.802 | 0.802 | ||
| Right Frontal CEN ROI | r | 0.215 | 0.227 | 0.311 | 0.064 | 0.024 | 0.045 | |
| p | 0.481 | 0.455 | 0.301 | 0.836 | 0.937 | 0.885 | ||
| pFDR | 0.937 | 0.937 | 0.937 | 0.937 | 0.937 | 0.937 | ||
| Right Parietal CEN ROI | r | 0.051 | 0.225 | 0.244 | 0.067 | −0.129 | −0.036 | |
| p | 0.869 | 0.459 | 0.422 | 0.829 | 0.676 | 0.907 | ||
| pFDR | 0.907 | 0.907 | 0.907 | 0.907 | 0.907 | 0.907 | ||
| SMN ROI | r | −0.313 | −0.120 | −0.206 | −0.475 | −0.465 | −0.485 | |
| p | 0.298 | 0.695 | 0.500 | 0.101 | 0.109 | 0.093 | ||
| pFDR | 0.447 | 0.695 | 0.600 | 0.218 | 0.218 | 0.218 | ||
| VN ROI | r | 0.226 | 0.183 | 0.172 | −0.216 | −0.415 | −0.331 | |
| p | 0.458 | 0.549 | 0.575 | 0.478 | 0.159 | 0.270 | ||
| pFDR | 0.575 | 0.575 | 0.575 | 0.575 | 0.575 | 0.575 | ||
| PD | Anterior DMN ROI | r | 0.002 | 0.467 | 0.339 | −0.169 | −0.274 | −0.235 |
| p | 0.995 | 0.079 | 0.216 | 0.548 | 0.323 | 0.399 | ||
| pFDR | 0.995 | 0.474 | 0.598 | 0.658 | 0.598 | 0.598 | ||
| Posterior DMN ROI | r | −0.187 | 0.229 | 0.107 | 0.021 | −0.082 | −0.036 | |
| p | 0.505 | 0.412 | 0.703 | 0.942 | 0.772 | 0.899 | ||
| pFDR | 0.942 | 0.942 | 0.942 | 0.942 | 0.942 | 0.942 | ||
| SN ROI | r | −0.668 | −0.661 | −0.747 | 0.677 | 0.579 | 0.653 | |
| p | 0.007 | 0.007 | 0.001 | 0.006 | 0.024 | 0.008 | ||
| pFDR | 0.010 | 0.010 | 0.006 | 0.010 | 0.024 | 0.010 | ||
| Left Frontal CEN ROI | r | 0.037 | 0.387 | 0.277 | −0.209 | −0.358 | −0.303 | |
| p | 0.895 | 0.155 | 0.317 | 0.454 | 0.190 | 0.273 | ||
| pFDR | 0.895 | 0.475 | 0.475 | 0.545 | 0.475 | 0.475 | ||
| Left Parietal CEN ROI | r | 0.054 | 0.207 | 0.156 | −0.392 | −0.285 | −0.350 | |
| p | 0.848 | 0.459 | 0.579 | 0.148 | 0.303 | 0.201 | ||
| pFDR | 0.848 | 0.688 | 0.695 | 0.603 | 0.606 | 0.603 | ||
| Right Frontal CEN ROI | r | 0.144 | 0.293 | 0.264 | −0.483 | −0.536 | −0.535 | |
| p | 0.610 | 0.289 | 0.342 | 0.068 | 0.039 | 0.040 | ||
| pFDR | 0.610 | 0.410 | 0.410 | 0.136 | 0.120 | 0.120 | ||
| Right Parietal CEN ROI | r | −0.113 | 0.401 | 0.298 | −0.179 | −0.479 | −0.357 | |
| p | 0.689 | 0.138 | 0.281 | 0.524 | 0.071 | 0.192 | ||
| pFDR | 0.689 | 0.384 | 0.421 | 0.629 | 0.384 | 0.384 | ||
| SMN ROI | r | −0.045 | −0.372 | −0.328 | −0.034 | 0.172 | 0.080 | |
| p | 0.875 | 0.172 | 0.232 | 0.904 | 0.539 | 0.776 | ||
| pFDR | 0.904 | 0.696 | 0.696 | 0.904 | 0.904 | 0.904 | ||
| VN ROI | r | −0.055 | 0.022 | −0.012 | 0.235 | −0.062 | 0.078 | |
| p | 0.845 | 0.939 | 0.967 | 0.400 | 0.826 | 0.782 | ||
| pFDR | 0.967 | 0.967 | 0.967 | 0.967 | 0.967 | 0.967 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Tella, S.; De Marco, M.; Anzuino, I.; Quaranta, D.; Baglio, F.; Silveri, M.C. The Contribution of Cognitive Control Networks in Word Selection Processing in Parkinson’s Disease: Novel Insights from a Functional Connectivity Study. Brain Sci. 2024, 14, 913. https://doi.org/10.3390/brainsci14090913
Di Tella S, De Marco M, Anzuino I, Quaranta D, Baglio F, Silveri MC. The Contribution of Cognitive Control Networks in Word Selection Processing in Parkinson’s Disease: Novel Insights from a Functional Connectivity Study. Brain Sciences. 2024; 14(9):913. https://doi.org/10.3390/brainsci14090913
Chicago/Turabian StyleDi Tella, Sonia, Matteo De Marco, Isabella Anzuino, Davide Quaranta, Francesca Baglio, and Maria Caterina Silveri. 2024. "The Contribution of Cognitive Control Networks in Word Selection Processing in Parkinson’s Disease: Novel Insights from a Functional Connectivity Study" Brain Sciences 14, no. 9: 913. https://doi.org/10.3390/brainsci14090913
APA StyleDi Tella, S., De Marco, M., Anzuino, I., Quaranta, D., Baglio, F., & Silveri, M. C. (2024). The Contribution of Cognitive Control Networks in Word Selection Processing in Parkinson’s Disease: Novel Insights from a Functional Connectivity Study. Brain Sciences, 14(9), 913. https://doi.org/10.3390/brainsci14090913






