Neonatal Markers of Prematurity as Predictors of Permanent Childhood Hearing Loss and Neurodevelopmental Impairment in Children Admitted to the Neonatal Intensive Care Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Cases
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Entry and Items
2.5. Statistical Analyses
3. Results
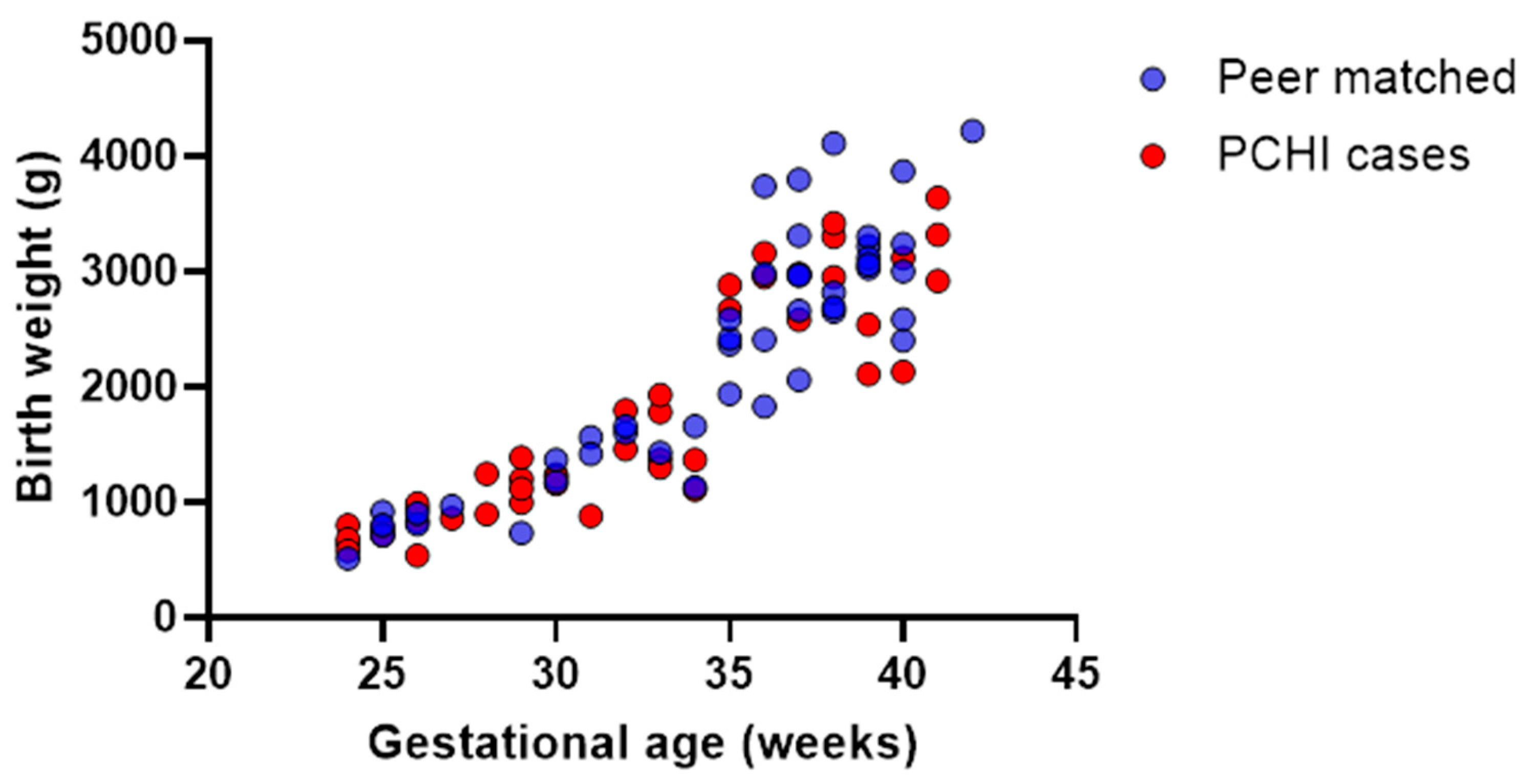
3.1. Demographics: Children with PCHI Were Significantly More Premature than Peer Controls and Spent More Time in the NICU
3.2. Time Spent in the NICU but Not Prematurity (z-Scores) Significantly Predicted PCHI
3.3. Children with PCHI Were Not All Born Premature or Small for Gestational Age
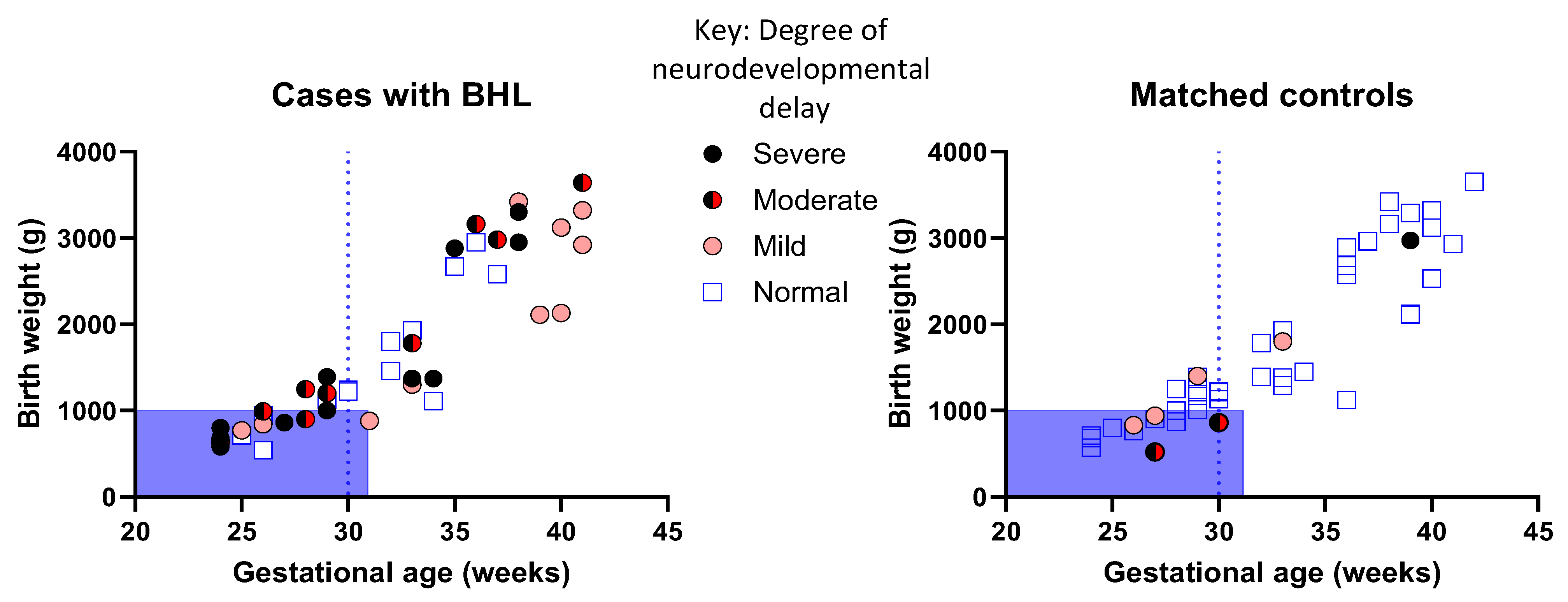
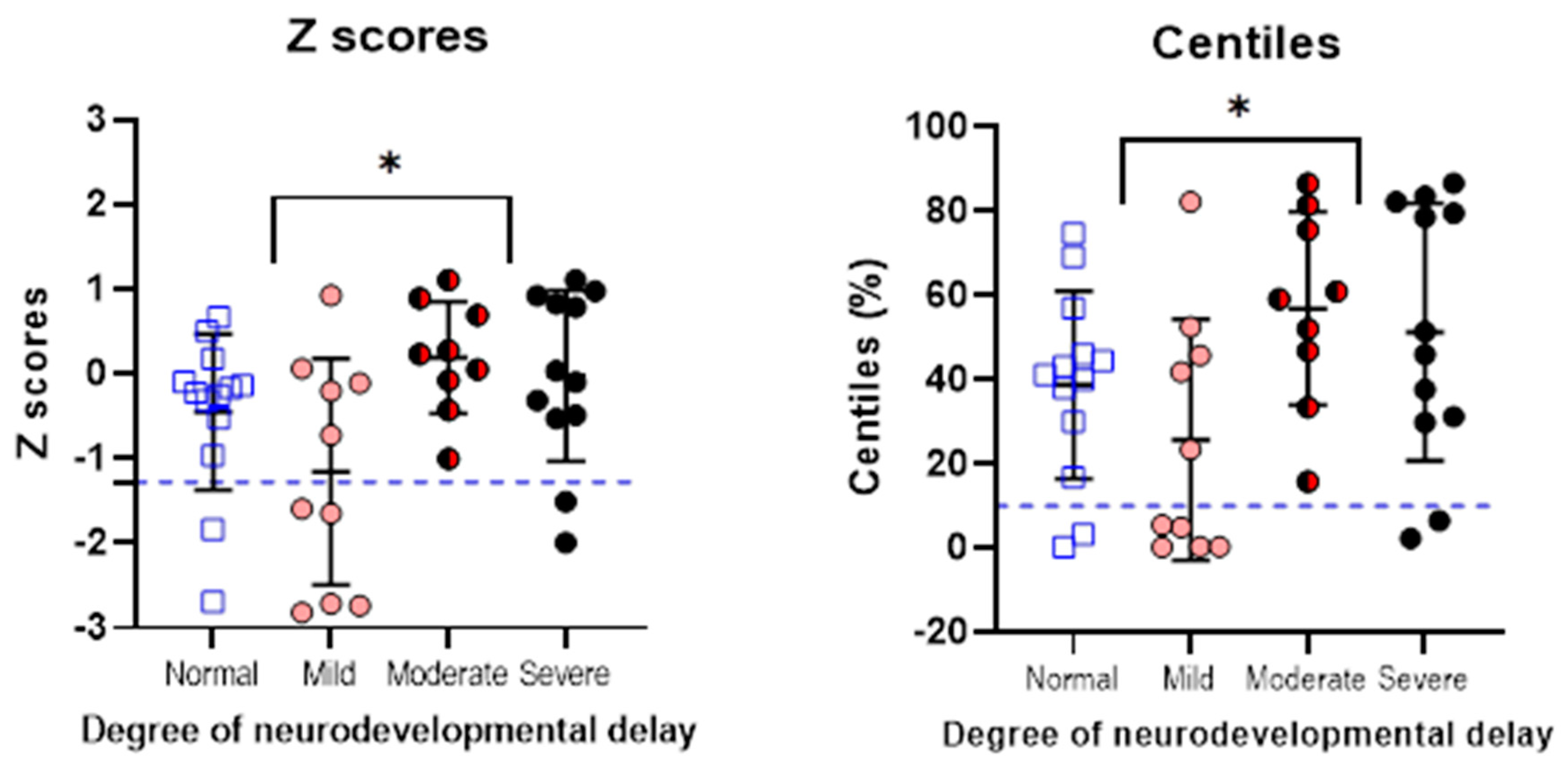
3.4. Children with PCHI Were More Likely to Have Moderate or Severe Neurodevelopmental Delay
3.5. Prematurity Is Not Prognostic of PCHI and Neurodevelopmental Outcomes in All Children with PCHI
3.6. Neurodevelopmental Outcomes Are Significantly Poorer in Children with PCHI Compared with Matched Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newsroom World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 2 February 2024).
- National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/hearing-loss-children/data/index.html (accessed on 15 May 2024).
- Korver, A.M.H.; Smith, R.J.H.; Van Camp, G.; Schleiss, M.R.; Bitner-Glindzicz, M.A.K.; Lustig, L.R.; Usami, S.-I.; Boudewyns, A.N. Congenital Hearing Loss HHS Public Access. Nat. Rev. Dis. Primers 2017, 3, 16094. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, M.; Ojala, R.; Korhonen, P.; Haataja, P.; Eriksson, K.; Gissler, M.; Luukkaala, T.; Tammela, O. Visual and Hearing Impairments after Preterm Birth. Pediatrics 2018, 142, e20173888. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Witte, J.; Hildmann, A.; Hennecke, K.H.; Schunck, K.U.; Maul, K.; Franke, U.; Fahnenstich, H.; Rabe, H.; Rossi, R.; et al. Neonatal Screening for Hearing Disorders in Infants at Risk: Incidence, Risk Factors, and Follow-Up. Pediatrics 1999, 104, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, V.; Marchand-Martin, L.; Marret, S.; Arnaud, C.; Benhammou, V.; Cambonie, G.; Debillon, T.; Dufourg, M.N.; Gire, C.; Goffinet, F.; et al. Neurodevelopmental Outcomes at Age 5 among Children Born Preterm: EPIPAGE-2 Cohort Study. BMJ 2021, 373, n741. [Google Scholar] [CrossRef] [PubMed]
- Marret, S.; Marchand-Martin, L.; Picaud, J.C.; Hascoët, J.M.; Arnaud, C.; Rozé, J.C.; Truffert, P.; Larroque, B.; Kaminski, M.; Ancel, P.Y. Brain Injury in Very Preterm Children and Neurosensory and Cognitive Disabilities during Childhood: The EPIPAGE Cohort Study. PLoS ONE 2013, 8, e62683. [Google Scholar] [CrossRef]
- Johnson, S.; Hennessy, E.; Smith, R.; Trikic, R.; Wolke, D.; Marlow, N. Academic Attainment and Special Educational Needs in Extremely Preterm Children at 11 Years of Age: The EPICure Study. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F283–F289. [Google Scholar] [CrossRef]
- Coenraad, S.; Goedegebure, A.; van Goudoever, J.B.; Hoeve, L.J. Risk Factors for Sensorineural Hearing Loss in NICU Infants Compared to Normal Hearing NICU Controls. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 999–1002. [Google Scholar] [CrossRef]
- Chant, K.; Bitner-Glindzicz, M.; Marlow, N. Cumulative Risk Factors Contributing to Hearing Loss in Preterm Infants. ADC Fetal Neonatal 2023, 108, 464–470. [Google Scholar] [CrossRef]
- Yates, A.M.; Jayasinghe, D.S.; Kitterick, P.T.; Thornton, S.K. Risk Factors for Hearing Loss for Babies Admitted to the Neonatal Intensive Care Unit. In Proceedings of the Fetal and Neonatal Neurology, Paris, France, 3–5 March 2021. Conference presentation. [Google Scholar]
- van Dommelen, P.; Mohangoo, A.D.; Verkerk, P.H.; van der Ploeg, C.P.; van Straaten, H.L.; Dutch NICU Neonatal Hearing Screening Working Group. Risk Indicators for Hearing Loss in Infants Treated in Different Neonatal Intensive Care Units. Acta Paediatr. 2010, 99, 344–349. [Google Scholar]
- Hille, E.T.M.; Van Straaten, H.L.M.; Verkerk, P.H.; Van Straaten, I.; Verkerk, P.; Hille, E.; Baerts, W.; Bunkers, C.; Smink, E.; Van Elburg, R.; et al. Prevalence and Independent Risk Factors for Hearing Loss in NICU Infants. Acta Paediatr. 2007, 96, 1155–1158. [Google Scholar] [CrossRef]
- Speleman, K.; Kneepkens, K.; Vandendriessche, K.; Debruyne, F.; Desloovere, C. Prevalence of Risk Factors for Sensorineural Hearing Loss in NICU Newborns. B-ENT 2012, 8, 1–6. [Google Scholar] [PubMed]
- Morimoto, N.; Taiji, H.; Tsukamoto, K.; Morimoto, Y.; Nakamura, T.; Hommura, T.; Ito, Y. Risk Factors for Elevation of ABR Threshold in NICU-Treated Infants. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, S.; Song, Y.; Sun, J.; Yan, W.; Wang, J.; Gao, X.; Li, X.; Ren, C.; Zhao, Q. Risk Factors for Infant Hearing Loss: A Meta-Analysis. Eur. J. Pediatr. 2024, 183, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Brandström, A.; Skenbäck, S.; Duan, M.; Uhlén, I. Risk Factors and Etiology of Childhood Hearing Loss: A Cohort Review of 296 Subjects. Acta Otolaryngol. 2020, 140, 668–674. [Google Scholar] [CrossRef]
- Howell, J.B.; Appelbaum, E.N.; Armstrong, M.F.; Chapman, D.; Dodson, K.M. An Analysis of Risk Factors in Unilateral versus Bilateral Hearing Loss. Ear Nose Throat J. 2019, 98, 330–333. [Google Scholar] [CrossRef]
- Bates, D.E.; Beaumont, S.J.; Baylis, B.W. Ototoxicity Induced by Gentamicin and Furosemide. Ann. Pharmacother. 2002, 36, 446–451. [Google Scholar] [CrossRef]
- Garinis, A.C.; Kemph, A.; Tharpe, A.M.; Weitkamp, J.H.; McEvoy, C.; Steyger, P.S. Monitoring Neonates for Ototoxicity. Int. J. Audiol. 2018, 57, S41–S48. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal Outcomes of Extremely Preterm Infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443. [Google Scholar] [CrossRef]
- Martínez-Cruz, C.F.; García Alonso-Themann, P.; Poblano, A.; Ochoa-López, J.M. Hearing Loss, Auditory Neuropathy, and Neurological Co-Morbidity in Children with Birthweight < 750 g. Arch. Med. Res. 2012, 43, 457–463. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Z.D. Brainstem Auditory Abnormality in Extremely Premature Babies and the Impact of Neonatal Bronchopulmonary Dysplasia. Acta Obstet. Gynecol. Scand. 2018, 97, 545–551. [Google Scholar] [CrossRef]
- Van Dommelen, P.; Verkerk, P.H.; Van Straaten, H.L.M.; Baerts, W.; Von Weissenbruch, M.; Duijsters, C.; Van Kaam, A.; Steiner, K.; De Vries, L.S.; Swarte, R.; et al. Hearing Loss by Week of Gestation and Birth Weight in Very Preterm Neonates. J. Pediatr. 2015, 166, 840–843.e1. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.; Foulon, I.; Van den Houte, K.; Heuninck, E.; Van Overmeire, B.; Gordts, F.; Topsakal, V. Analysis of Congenital Hearing Loss after Neonatal Hearing Screening. Front. Pediatr. 2023, 11, 1153123. [Google Scholar] [CrossRef]
- AL-Harbi, M.; Barakat, N.; AL-Khandary, M. Hearing Screening in at Risk Newborn. J. Med. Sci. 2008, 8, 648–653. [Google Scholar] [CrossRef]
- Lieu, J.E.C.; Kenna, M.; Anne, S.; Davidson, L. Hearing Loss in Children A Review. JAMA 2020, 324, 2195–2205. [Google Scholar] [CrossRef]
- Lieu, J.E.C. Permanent Unilateral Hearing Loss (UHL) and Childhood Development. Curr. Otorhinolaryngol. Rep. 2018, 6, 74–81. [Google Scholar] [CrossRef]
- Netten, A.P.; Rieffe, C.; Theunissen, S.C.P.M.; Soede, W.; Dirks, E.; Korver, A.M.H.; Konings, S.; Oudesluys-Murphy, A.M.; Dekker, F.W.; Frijns, J.H.M. Early Identification: Language Skills and Social Functioning in Deaf and Hard of Hearing Preschool Children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2221–2226. [Google Scholar] [CrossRef]
- June Holstrum, W.; Gaffney, M.; Gravel, J.S.; Oyler, R.F.; Ross, D.S. Early Intervention for Children with Unilateral and Mild Bilateral Degrees of Hearing Loss. Trends Amplif. 2008, 12, 35–41. [Google Scholar] [CrossRef]
- Ching, T.Y.C. Is Early Intervention Effective in Improving Spoken Language Outcomes of Children with Congenital Hearing Loss? Am. J. Audiol. 2015, 24, 345–348. [Google Scholar] [CrossRef]
- Horrocks, L.M.; Kitterick, P.T.; Jayasinghe, D.S.; Willis, K.R.; Martin, K.R.M.; Dixit, A.; Thornton, S.K. Multiple Congenital Anomalies and Adverse Developmental Outcomes Are Associated with Neonatal Intensive Care Admission and Unilateral Hearing Loss. Front. Pediatr. 2022, 10, 1068884. [Google Scholar] [CrossRef]
- Holden-Pitt, L.; Diaz, J.A. Thirty Years of the Annual Survey of Deaf and Hard-of-Hearing Children & Youth: A Glance over the Decades. Am. Ann. Deaf 1998, 143, 71–76. [Google Scholar] [CrossRef]
- Holseth, K.; Mattson, T.S. Children with Congenital Hearing Loss—A Vulnerable Group. Tidsskr. Nor. Legeforening 2019, 139. [Google Scholar] [CrossRef]
- Ding, S.; Lemyre, B.; Daboval, T.; Barrowman, N.; Moore, G.P. A Meta-Analysis of Neurodevelopmental Outcomes at 4–10 Years in Children Born at 22–25 Weeks Gestation. Acta Paediatr. 2019, 108, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.M.; Pedersen, M.V.; Milidou, I.; Glavind, J.; Henriksen, T.B. Long-Term Cognition and Behavior in Children Born at Early Term Gestation: A Systematic Review. Acta Obstet. Gynecol. Scand. 2019, 98, 1227–1234. [Google Scholar] [CrossRef]
- Pascal, A.; Govaert, P.; Oostra, A.; Naulaers, G.; Ortibus, E.; Van den Broeck, C. Neurodevelopmental Outcome in Very Preterm and Very-Low-Birthweight Infants Born over the Past Decade: A Meta-Analytic Review. Dev. Med. Child Neurol. 2018, 60, 342–355. [Google Scholar] [CrossRef]
- Wood, S.A.; Sutton, G.J.; Davis, A.C. International Journal of Audiology Performance and Characteristics of the Newborn Hearing Screening Programme in England: The First Seven Years. Int. J. Audiol. 2015, 54, 353–358. [Google Scholar] [CrossRef]
- Hemmingsen, D.; Moster, D.; Engdahl, B.L.; Klingenberg, C. Sensorineural Hearing Impairment among Preterm Children: A Norwegian Population-Based Study. Arch. Dis. Child. Fetal Neonatal Ed. 2024, F1–F7. [Google Scholar] [CrossRef]
- Neumann, K.; Mathmann, P.; Chadha, S.; Euler, H.A.; White, K.R. Newborn Hearing Screening Benefits Children, but Global Disparities Persist. J. Clin. Med. 2022, 11, 271. [Google Scholar] [CrossRef]

| PCHI Cases (N = 46) | Matched Controls (N = 46) | Peer Controls (N = 46) | ||||
|---|---|---|---|---|---|---|
| Median | Range/ IQR | Median | Range/ IQR | Median | Range/ IQR | |
| Birth weight (g) | 1370 | 540–3420/ 960, 2648 | 1385 | 521–3650/ 955, 2653 | 2503 * | 514–4220/ 1464, 3051 |
| Gestational age (weeks) | 32 | 24–41/ 28, 37 | 32 | 24–41/ 28, 37 | 36 * | 25–42/ 31, 38 |
| Time on NICU (days) | 44 | 0–152/ 14, 100 | 25 | 1–140/ 7, 71 | 8 * | 2–141/ 3, 31 |
| Developmental Outcome Data (N = 44) | PCHI | Matched Controls | Chi-Squared Value | p Value |
|---|---|---|---|---|
| Speech and language | 26/44 (59%) | 5/44 (11%) | 22 | <0.0001 * |
| Emotional-social difficulties | 9/44 (20%) | 4/44 (9%) | 2 | 0.1 * |
| Cerebral palsy | 13/44 (30%) | 1/44 (2%) | 12 | 0.0005 * |
| Bilateral cerebral palsy | 11/44 (25%) | 1/44 (2%) | 10 | 0.002 * |
| Neurodevelopmental impairment (total) | 31/44 (70%) | 7/44 (16%) | 23 | <0.00001 |
| Degree of Neurodevelopmental Impairment (N = 44) | PCHI | Matched Controls | Chi-Squared Value | p Value |
| Mild | 10/44 (23%) | 4/44 (9%) | 3 | 0.080 * |
| Moderate | 9/44 (20%) | 2/44 (5%) | 5 | 0.02 * |
| Severe | 12/44 (27%) | 1/44 (2%) | 11 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moosan, H.; Hoare, D.J.; Jayasinghe, D.; Willis, K.R.; Martin, K.; Thornton, S.K. Neonatal Markers of Prematurity as Predictors of Permanent Childhood Hearing Loss and Neurodevelopmental Impairment in Children Admitted to the Neonatal Intensive Care Unit. Brain Sci. 2024, 14, 926. https://doi.org/10.3390/brainsci14090926
Moosan H, Hoare DJ, Jayasinghe D, Willis KR, Martin K, Thornton SK. Neonatal Markers of Prematurity as Predictors of Permanent Childhood Hearing Loss and Neurodevelopmental Impairment in Children Admitted to the Neonatal Intensive Care Unit. Brain Sciences. 2024; 14(9):926. https://doi.org/10.3390/brainsci14090926
Chicago/Turabian StyleMoosan, Hayma, Derek J. Hoare, Dulip Jayasinghe, Karen R. Willis, Katherine Martin, and Sally K. Thornton. 2024. "Neonatal Markers of Prematurity as Predictors of Permanent Childhood Hearing Loss and Neurodevelopmental Impairment in Children Admitted to the Neonatal Intensive Care Unit" Brain Sciences 14, no. 9: 926. https://doi.org/10.3390/brainsci14090926
APA StyleMoosan, H., Hoare, D. J., Jayasinghe, D., Willis, K. R., Martin, K., & Thornton, S. K. (2024). Neonatal Markers of Prematurity as Predictors of Permanent Childhood Hearing Loss and Neurodevelopmental Impairment in Children Admitted to the Neonatal Intensive Care Unit. Brain Sciences, 14(9), 926. https://doi.org/10.3390/brainsci14090926






