Abstract
Background: In motor imagery brain–computer interface (MI-BCI) research, electroencephalogram (EEG) signals are complex and nonlinear. This complexity and nonlinearity render signal processing and classification challenging when employing traditional linear methods. Information entropy, with its intrinsic nonlinear characteristics, effectively captures the dynamic behavior of EEG signals, thereby addressing the limitations of traditional methods in capturing linear features. However, the multitude of entropy types leads to unclear application scenarios, with a lack of systematic descriptions. Methods: This study conducted a review of 63 high-quality research articles focused on the application of entropy in MI-BCI, published between 2019 and 2023. It summarizes the names, functions, and application scopes of 13 commonly used entropy measures. Results: The findings indicate that sample entropy (16.3%), Shannon entropy (13%), fuzzy entropy (12%), permutation entropy (9.8%), and approximate entropy (7.6%) are the most frequently utilized entropy features in MI-BCI. The majority of studies employ a single entropy feature (79.7%), with dual entropy (9.4%) and triple entropy (4.7%) being the most prevalent combinations in multiple entropy applications. The incorporation of entropy features can significantly enhance pattern classification accuracy (by 8–10%). Most studies (67%) utilize public datasets for classification verification, while a minority design and conduct experiments (28%), and only 5% combine both methods. Conclusions: Future research should delve into the effects of various entropy features on specific problems to clarify their application scenarios. As research methodologies continue to evolve and advance, entropy features are poised to play a significant role in a wide array of fields and contexts.
1. Introduction
Brain–Computer Interface (BCI) technology facilitates interaction between the human brain and external devices by recording and decoding neurophysiological signals generated by brain activity. These signals are processed into formats that can be interpreted by external devices, such as computers, enabling communication and control [1]. Common paradigms employed in BCI systems include Steady-State Visual Evoked Potential (SSVEP), P300, and Motor Imagery (MI).
MI is categorized into visual MI and kinesthetic MI. Visual MI involves imagining oneself as an observer watching specific movements from a distance, while kinesthetic MI involves imagining oneself performing specific movements from a first-person perspective [2]. MI signals are bioelectrical signals generated by the brain during the mental simulation of movement, representing an endogenous and spontaneous activity. Different MI tasks induce various brain signals that encompass distinct sensory motor rhythms (SMRs), characterized by increases or decreases in the power of specific frequency bands across different brain regions [3,4]. Compared with P300 and SSVEP, MI only requires the user’s own motor imagery without external stimuli, making the interaction more natural. Furthermore, MI is based on the user’s subjective brain activity, making it more suitable for long-term use and avoiding visual fatigue.
The Common Spatial Pattern (CSP) and its derivatives are among the most prevalent feature-extraction methods employed in MI-BCI data processing [5,6,7,8,9]. CSP extracts feature vectors from SMRs, which are then input into various classifiers for training and classification [10]. However, MI-BCI signals often exhibit significant inter-trial and inter-individual variability, manifesting as complex nonlinear dynamic characteristics. These traditional linear feature-extraction methods struggle to effectively capture the complex characteristics of these signals [11], so the classification of MI-BCI signals is more challenging [12].
Entropy is a measure of the disorder and randomness of a system. In the mid-19th century, Clausius introduced the concept of entropy in the study of the second law of thermodynamics, which is known as thermodynamic entropy [13]. Later, Boltzmann enriched its physical meaning by providing a statistical mechanics interpretation and derived the statistical expression of entropy, known as the Boltzmann relation, which is the entropy in statistical mechanics [13]. Shannon, in 1948, defined a quantity that measures the uncertainty of information and called it information entropy [14]. As research on information entropy deepened, researchers later proposed approximate entropy, sample entropy, permutation entropy, fuzzy entropy, and other types of information entropy. “Information entropy” thus became a general term for this class of computational methods, with Shannon’s originally proposed information entropy being renamed as Shannon entropy. Information entropy, due to its nonlinear characteristics, can effectively describe the complex dynamic behavior of EEG signals and can compensate for the shortcomings of traditional linear feature-extraction methods in MI classification, which is widely used in the feature extraction of physiological signals such as ECG and EEG [14]. The application of information entropy in EEG has mainly focused on the diagnosis of neurological diseases [15], with relatively fewer applications in motor imagery paradigms in brain–computer interfaces. Depending on the type and definition of information entropy, it can be used to measure different aspects of signal characteristics [16]. Using information entropy for feature extraction in motor imagery signals can improve the efficiency of feature extraction and enhance the accuracy of signal classification.
In recent years, although the application of information entropy in MI has increased, related application scenarios remain unclear due to the variety of information entropy types and lack of systematic review. Additionally, comparative studies on different types of entropy in MI (e.g., in neuroscience, motor science, etc.) are relatively scarce, making it difficult to precisely select the most suitable entropy metric in specific scenarios. Therefore, the applicability of entropy in MI remains somewhat limited. This study systematically reviewed articles about entropy applied for MI-BCI classification, summarizing the physical significance and mathematical formulas of commonly used entropy indices in different application scenarios of MI-BCI. This study aimed to help future researchers select the most appropriate entropy to process MI-BCI signals in different scenarios.
2. Materials and Methods
This study used keywords such as “MI”, “EEG”, “motor imagery”, “entropy”, and “information entropy” to search for relevant studies in the “Web of Science” and “PubMed” databases from 2019 to 2023. Selection criteria: ① Entropy is used for MI signal-feature extraction; ② Entropy is used to optimize MI classification algorithms; ③ The research topic aligns with the application of entropy in MI; ④ The language is English; ⑤ The paper is a research paper.
Exclusion criteria: ① Duplicate literature; ② Unofficially published papers, such as master’s and doctoral dissertations, conference papers, advertisements, etc.; ③ Unable to obtain the full text of the paper; ④ Review articles.
Our latest search work was performed on 31 December 2023. The titles and abstracts were screened by two independent reviewers. In the second filtering, these full-text articles were then analyzed and evaluated independently by the reviewers. In the case of discrepancies between the two reviewers, a third reviewer decided whether the article should be included. During this process, related studies were progressively included in our final database.
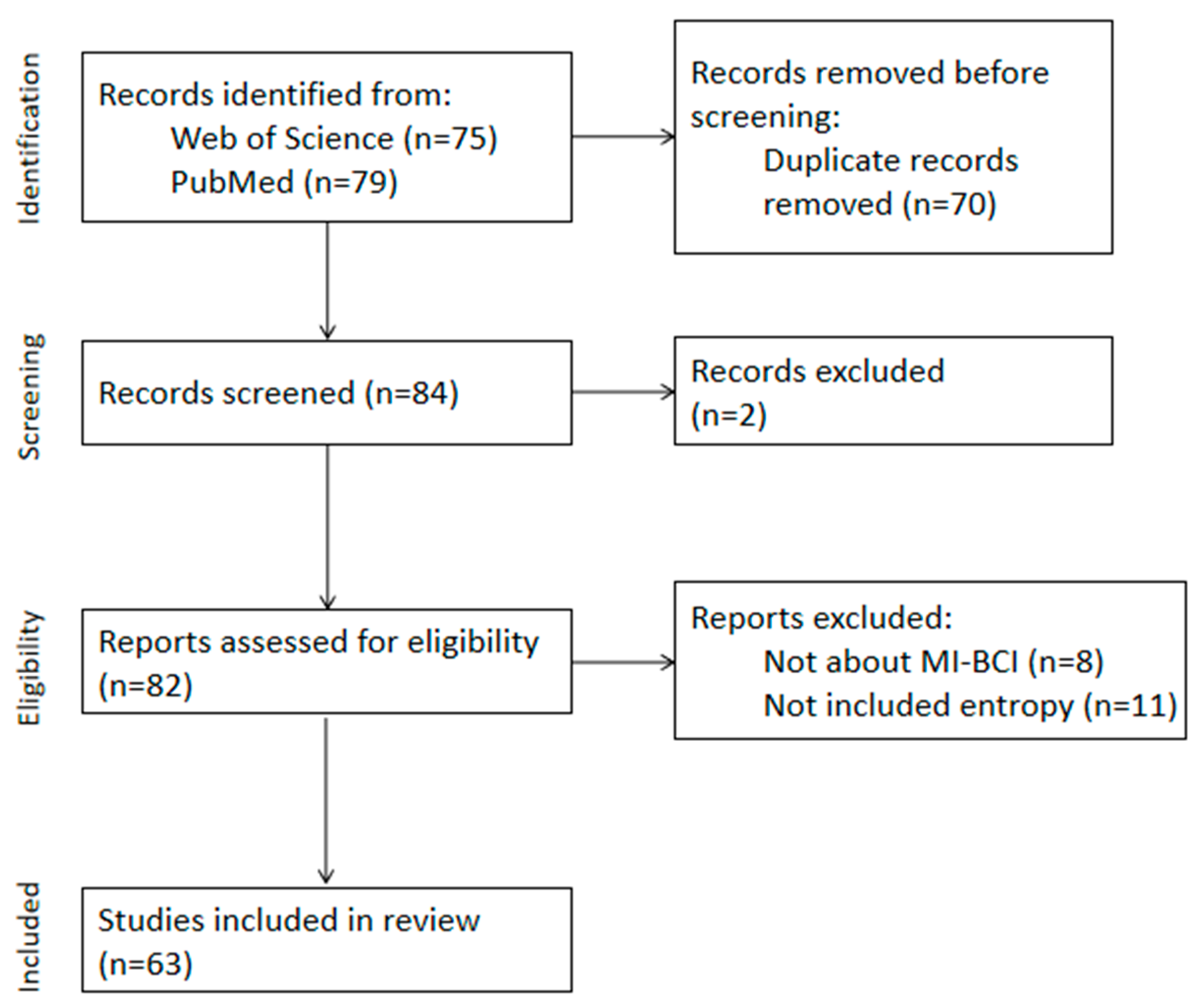
Ultimately, 63 studies meeting the criteria from the past five years (2019–2023) were included, with the screening process illustrated in Figure 1. We will list all the information in Appendix B. And we have summarized the journal sources in Appendix C.
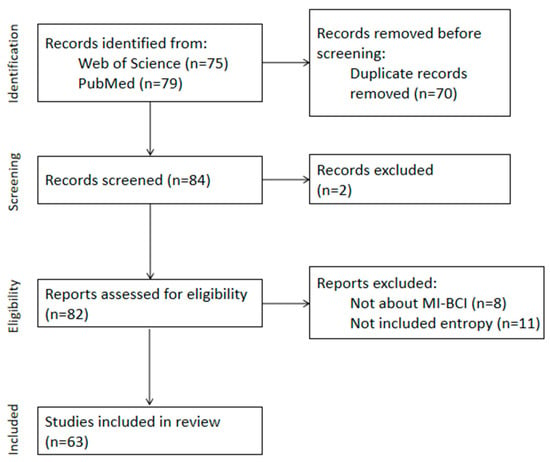
Figure 1.
Flow diagram of PRISMA approach used for this systematic review.
3. Result
3.1. Physical Significance and Calculation Methods of Entropy
This study summarizes the names, functions, and application ranges of 13 commonly used information entropies in these 63 journal articles (Table 1).

Table 1.
The name, function, and scope of application of information entropy appearing in 63 research papers.
3.2. Application of Entropy in MI
After preprocessing the EEG signals of the motor imagery paradigm, feature-extraction algorithms need to be applied to accurately extract key features from the signals. These extracted features are then transformed into feature vectors or feature matrices, which are input into classifiers for systematic training and classification. Finally, classification results are obtained through this process. It is worth noting that the final classification results are often influenced by the choice of feature-extraction methods and the performance of classifiers. Therefore, choosing an appropriate feature-extraction method or improving classifier efficiency can lead to better classification results. This section will introduce the application of entropy in MI from three perspectives: entropy for feature extraction, entropy for optimizing classification algorithms, and multiple entropy fusion classification.
3.2.1. Entropy for Feature Extraction
Entropy, as a nonlinear feature, can be directly used for feature representation of MI signals. Previous research has used seven types of entropy to directly extract features from EEG signals of four different grip forces, combining the obtained entropy values (power spectral entropy, singular spectral entropy, logarithmic energy entropy, sample entropy, fuzzy entropy, permutation entropy, and envelope entropy) into feature vectors and using support vector machines (SVM) for classification [16]. The classification accuracy reached 91.73% in binary classification and 69.59% in four-class classification.
Entropy can also be combined with other feature-extraction methods, and the combination of multiple classification methods can more comprehensively extract signal features, thereby improving classification results. Yang et al. [40] used CSP and improved multiscale permutation entropy fusion methods to extract MI features. Under the same classifier, the average classification accuracy of multi-domain feature extraction increased by 1.52% compared to CSP features alone, and by 32.87% compared to using improved multiscale permutation entropy features alone.
Hou et al. [41] used a new framework based on high-order spectral entropy and CSP to extract features from BCI Competition IV dataset IIa and BCI Competition III dataset IVa, using an SVM algorithm based on RBF kernel function for classification. The highest accuracy on the dataset 2a reached 85%, and the experiment on dataset IVa also achieved good results.
Saha et al. [42] used wavelet-based mean maximum (wMEM), CSP, and regularized common spatial pattern (RCSP) to judge the sensorimotor tasks of the right hand and right foot using the BCI Competition III dataset IVIA and task-specific EEG channels.
Ji et al. [43] proposed a feature-extraction method based on discrete wavelet transform (DWT), empirical mode decomposition (EMD), and approximate entropy. They decomposed the EEG signals into a series of narrowband signals, then used EMD to decompose the sub-band signals, obtaining a set of stationary time series called intrinsic mode functions (IMFs). They then reconstructed the IMFs with appropriate signal selection to obtain the approximate entropy of the reconstructed signal as the corresponding feature vector, and finally used SVM for classification.
Wang et al. [44] mapped EEG signals to feature spaces using time-domain information, statistical measures, wavelet coefficients, mean power, sample entropy, and CSP, using SVM as the intelligent classifier model and sparse logistic regression as the feature selection technique, achieving an accuracy of 79.51%.
Entropy can also participate in channel selection, removing redundant information from EEG signals to improve classification results. Sun et al. [45] calculated the weighted permutation entropy of all sampled electrode channels, selected half of the channels with higher weighted permutation entropy values, then used a binary gravitational search algorithm to search and determine an optimal channel combination. Finally, CSP was used to extract features from the selected channels, and SVM was used to train the classifier. The method achieved accuracies of 88.0% (compared to 57.5% using all channels) and 91.1% (compared to 79.4% using all channels) on two datasets, respectively.
Entropy provides efficient feature-extraction methods, effectively extracting features from different MI signals. Combining entropy with traditional feature-extraction methods can further improve classification accuracy, indicating the necessity of exploring fusion-feature-extraction methods involving entropy. After extracting MI features using entropy or entropy-involved fusion algorithms, most studies employ traditional machine learning classifiers or neural network classifiers for classification.
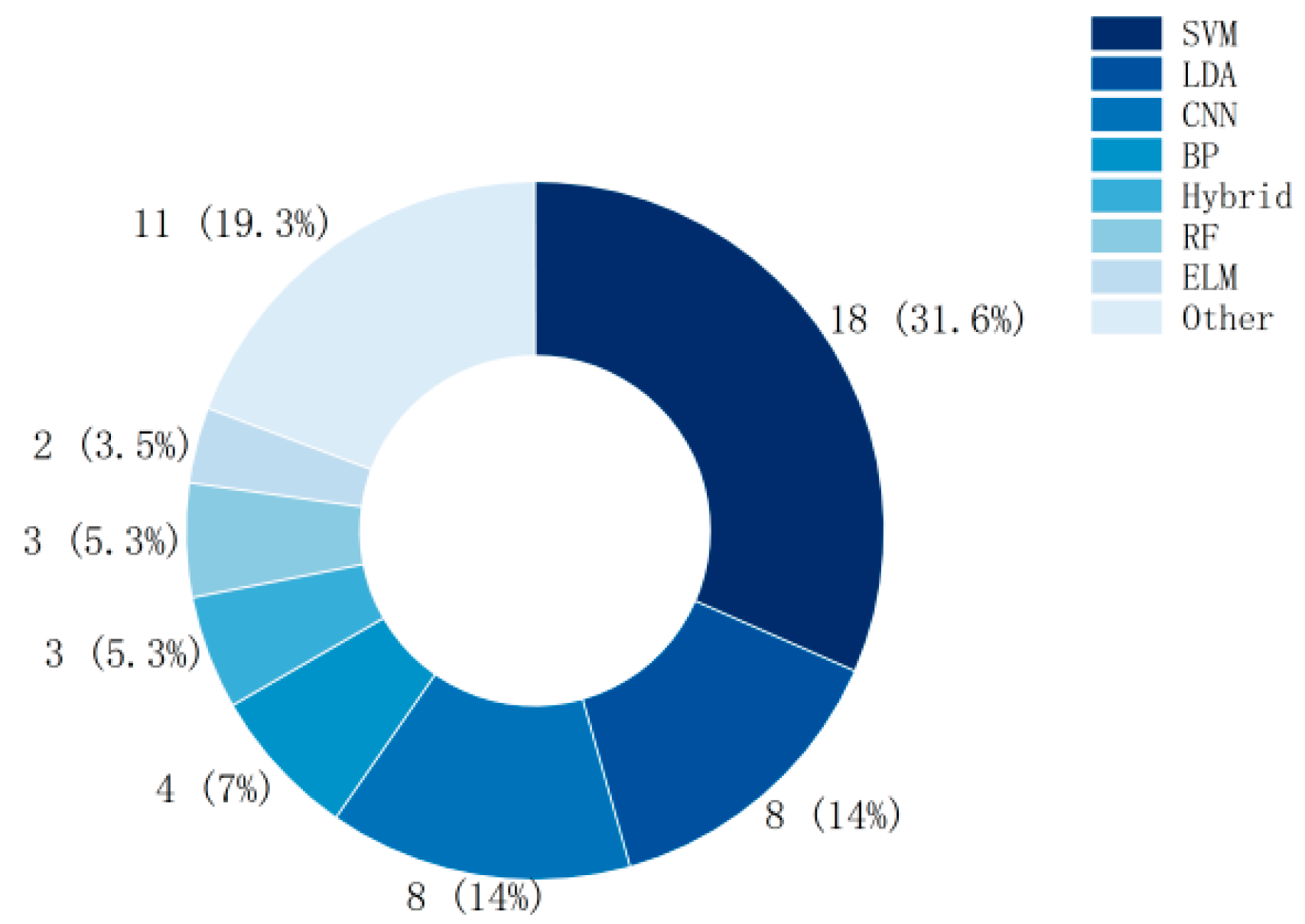
As can be seen from Figure 2, SVM is one of the most widely used classification algorithms, Linear Discriminant Analysis (LDA) and Convolutional Neural Network (CNN) are also frequently used in the field of MI-BCI.

Figure 2.
Number of studies using classifiers (BP: Back Propagation Neural Network, RF: Random Forest, ELM: Extreme Learning Machine).
Khan et al. [46] compared the accuracies of different classifiers in the same scenario. The tests were carried out on two datasets, BCI Competition IV dataset 1 and BCI Competition III dataset 4a, using 10-fold cross-validation. On BCI Competition IV dataset 1, the average classification accuracy of the logistic regression classifier reached 90.42%, outperforming other classifiers (such as 89.78% for LDA, 89.20% for Gaussian Naive Bayes, 88.14% for Fine Gaussian SVM, 86.21% for Fine KNN, and 85.57% for Complex Tree). On BCI Competition III dataset 4a, the average classification accuracy of the logistic regression was 95.42%, which was also higher than that of other classifiers (95.06% for LDA, 94.44% for Gaussian Naive Bayes, 92.72% for Fine KNN, 90.66% for Complex Tree, and 87.78% for SVM).
But, there are few direct comparisons of the efficiency differences of different classifiers in processing the same entropy feature-extraction task in current studies, and due to the diversity of data sets adopted by different studies, it has not yet been clearly determined which classifier best matches the specific entropy feature to achieve the best classification effect. Further in-depth research is needed to explore and solve the problem of how to select the most suitable classifier for a specific entropy feature.
3.2.2. Entropy for Optimizing Classification Algorithms
Tang et al. [47] proposed a Conditional Adversarial Domain Adaptation Neural Network (CDAN) that applies densely connected convolutional neurons to obtain high-level discriminative features from the original EEG time series. On this basis, a new cross-entropy-based conditional domain discriminator is introduced to confront the label classifier, learning common subject–invariant EEG features. Alan Preciado-Grijalva et al. [48] explored the application of CDAN and its entropy variant CDAN + E in domain adaptation, reducing the uncertainty between the source domain and target domain through entropy conditioning, thereby improving classification performance. Yu Xie et al. [49] proposed an MI EEG signal classification method based on data augmentation and convolutional neural networks, combined with a cross-entropy loss function, which significantly improved classification accuracy. Rabia Avais Khan et al. [46] proposed a novel framework that enhances MI EEG signal classification accuracy and efficiency through channel selection techniques and entropy-based optimization methods.
Optimizing classification algorithms using entropy is an emerging research direction, with limited research in this area so far. This may be due to the fact that both entropy and neural networks lack good interpretability.
3.2.3. Multiple Entropy Fusion Classification
When using information entropy, multiple entropies can be selected simultaneously, and their results can be fused into a single-feature quantity as a basis for classification. Li et al. [50] used sample entropy, permutation entropy, and CSP to study the impact of massage on decoding EEG rhythms of left/right motor imagery and motor execution (ME) in patients with skeletal muscle pain. Al-Qazzaz et al. [51] combined various entropies in an MI dataset of stroke patients with upper limb hemiparesis. All patients underwent 25 MI-based BCI rehabilitation sessions, followed by assessments to examine EEG neuro-rehabilitation before and after functional changes. Conventional filters and automatic independent component analysis with wavelet transform (AICAWT) techniques were first used for denoising. Then, time, entropy, and frequency-domain attributes were calculated, and effective features were combined into time-entropy-frequency (TEF) attributes. Therefore, the AICAWT and TEF fusion feature set were used to develop an AICA-WT-TEF framework. Then, the SVM, k-nearest neighbor (kNN), and RF classification techniques were tested for MI-based BCI rehabilitation. The study used multiple entropies, namely sample entropy, fuzzy entropy, Tsallis entropy, improved multiscale permutation entropy, multiscale fuzzy entropy, and fine composite multiscale fuzzy entropy.
Khare et al. [52] proposed a simple and flexible MI classification method that uses single-channel adaptive decomposition methods and EEG signal classification methods, employing a multi-cluster unsupervised learning method for channel selection, and flexible variational mode decomposition (F-VMD) for adaptive signal decomposition. The F-VMD decomposition results were feature-extracted using entropy and quartile methods, including approximate entropy, sample entropy, Shannon entropy, logarithmic entropy, permutation entropy, Renyi entropy, and Tsallis entropy. These features were then classified using a flexible extreme learning machine (F-ELM).
Wu et al. [53] used multivariate empirical mode decomposition (MEMD) to decompose EEG signals into multiple intrinsic mode functions (IMFs). CSP was applied to highly correlated IMF functions to extract AR coefficients and energy entropy, fuzzy entropy, and multiscale entropy results as classification features. After optimization using genetic algorithms, classification was performed.
Chen et al. [54] extracted four types of entropy from the collected EEG signals, including Shannon entropy amplitude measurement, Shannon entropy phase synchronization measurement, wavelet entropy, and sample entropy. Principal component analysis was then used for dimensionality reduction, and SVM was used for classification. The method was evaluated using the BCI Competition 2003 Dataset III. The experimental results showed that the classification results of using Shannon entropy amplitude measurement, Shannon entropy phase synchronization measurement, wavelet entropy, and sample entropy alone were 81.05%, 78.91%, 80.97%, and 81.52%, respectively. The fusion method based on these four entropies achieved better classification performance, with an accuracy of approximately 88.36%. The results showed that multiple entropy fusion classification results in higher accuracy compared to single entropy.
Different entropies have unique calculation methods and intrinsic meanings. The comprehensive application of multiple entropy features allows for a multidimensional characterization of signals [51], thereby improving classification efficiency.
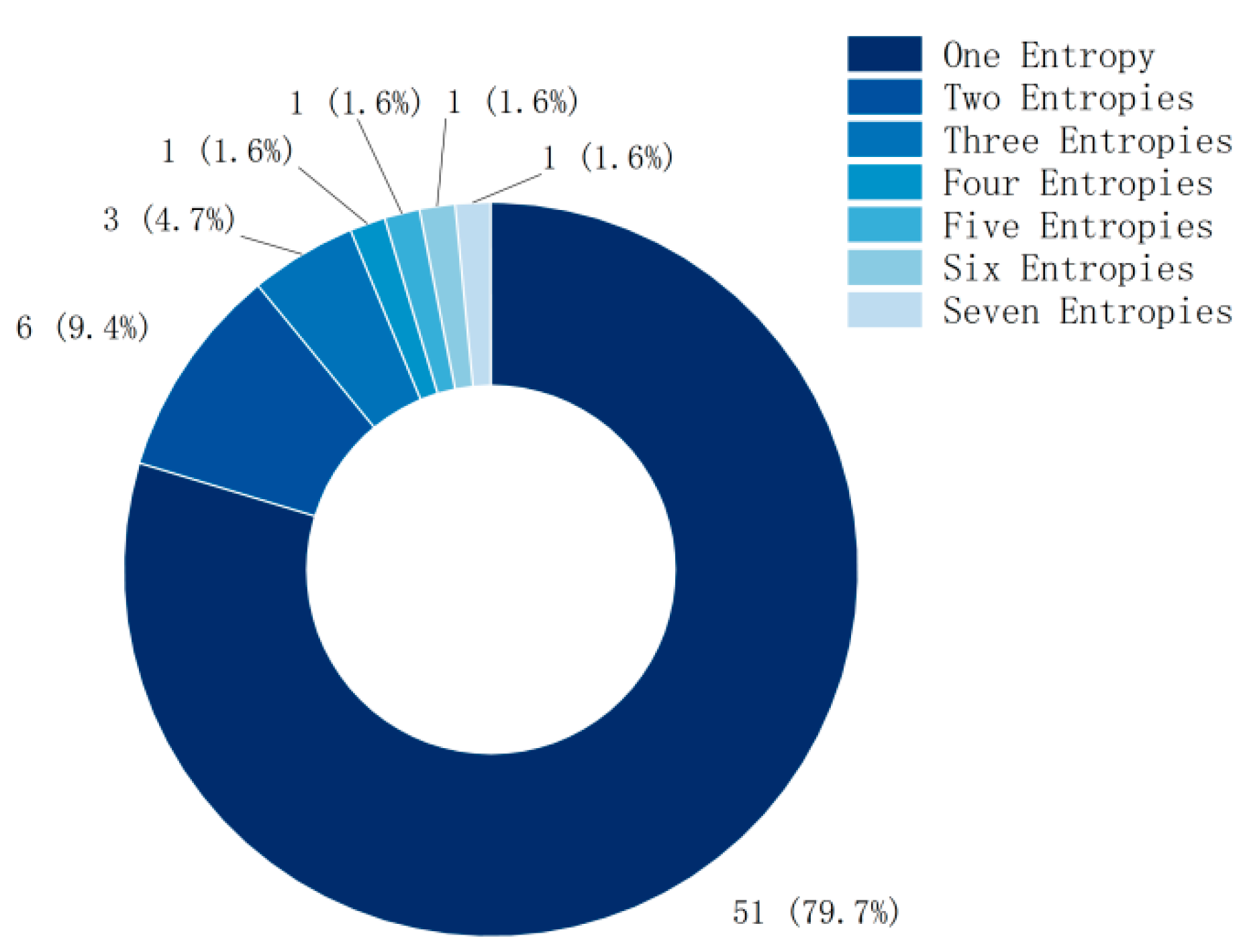
Although the method of combining multiple entropies has achieved better feature- extraction performance in MI classification, nearly 80% of the current research still focuses on a single entropy (as shown in Figure 3), and the research using multiple entropies also focuses on two or three entropies.
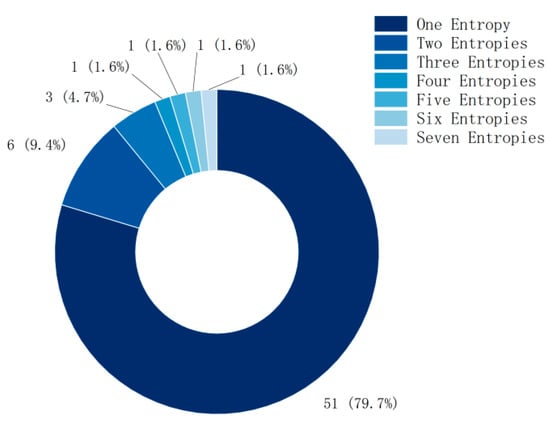
Figure 3.
The number of entropies used in each study.
Subsequent research should use more entropies for feature extraction and classification of MI.
3.3. Number of Research of Entropy Features in MI-BCI
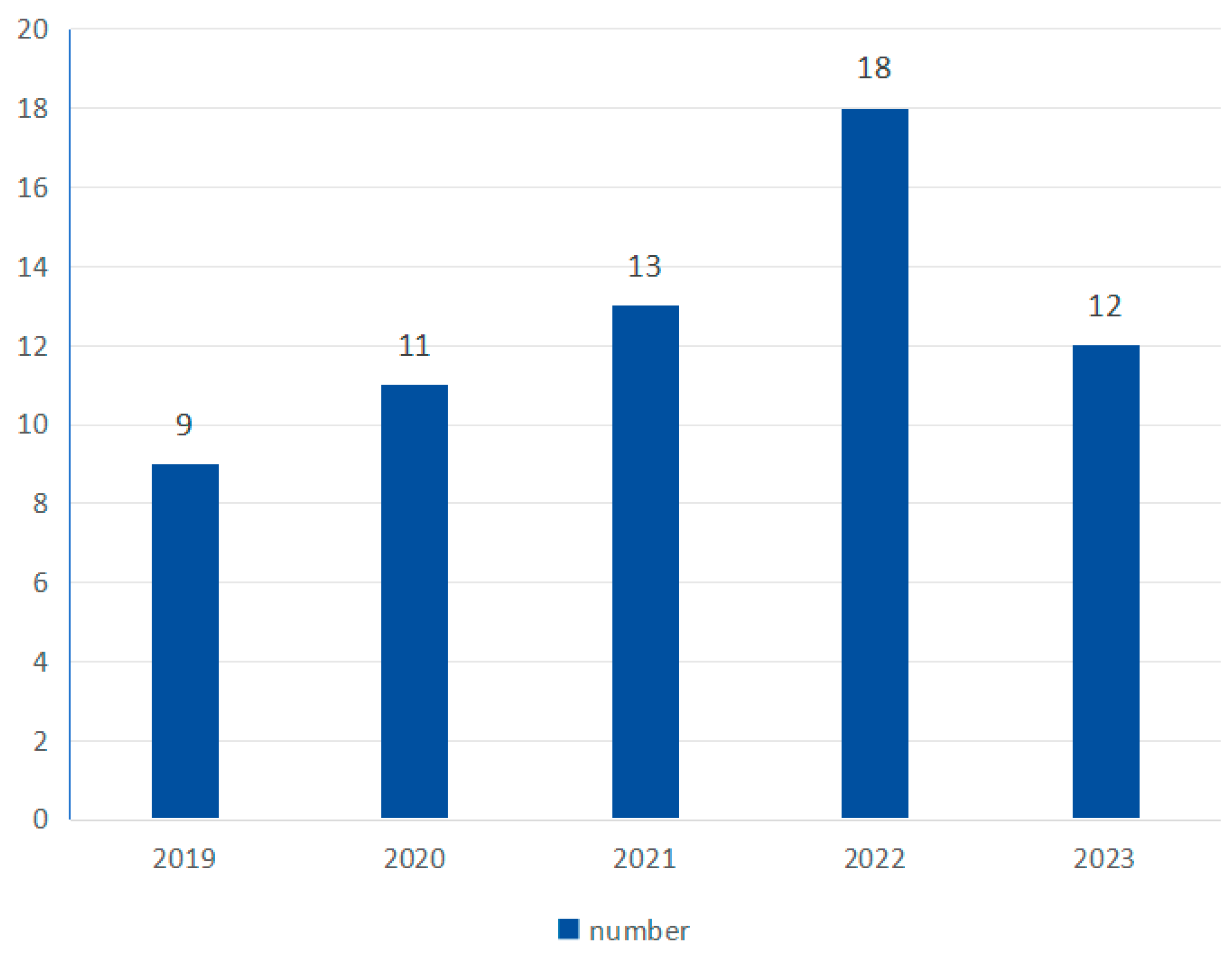
As shown in Figure 4, the number of studies shows an overall upward trend, with 9 in 2019, 11 in 2020, 13 in 2021, 18 in 2022, and 12 in 2023.
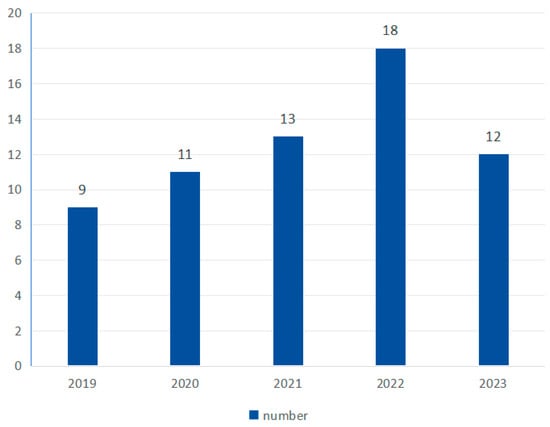
Figure 4.
Yearly research number.
3.4. Accuracy of Entropy Features in MI-BCI
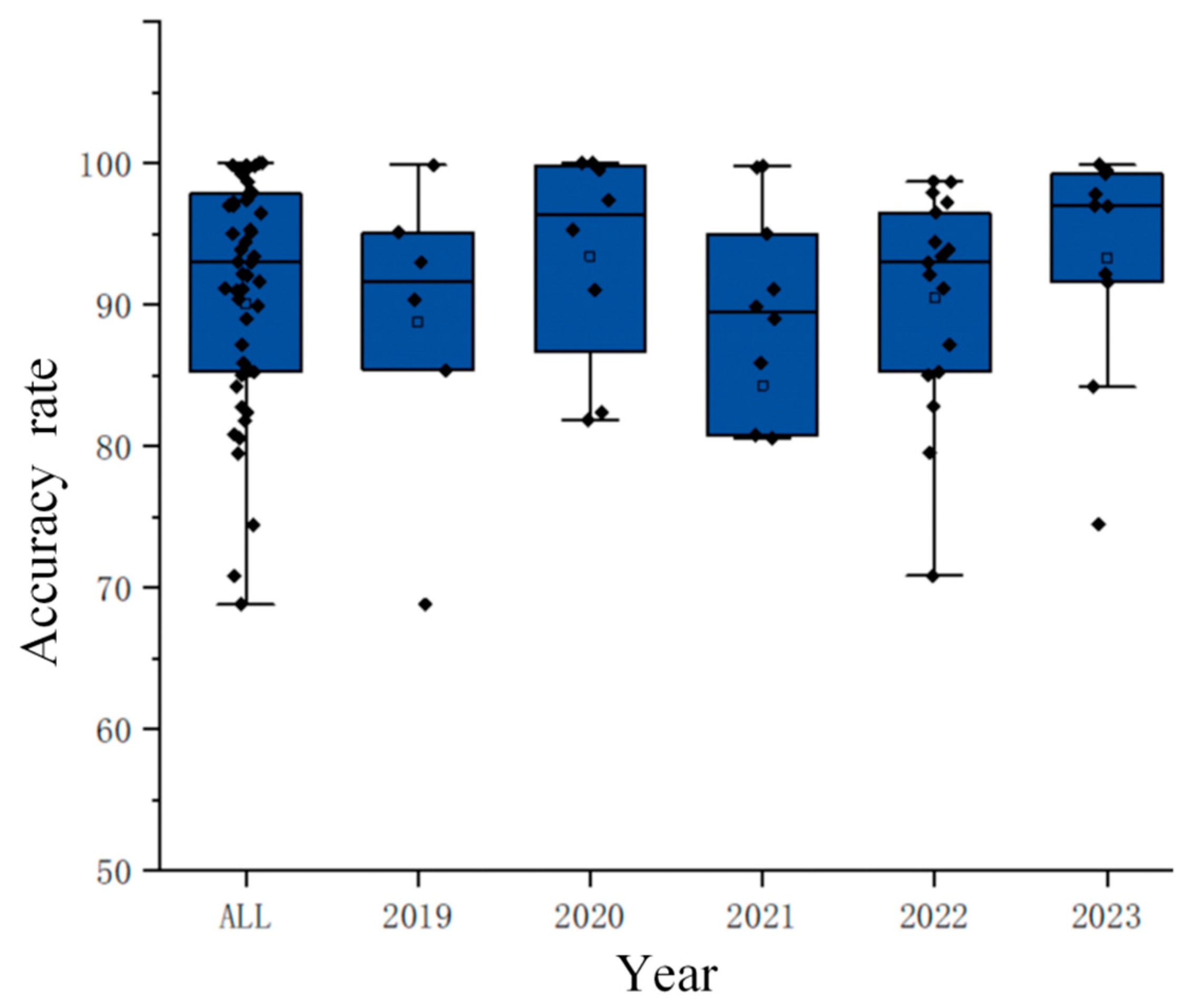
As shown in Figure 5, most studies have achieved accuracy rates above 80%, with more than half reaching accuracy rates of over 90%, and some studies even achieving peak accuracy rates exceeding 99%. This fully demonstrates that entropy has high classification efficiency in motor imagery paradigms.
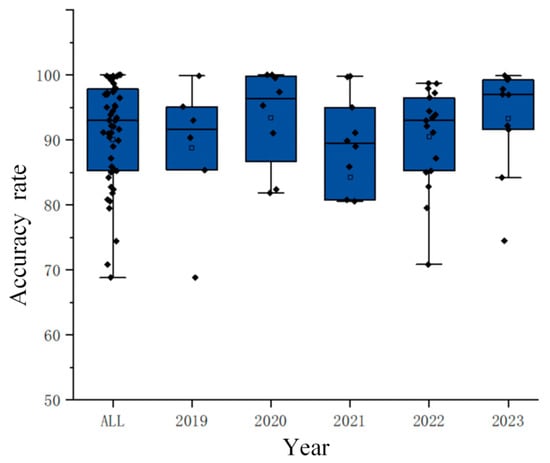
Figure 5.
Yearly accuracy distribution chart. (The diamond represents the accuracy of each study, and the hollow square represents the average value of the data in this group).
3.5. Entropy Type in MI-BCI
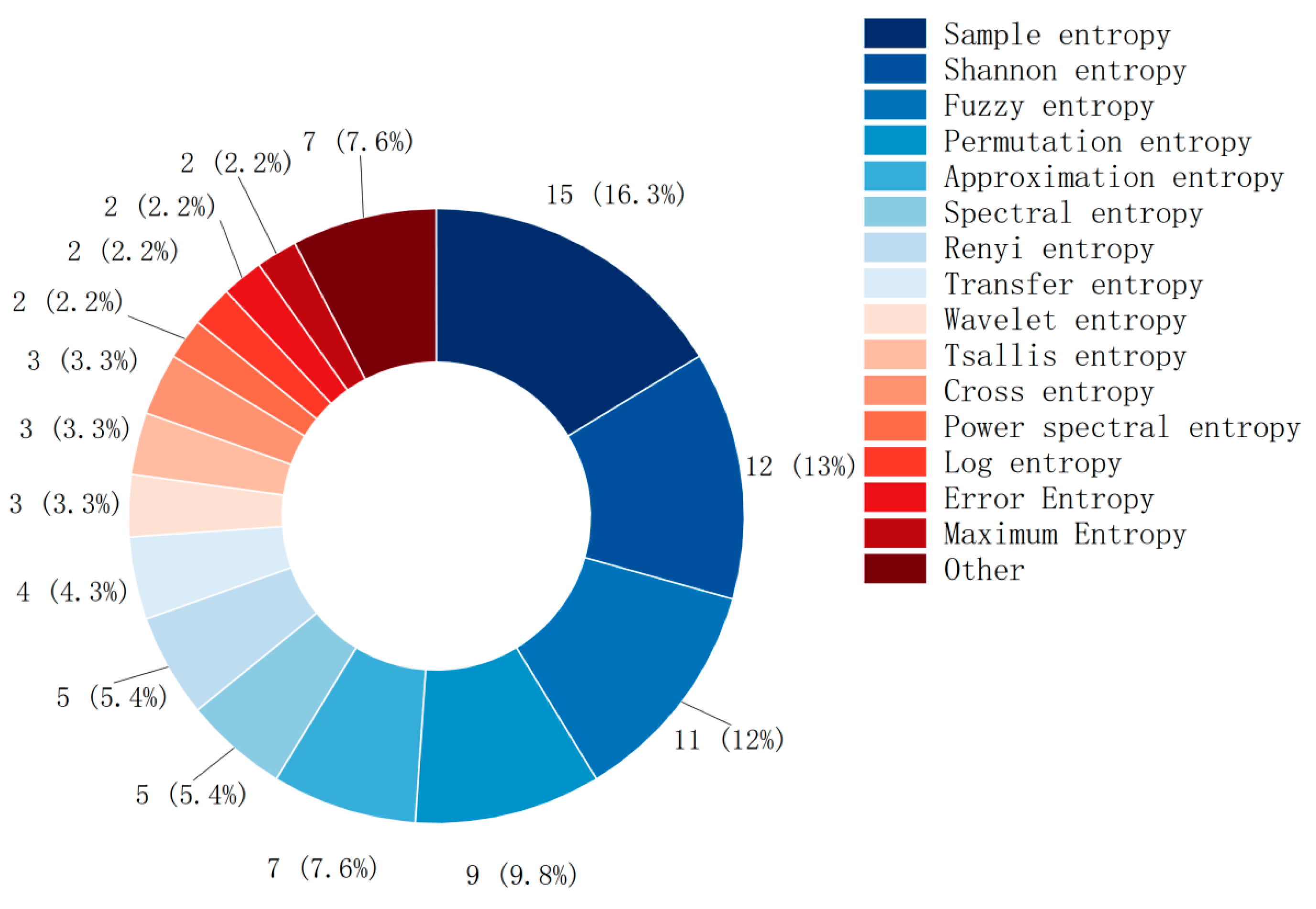
This study conducted a statistical analysis of the most commonly used entropy fea-tures in the literature. Most MI-BCI studies use simple and well-defined entropy features, as shown in Figure 6. The usage frequency in descending order is: sample entropy (15), Shannon entropy (12), fuzzy entropy (10), permutation entropy (8), approximate entropy (7), spectral entropy (5), and Renyi entropy (5).
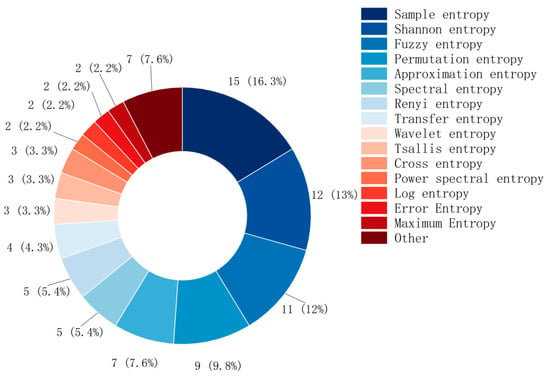
Figure 6.
Types of entropy and their usage.
3.6. Selection of Datasets
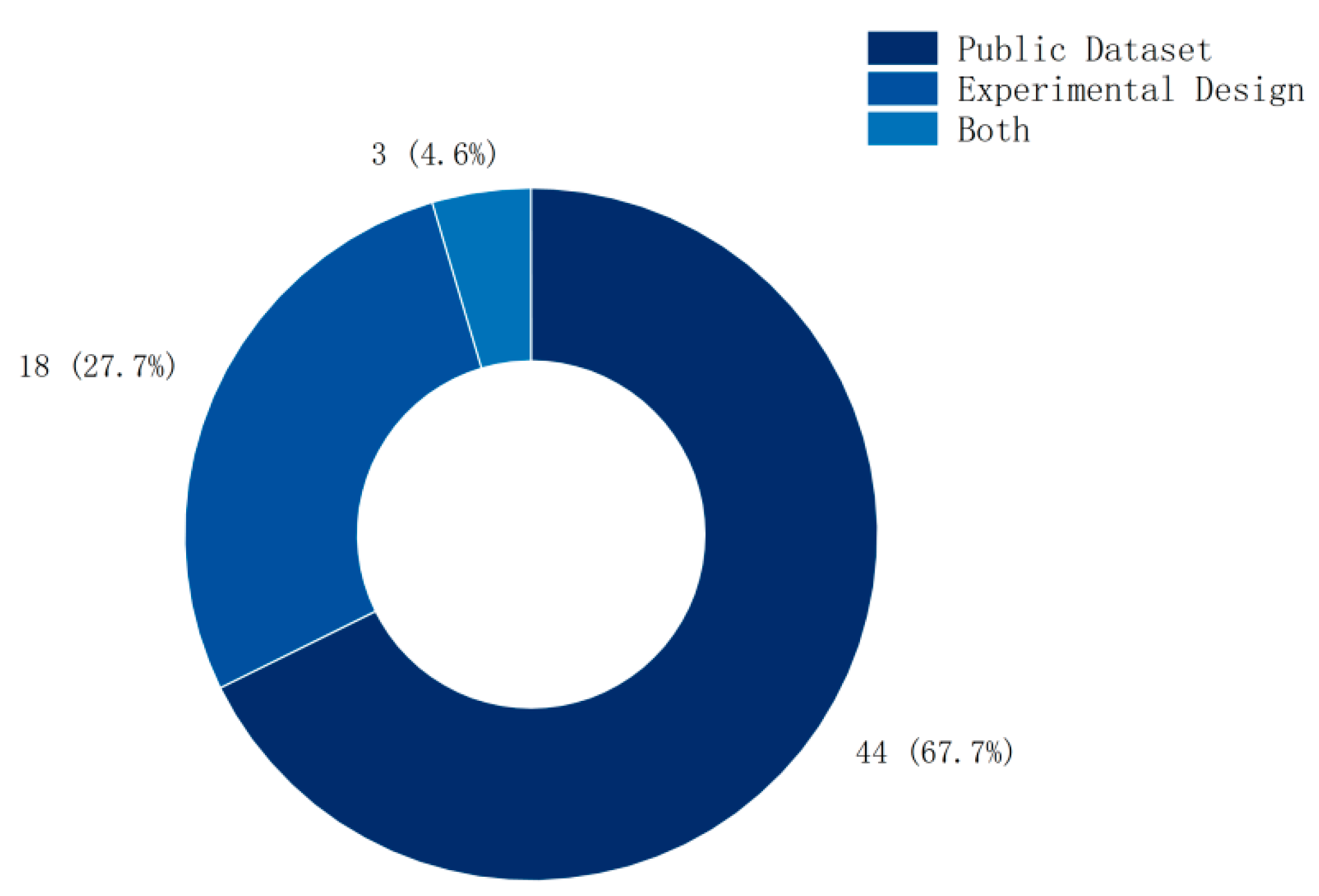
As shown in Figure 7, among the methods for validating entropy features, public dataset validation accounts for 67%, experimental design accounts for 28%, and only 5% combine both.
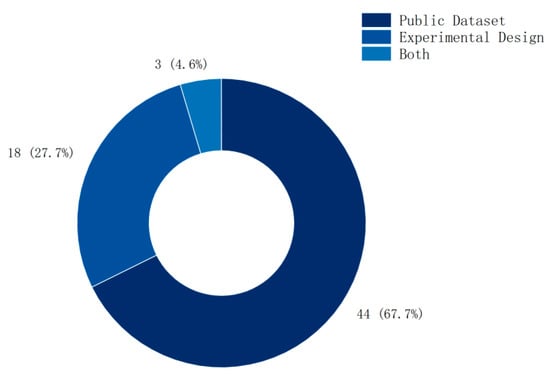
Figure 7.
Dataset distribution.
4. Discussion
Entropy-based feature-extraction and classification methods have been widely used in the field of physiological signal feature extraction due to their simplicity and efficiency. Applying entropy to motor imagery signal classification is an emerging method with broad application prospects. As shown in Figure 4, in recent years (2019–2023), this research direction has gradually become very popular.
4.1. Comparison with Other Methods
Currently, the most widely used feature-extraction method for MI-BCI data is the common spatial pattern (CSP) and its derivative methods [5,6,7,8,9]. Since the CSP algorithm adopts a strict linear-mode assumption relationship in the acquisition of source signals and the recording of electroencephalogram (EEG) signals, it is overly restricted by the original recording parameters of the subjects, such as the signal time period, filtering frequency band, number of electrode channels, etc. EEG signals are non-stationary and non-linear, so the CSP algorithm cannot accurately and effectively describe the characteristic signals of the brain. As a non-linear measurement method, entropy is more friendly to non-linear signals.
CSP mainly conducts feature extraction based on spatial filters. Therefore, for movements with significantly different movement areas, CSP can quantify feature quantities with large differences. However, for fine movements with similar movement areas, it is difficult to distinguish them using CSP. Entropy is not affected by spatial position.
The CSP algorithm can only consider the EEG signals of two categories. Thus, when dealing with multi-classification problems, one vs. rest or one vs. one methods need to be used to perform CSP for feature extraction multiple times. In multi-classification, entropy can extract the entropy values of multiple states at once, reducing the calculation steps.
In terms of classification accuracy, Li et al. [55] use fuzzy entropy to extract MI information to construct feature vectors, and use SVM classifier for classification, the final result of which (99.43%) is higher than the traditional method (CSP: 86.67%, FBCSP: 90.50%, DFBCSP: 91.40%, SFBCSP: 92.05%, SWCSP: 93.0%).Yang et al. [40] found that for the same subject and the same classifier, the average classification accuracy of multi-domain feature extraction increased by 1.52% compared to the CSP feature results, and the average classification accuracy increased by 32.87% compared to the IMPE feature classification results. Tang et al. [47] found that, on the High Gamma Dataset, the accuracy of the entropy method (95.3%) was higher than that of FB-CSP (91.2%). Lv et al. [56] found that, compared with the common spatial pattern (CSP) algorithm, the entropy method has significant advantages.
This shows that in some scenarios, compared with CSP, entropy has the characteristics of simple calculation and high accuracy.
4.2. Frequently Used Entropy Features in MI-BCI
Compared to other entropy metrics, sample entropy is less sensitive to noise and changes in data length, making it more reliable and stable when processing real EEG signals. In MI-BCI experiments, EEG signals are often disturbed by various noises, and the anti-interference ability of sample entropy makes it an ideal tool for analyzing these signals, which is why it is frequently used. Shannon entropy is a basic concept in information theory used to quantify uncertainty or the amount of information. Its intuitive definition and relatively simple calculation make it easily accepted and applied by researchers in MI-BCI studies. Similar to sample entropy, fuzzy entropy is also suitable for analyzing nonlinear signals. In MI-BCI, this capability allows fuzzy entropy to more accurately capture the nonlinear characteristics of brain activity. The frequent use of sample entropy, Shannon entropy, and fuzzy entropy in MI-BCI literature is mainly due to their advantages in processing complex and nonlinear signals, anti-interference capability, information theory foundation, intuitive understanding, broad application scenarios, fuzziness and uncertainty, and parameter flexibility.
4.3. Single- or Multiple-Entropy Features in MI-BCI
The combination of multiple entropies in MI classification indeed achieves better feature-extraction performance, reflecting the advantages of different entropy metrics in capturing different aspects of EEG signals. However, in the current literature review, most studies (79.7%) still focus on single entropy, possibly because single entropy is relatively simple to calculate and intuitive, and, in some cases, already meets the research needs. Nevertheless, a portion of the studies (about 14.1%, i.e., 9.4% using two entropies and 4.7% using three entropies) explored methods of combining multiple entropies and found that this approach significantly improves classification accuracy in different paradigms (8–10%).
Multiple-entropy features have certain advantages in fusion, as different entropy metrics (e.g., sample entropy, Shannon entropy, fuzzy entropy) focus on different aspects of EEG signal complexity, information content, fuzziness, etc. Their combination allows for a more comprehensive extraction of EEG signal features, providing good complementarity. Additionally, the combination of multiple entropies can offer richer feature information, helping to improve classifier performance and thereby enhance classification accuracy. Particularly in complex MI-BCI tasks, the combination of multiple entropies can more accurately reflect the state of brain activity. Furthermore, different MI task paradigms may require different feature-extraction methods. The combination of multiple entropies can be flexibly adjusted to suit the needs of different paradigms, achieving high classification accuracy in various paradigms.
Different entropies have different calculation methods, and using multiple entropies will increase the computational cost and time more than using a single entropy. The single entropy calculation is relatively simple, easy to implement and deploy. In resource-limited or time-sensitive situations, researchers are more inclined to choose single entropy for analysis. Moreover, single entropy has more research basis than multiple entropy. Although the combination of multiple entropies is gaining attention with the deepening of research, single entropy is still the main choice.
To further improve the performance and application effectiveness of MI-BCI systems, actively exploring the combination of multiple entropies may be a future research direction. With the continuous development of computing technology and the increasing availability of computing resources, the combination of multiple entropies will also become easier to implement and deploy.
4.4. Application Scenarios of Entropy Features in MI-BCI
Most of the research remains focused on the study of feature-extraction and classification methods, with only a few studies conducted in real-world environments. Choy et al. [35] applied entropy in the motor imagery of stroke patients in order to explore whether motor imagery and virtual reality can help activate the motor cortex of stroke patients. Li et al. [57] used entropy to explore the relationship between motor imagery and age-related fatigue in the CNN classification of EEG data. Al-Qazzaz et al. [51] used entropy to explore a motor-imagery-based rehabilitation assessment scheme for stroke patients.
The dominance of public dataset validation in current research reflects researchers’ pursuit of reproducibility and convenience. However, this does not mean that these studies are diverse, as most studies focus on a small subset of publicly available datasets, or use only one publicly available dataset. However, to clarify the application scenarios of entropy features more precisely, researchers need to design experiments of different types and simultaneously compare the classification results of public datasets to explore the application scenarios of different entropies. With the continuous innovation and improvement of research methods, entropy features will play an important role in disease diagnosis and rehabilitation.
5. Conclusions
For subsequent researchers to use entropy features, they can prioritize the use of multiple common entropies (Sample entropy, Shannon entropy, Fuzzy entropy, Permutation entropy, Approximate entropy, Spectral entropy, and Renyi entropy) in combination, and use multiple common classifiers (SVM, LDA, CNN, BP Neural Network, RF, ELM) to classify simultaneously in order to find the best classification results.
Studies have been carried out to compare the feature-extraction and classification performance of different entropies on the same research object in order to explore the best matching entropy of different research objects. Based on this, it is expected to establish a motor imagery paradigm brain–computer interface feature-extraction and classification scheme that integrates multiple feature-extraction methods and adopts multiple classifiers for weighted classification. At the same time, the exploration of the applicable scope of different entropy features needs to continue, aiming to form a consensus on entropy features in motor imagery.
6. Limitation
This study has the following limitations due to the focus and inherent shortcomings of the articles in the areas reviewed.
In terms of application scenarios, we did not explore the sociodemographic characteristics (gender, age, type of neurological pathology) of individuals with EEG, the time of EEG recording, or the number of EEG electrodes used. This is because most of the retrieved studies focused on data processing and did not specify these details.
In terms of computing entropy, we did not explore the computational cost of different entropies and the cost of implementing multi-entropy, because this is difficult to compare due to the varying amounts of data and computing equipment used in different studies. We also did not explore the impact of entropy parameters on classification performance, because different studies used different datasets and classifiers, and comparing them without controlling for variables is unscientific.
In terms of classifier selection, we did not explore how to improve the interpretability of entropy and neural networks.
We hope that future studies will focus on these points and improve upon the research in this field.
Funding
This study was supported by Key Project of Construction of Drug Regulatory Science System (RS2024X007), STI 2030-MajorProjects under grant 2021ZD0200406, the Medical and Health Innovation Project (Grant No. 2021-I2M-1-042, 2021-I2M-1-058), the Tianjin Outstanding Youth Fund Project (Grant No. 20JCJQIC00230).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Entropy Calculation Method
- Shannon entropy [17]
- 2.
- Power spectrum entropy [18,19,20,21]
- 3.
- Singular spectrum entropy [22]
- 4.
- Log energy entropy [23,24]
- 5.
- Approximate entropy [15,25]
- 6.
- Sample entropy [26]
- 7.
- fuzzy entropy [27,28,29,30]
- 8.
- Permutation entropy [20,27]
- 9.
- Transfer entropy [31]
- 10.
- Renyi entropy [32,33,34]
- 11.
- Spectral entropy [35]
- 12.
- Wavelet entropy [36]
- 13.
- Tsallis entropy [37,38,39]
Appendix B. Research Statistical Table

| Years | Authors | Entropy Type | Classifier Type | Dataset * | Accuracy |
|---|---|---|---|---|---|
| 2023 | [58] | Differential entropy | GBDT | 0 | 96.96 |
| 2023 | [59] | Wavelet entropy | RF | 0 | 99.26 |
| 2023 | [60] | Spectral entropy | SVM | 0 | 84.19 |
| 2023 | [61] | Shannon entropy | DRN | 0 | 91.6 |
| 2023 | [62] | Wavelet entropy | SVM | 0 | 97 |
| 2023 | [63] | Shannon entropy, Renyi entropy, Sample entropy, Permutation entropy, Approximate entropy | NB, SVM, RF, KNN | 0 | 97.83 |
| 2023 | [30] | Fuzzy entropy | LDA | 0 | 92.14 |
| 2023 | [35] | Spectral entropy | LDA | 1 | / |
| 2023 | [64] | Renyi entropy, Shannon entropy | LDA | 0 | 99.87 |
| 2023 | [17] | Shannon entropy | ELM | 0 | 99.48 |
| 2023 | [65] | Tsallis Entropy, Dispersion Entropy, Shannon entropy | RF, KNN | 0 | 74.48 |
| 2023 | [49] | Cross entropy | CNN | 0 | 97.61 |
| 2022 | [66] | Transfer entropy | CNN-LSTM | 1 | 97.21 |
| 2022 | [40] | Permutation entropy | Decision Tree, LDA, Naive Bayes, SVM, KNN, ECPA | 1 | 91.15 |
| 2022 | [67] | Sample entropy | BP network | 0 | 93 |
| 2022 | [19] | Power spectral entropy | SVM | 0 | 85.24 |
| 2022 | [44] | Sample entropy | SVM | 1 | 79.51 |
| 2022 | [68] | Approximate entropy | XGBO | 0 | 94.44 |
| 2022 | [69] | Spectral entropy | LDA | 0 | 98.67 |
| 2022 | [70] | Spectral entropy | Decision Tree | 0 | 98.71 |
| 2022 | [56] | Approximate entropy, Sample entropy, Fuzzy entropy, Multiscale entropy | SVM | 0 | 97.88 |
| 2022 | [71] | Sample entropy | SVM | 1 | 93.89 |
| 2022 | [57] | Rhythm entropy | CNNs | 1 | 82.81 |
| 2022 | [72] | Transfer entropy | LDA | 1 | 92.1 |
| 2022 | [73] | Shannon entropy, Correlation entropy, Conditional entropy | Ego-CNNs | 0 | 93.4 |
| 2022 | [74] | Rayleigh entropy | SLDA | 0 | 96.5 |
| 2022 | [41] | Shannon entropy | SVM | 0 | 85 |
| 2022 | [75] | Information entropy | MSTL-MCF | 0 | 70.86 |
| 2022 | [76] | Shannon entropy | 3-D-CNN | 0 | 87.15 |
| 2022 | [46] | Shannon entropy | LRC | 0 | 95.42 |
| 2021 | [77] | Fuzzy entropy | SVM | 1 | 80.56 |
| 2021 | [78] | Sample entropy | / | 1 | / |
| 2021 | [79] | Sample entropy | SNN | 1 | 89.9 |
| 2021 | [45] | Permutation entropy, Weighted permutation entropy | SVM | 0 | 91.1 |
| 2021 | [80] | Renyi entropy | SVM | 0 | 31.14 |
| 2021 | [81] | Sample entropy | / | 1 | / |
| 2021 | [50] | Sample entropy, Permutation entropy | BiLSTM | 1 | 89 |
| 2021 | [82] | Sample entropy, Permutation entropy | CNN | 0 | / |
| 2021 | [83] | Shannon entropy | LDA | 0 | 99.8 |
| 2021 | [84] | Cross entropy | CNN | 0 | 80.8 |
| 2021 | [85] | Multiscale entropy | FLDA | 2 | 85.89 |
| 2021 | [86] | Renyi entropy | TE-base | 0 | 95 |
| 2021 | [51] | Sample entropy, Fuzzy entropy, Tsallis entropy, Improved Multiscale Permutation entropy, Multiscale Fuzzy Entropy, Refined Composite Multiscale Fuzzy entropy | RF | 1 | 99.66 |
| 2020 | [87] | Maximum entropy | SVM | 0 | 81.83 |
| 2020 | [88] | Sample entropy | ELM | 1 | 91 |
| 2020 | [89] | Approximate entropy, Fuzzy entropy | / | 0 | 97.4 |
| 2020 | [90] | Transfer entropy | / | 2 | / |
| 2020 | [47] | Cross entropy | CDAN | 0 | 95.3 |
| 2020 | [91] | Sample entropy | SVM | 1 | 99.5 |
| 2020 | [92] | Multiscale Fuzzy entropy | BP | 0 | 100 |
| 2020 | [93] | Spectral entropy | / | 1 | / |
| 2020 | [52] | Approximate entropy, Sample entropy, Shannon entropy, Logarithmic entropy, Permutation entropy, Renyi entropy, Tsallis entropy | FFMIC | 0 | 100 |
| 2020 | [94] | Cross entropy | FBSF-TSCNN | 0 | 82.4 |
| 2020 | [47] | Shannon entropy | CDAN | 0 | 94.3 |
| 2019 | [53] | Energy entropy, Fuzzy entropy, Multiscale entropy | KNN | 0 | 85.36 |
| 2019 | [42] | Wavelet-based average maximum entropy | / | 0 | 90.36 |
| 2019 | [95] | Shannon wavelet entropy, Logarithmic entropy | LS-SVM | 0 | 93 |
| 2019 | [55] | Improved refined composite multivariate multiscale fuzzy entropy | SVM | 0 | 99.86 |
| 2019 | [43] | Approximate entropy | SVM | 0 | 95.1 |
| 2019 | [96] | Approximate entropy | / | 1 | / |
| 2019 | [97] | Symbolic transfer entropy | LDA | 0 | 68.8 |
| 2019 | [98] | Minimum error entropy | / | 0 | / |
| 2019 | [48] | Shannon entropy | CDAN | 0 | Under 70 |
*: In the dataset column, 0 represents public datasets, 1 represents private datasets, and 2 represents both.
Appendix C. Journals Used in This Review

| Journals | Number |
|---|---|
| Entropy | 8 |
| Frontiers in Neuroscience | 6 |
| BIOMEDICAL SIGNAL PROCESSING AND CONTROL | 4 |
| IEEE Transactions on Neural Systems and Rehabilitation Engineering | 3 |
| Asian Journal of Control | 2 |
| BioMedical Engineering OnLine | 2 |
| Electronics | 2 |
| IEEE Journal of Translational Engineering in Health and Medicine | 2 |
| Journal of Neuroscience Methods | 2 |
| Sensors | 2 |
| Applied Sciences | 1 |
| arXiv | 1 |
| Behavioral Brain Research | 1 |
| Biocybernetics and Biomedical Engineering | 1 |
| Biosensors | 1 |
| Brain Sciences | 1 |
| Cognitive Neurodynamics | 1 |
| Computational Intelligence and Neuroscience | 1 |
| Computer Methods in Biomechanics and Biomedical Engineering | 1 |
| Computers in Biology and Medicine | 1 |
| Evolving Systems | 1 |
| Expert Systems with Applications | 1 |
| Frontiers in Aging Neuroscience | 1 |
| Frontiers in Human Neuroscience | 1 |
| Frontiers in Neuroinformatics | 1 |
| IEEE Access | 1 |
| IEEE Sensors Journal | 1 |
| IEEE Signal Processing Letters | 1 |
| IEEE Transactions on Computational Social Systems | 1 |
| IEEE Transactions on Neural Networks and Learning Systems | 1 |
| International Journal of Pattern Recognition and Artificial Intelligence | 1 |
| Journal of Neural Engineering | 1 |
| Mathematical Biosciences and Engineering | 1 |
| Multimedia Tools and Applications | 1 |
| Neuroscience Letters | 1 |
| Neuroscience Research | 1 |
| PLOS ONE | 1 |
| Power and Entropy | 1 |
| Signal | 1 |
| Wireless Communications and Mobile Computing | 1 |
References
- Liu, H.; Du, Y.; Peng, J. A review of brain-computer interface development. Electron. Sci. Technol. 2011, 24, 116–119. [Google Scholar]
- Wang, H.; Jia, J.; Sun, L. Application of motor imagery therapy in upper limb rehabilitation of stroke patients and research progress of its neural mechanism. Chin. J. Phys. Med. Rehabil. 2019, 41, 473–476. [Google Scholar]
- Tariq, M.; Trivailo, P.M.; Simic, M. Mu-Beta event-related (de)synchronization and EEG classification of left-right foot dorsiflexion kinaesthetic motor imagery for BCI. PLoS ONE 2020, 15, e0230184. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, M.; Wang, Y.; Zhang, S.; Chen, L.; Ming, D. Enhance decoding of pre-movement EEG patterns for brain–computer interfaces. J. Neural Eng. 2019, 17, 016033. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, D.; Li, C.; Zhu, R. Ensemble classifier based on optimized extreme learning machine for motor imagery classification. J. Neural Eng. 2020, 17, 026004. [Google Scholar] [CrossRef]
- Wang, K.; Zhai, D.-H.; Xia, Y. Motor Imagination EEG Recognition Algorithm based on DWT, CSP and Extreme Learning Machine. In Proceedings of the 2019 Chinese Control Conference (CCC), Guangzhou, China, 27–30 July 2019; pp. 4590–4595. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, G.; Gao, D.; Zhang, Y. EEG classification using sparse Bayesian extreme learning machine for brain–computer interface. Neural Comput. Appl. 2018, 32, 6601–6609. [Google Scholar] [CrossRef]
- Ang, K.K.; Chin, Z.Y.; Wang, C.; Guan, C.; Zhang, H. Filter Bank Common Spatial Pattern Algorithm on BCI Competition IV Datasets 2a and 2b. Front. Neurosci. 2012, 6, 39. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wu, C.-W.; Lin, C.-T.; Chen, S.-A. A novel classification method for motor imagery based on Brain-Computer Interface. In Proceedings of the 2014 International Joint Conference on Neural Networks (IJCNN), Beijing, China, 6–11 July 2014; pp. 4099–4102. [Google Scholar] [CrossRef]
- Lotte, F.; Bougrain, L.; Cichocki, A.; Clerc, M.; Congedo, M.; Rakotomamonjy, A.; Yger, F. A review of classification algorithms for EEG-based brain–computer interfaces: A 10 year update. J. Neural Eng. 2018, 15, 031005. [Google Scholar] [CrossRef] [PubMed]
- Kevric, J.; Subasi, A. Comparison of signal decomposition methods in classification of EEG signals for motor-imagery BCI system. Biomed. Signal Process. Control 2017, 31, 398–406. [Google Scholar] [CrossRef]
- Park, S.; Ha, J.; Kim, D.-H.; Kim, L. Improving Motor Imagery-Based Brain-Computer Interface Performance Based on Sensory Stimulation Training: An Approach Focused on Poorly Performing Users. Front. Neurosci. 2021, 15, 732545. [Google Scholar] [CrossRef]
- Xu, P.; Wang, D. Research progress of information entropy in hydrology and water resources science. J. North China Univ. Water Resour. Hydropower (Nat. Sci. Ed.) 2017, 38, 3015–3028. [Google Scholar]
- Shannon, C.E. The mathematical theory of communication. 1963. M.D. Comput. Comput. Med. Pract. 1997, 14, 306–317. [Google Scholar]
- Jui, S.J.J.; Deo, R.C.; Barua, P.D.; Devi, A.; Soar, J.; Acharya, U.R. Application of Entropy for Automated Detection of Neurological Disorders With Electroencephalogram Signals: A Review of the Last Decade (2012–2022). IEEE Access 2023, 11, 71905–71924. [Google Scholar] [CrossRef]
- Yao, B.; Wu, C.; Zhang, X.; Yao, J.; Xue, J.; Zhao, Y.; Li, T.; Pu, J. The EEG-Based Fusion Entropy-Featured Identification of Isometric Contraction Forces Under the Same Action. Sensors 2024, 24, 2323. [Google Scholar] [CrossRef]
- Balmuri, K.R.; Madala, S.R.; Divakarachari, P.B.; de Prado, R.P.; Frnda, J. Enhanced grasshopper optimization algorithm with extreme learning machines for motor-imagery classification. Asian J. Control 2022, 25, 3015–3028. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, B.; Huang, L. Feature Extraction of EEG Signals Using Power Spectral Entropy. In Proceedings of the 2008 International Conference on BioMedical Engineering and Informatics, Sanya, China, 27–30 May 2008; pp. 435–439. [Google Scholar]
- Wang, K.; Tian, F.; Xu, M.; Zhang, S.; Xu, L.; Ming, D. Resting-State EEG in Alpha Rhythm May Be Indicative of the Performance of Motor Imagery-Based Brain–Computer Interface. Entropy 2022, 24, 1556. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, T.; Li, T. Detecting Epileptic Seizures in EEG Signals with Complementary Ensemble Empirical Mode Decomposition and Extreme Gradient Boosting. Entropy 2020, 22, 140. [Google Scholar] [CrossRef]
- Kojima, M.; Obuchi, S.; Mizuno, K.; Henmi, O.; Ikeda, N. Power Spectrum Entropy of Acceleration Time-series During Movement as an Indicator of Smoothness of Movement. J. Physiol. Anthr. 2008, 27, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.T.; Raj, A.N.J.; Balasubramanian, P.; Chen, Y. Schizophrenia detection using MultivariateEmpirical Mode Decomposition and entropy measures from multichannel EEG signal. Biocybern. Biomed. Eng. 2020, 40, 1124–1139. [Google Scholar] [CrossRef]
- Das, A.B.; Bhuiyan, M.I.H. Discrimination and classification of focal and non-focal EEG signals using entropy-based features in the EMD-DWT domain. Biomed. Signal Process. Control 2016, 29, 11–21. [Google Scholar] [CrossRef]
- Aydın, S.; Saraoğlu, H.M.; Kara, S. Log Energy Entropy-Based EEG Classification with Multilayer Neural Networks in Seizure. Ann. Biomed. Eng. 2009, 37, 2626–2630. [Google Scholar] [CrossRef]
- Delgado-Bonal, A.; Marshak, A. Approximate Entropy and Sample Entropy: A Comprehensive Tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Simons, S.; Espino, P.; Abásolo, D. Fuzzy Entropy Analysis of the Electroencephalogram in Patients with Alzheimer’s Disease: Is the Method Superior to Sample Entropy? Entropy 2018, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Zanin, M.; Zunino, L.; Rosso, O.A.; Papo, D. Permutation Entropy and Its Main Biomedical and Econophysics Applications: A Review. Entropy 2012, 14, 1553–1577. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Xie, H.; Yu, W. Characterization of Surface EMG Signal Based on Fuzzy Entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 266–272. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, W.; Wang, Y.-K.; Hussain, F.K.; Al-Jumaily, A.; Lin, C.-T. Effects of repetitive SSVEPs on EEG complexity using multiscale inherent fuzzy entropy. Neurocomputing 2019, 389, 198–206. [Google Scholar] [CrossRef]
- Li, L.; Chen, W.; Li, M. The application of hybrid feature based on local mean decomposition for motor imagery electroencephalogram signal classification. Asian J. Control 2023, 25, 3305–3317. [Google Scholar] [CrossRef]
- Schreiber, T. Measuring Information Transfer. Phys. Rev. Lett. 2000, 85, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Renyi, A. On measures of entropy and information. In Proceedings of the Fourth Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, CA, USA, 20 June–30 July 1960. [Google Scholar]
- Safara, F.; Ramaiah, A.R.A. RenyiBS: Renyi entropy basis selection from wavelet packet decomposition tree for phonocardiogram classification. J. Supercomput. 2020, 77, 3710–3726. [Google Scholar] [CrossRef]
- Xu, L.; Bai, L.; Jiang, X.; Tan, M.; Zhang, D.; Luo, B. Deep Rényi entropy graph kernel. Pattern Recognit. 2020, 111, 107668. [Google Scholar] [CrossRef]
- Choy, C.S.; Fang, Q.; Neville, K.; Ding, B.; Kumar, A.; Mahmoud, S.S.; Gu, X.; Fu, J.; Jelfs, B. Virtual reality and motor imagery for early post-stroke rehabilitation. Biomed. Eng. Online 2023, 22, 66. [Google Scholar] [CrossRef]
- Nikias, C.L.; Mendel, J.M. Signal processing with higher-order spectra. IEEE Signal Process. Mag. 1993, 10, 10–37. [Google Scholar] [CrossRef]
- Tsallis, C. Possible generalization of Boltzmann-Gibbs statistics. J. Stat. Phys. 1988, 52, 479–487. [Google Scholar] [CrossRef]
- Wu, D.; Jia, H.; Abualigah, L.; Xing, Z.; Zheng, R.; Wang, H.; Altalhi, M. Enhance Teaching-Learning-Based Optimization for Tsallis-Entropy-Based Feature Selection Classification Approach. Processes 2022, 10, 360. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, B.; Zou, G.; Yue, X.; Xu, Z.; Li, H. Domain Adaptation with Data Uncertainty Measure Based on Evidence Theory. Entropy 2022, 24, 966. [Google Scholar] [CrossRef]
- Yang, L.; Shi, T.; Lv, J.; Liu, Y.; Dai, Y.; Zou, L. A multi-feature fusion decoding study for unilateral upper-limb fine motor imagery. Math. Biosci. Eng. 2022, 20, 2482–2500. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, T.; Lun, X.; Wang, F. A novel method for classification of multi-class motor imagery tasks based on feature fusion. Neurosci. Res. 2021, 176, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Hossain, S.; Ahmed, K.; Mostafa, R.; Hadjileontiadis, L.; Khandoker, A.; Baumert, M. Wavelet Entropy-Based Inter-subject Associative Cortical Source Localization for Sensorimotor BCI. Front. Neurosci. 2019, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Ma, L.; Dong, H.; Zhang, X. EEG Signals Feature Extraction Based on DWT and EMD Combined with Approximate Entropy. Brain Sci. 2019, 9, 201. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.-H.; Yang, J.; Sawan, M. Intelligent Classification Technique of Hand Motor Imagery Using EEG Beta Rebound Follow-Up Pattern. Biosensors 2022, 12, 384. [Google Scholar] [CrossRef]
- Sun, H.; Jin, J.; Kong, W.; Zuo, C.; Li, S.; Wang, X. Novel channel selection method based on position priori weighted permutation entropy and binary gravity search algorithm. Cogn. Neurodyn. 2020, 15, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Rashid, N.; Shahzaib, M.; Malik, U.F.; Arif, A.; Iqbal, J.; Saleem, M.; Khan, U.S.; Tiwana, M. A novel framework for classification of two-class motor imagery EEG signals using logistic regression classification algorithm. PLoS ONE 2023, 18, e0276133. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, X. Conditional Adversarial Domain Adaptation Neural Network for Motor Imagery EEG Decoding. Entropy 2020, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Grijalva, A.; Muthireddy, V.S.S.R. Evaluation of Deep Neural Network Domain Adaptation Techniques for Image Recognition. arXiv 2021. [Google Scholar] [CrossRef]
- Xie, Y.; Oniga, S. Classification of Motor Imagery EEG Signals Based on Data Augmentation and Convolutional Neural Networks. Sensors 2023, 23, 1932. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, K.; Ma, J.; Wang, B.; Qiao, X.; Yan, Y.; Du, W.; Wanga, L. Massage Therapy’s Effectiveness on the Decoding EEG Rhythms of Left/Right Motor Imagery and Motion Execution in Patients With Skeletal Muscle Pain. IEEE J. Transl. Eng. Health Med. 2021, 9, 2100320. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazzaz, N.K.; Alyasseri, Z.A.A.; Abdulkareem, K.H.; Ali, N.S.; Al-Mhiqani, M.N.; Guger, C. EEG feature fusion for motor imagery: A new robust framework towards stroke patients rehabilitation. Comput. Biol. Med. 2021, 137, 104799. [Google Scholar] [CrossRef]
- Khare, S.K.; Bajaj, V. A facile and flexible motor imagery classification using electroencephalogram signals. Comput. Methods Programs Biomed. 2020, 197, 105722. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, T.; Wang, Q.; Zhu, Q.; Chen, J. EEG Signal Processing Based on Multivariate Empirical Mode Decomposition and Common Spatial Pattern Hybrid Algorithm. Int. J. Pattern Recognit. Artif. Intell. 2019, 33, 1959030. [Google Scholar] [CrossRef]
- Chen, S.; Luo, Z.; Gan, H. An entropy fusion method for feature extraction of EEG. Neural Comput. Appl. 2016, 29, 857–863. [Google Scholar] [CrossRef]
- Li, M.; Wang, R.; Yang, J.; Duan, L. An Improved Refined Composite Multivariate Multiscale Fuzzy Entropy Method for MI-EEG Feature Extraction. Comput. Intell. Neurosci. 2019, 2019, 7529572. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Qiao, L.; Lv, H. Cognitive Computing for Brain–Computer Interface-Based Computational Social Digital Twins Systems. IEEE Trans. Comput. Soc. Syst. 2022, 9, 1635–1643. [Google Scholar] [CrossRef]
- Li, X.; Chen, P.; Yu, X.; Jiang, N. Analysis of the Relationship Between Motor Imagery and Age-Related Fatigue for CNN Classification of the EEG Data. Front. Aging Neurosci. 2022, 14, 909571. [Google Scholar] [CrossRef]
- Wang, Y.; Song, C.; Zhang, T.; Yao, Z.; Chang, Z.; Wang, D. Feature Extraction of Motor Imagery EEG via Discrete Wavelet Transform and Generalized Maximum Fuzzy Membership Difference Entropy: A Comparative Study. Electronics 2023, 12, 2207. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, H.; Li, X.; Chen, S.; Gao, D.; Zhang, Y. Motor imagery classification method based on relative wavelet packet entropy brain network and improved lasso. Front. Neurosci. 2023, 17, 1113593. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Chaturvedi, A. Automatic EEG channel selection for multiclass brain-computer interface classification using multiobjective improved firefly algorithm. Multimed. Tools Appl. 2022, 82, 5405–5433. [Google Scholar] [CrossRef]
- Kumar, T.R.; Mahalaxmi, U.; Mm, R.; Bhatt, D. Optimization enabled deep residual neural network for motor imagery EEG signal classification. Biomed. Signal Process. Control 2022, 80, 104317. [Google Scholar] [CrossRef]
- Phadikar, S.; Sinha, N.; Ghosh, R. Unsupervised feature extraction with autoencoders for EEG based multiclass motor imagery BCI. Expert Syst. Appl. 2022, 213, 118901. [Google Scholar] [CrossRef]
- Maher, A.; Qaisar, S.M.; Salankar, N.; Jiang, F.; Tadeusiewicz, R.; Pławiak, P.; El-Latif, A.A.A.; Hammad, M. Hybrid EEG-fNIRS brain-computer interface based on the non-linear features extraction and stacking ensemble learning. Biocybern. Biomed. Eng. 2023, 43, 463–475. [Google Scholar] [CrossRef]
- Batistić, L.; Lerga, J.; Stanković, I. Detection of motor imagery based on short-term entropy of time–frequency representations. Biomed. Eng. Online 2023, 22, 41. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Aldoori, A.A.; Ali, S.H.B.M.; Ahmad, S.A.; Mohammed, A.K.; Mohyee, M.I. EEG Signal Complexity Measurements to Enhance BCI-Based Stroke Patients’ Rehabilitation. Sensors 2023, 23, 3889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Dong, R.; Lu, Z.; Li, C. Electroencephalogram and surface electromyogram fusion-based precise detection of lower limb voluntary movement using convolution neural network-long short-term memory model. Front. Neurosci. 2022, 16, 954387. [Google Scholar] [CrossRef]
- Wang, X.; Lu, H.; Shen, X.; Ma, L.; Wang, Y. Prosthetic control system based on motor imagery. Comput. Methods Biomech. Biomed. Eng. 2021, 25, 764–771. [Google Scholar] [CrossRef]
- Thenmozhi, T.; Helen, R. Feature Selection Using Extreme Gradient Boosting Bayesian Optimization to upgrade the Classification Performance of Motor Imagery signals for BCI. J. Neurosci. Methods 2021, 366, 109425. [Google Scholar] [CrossRef]
- Roy, G.; Bhoi, A.K.; Das, S.; Bhaumik, S. Cross-correlated spectral entropy-based classification of EEG motor imagery signal for triggering lower limb exoskeleton. Signal Image Video Process. 2022, 16, 1831–1839. [Google Scholar] [CrossRef]
- Roy, G.; Bhoi, A.; Bhaumik, S. A Comparative Approach for MI-Based EEG Signals Classification Using Energy, Power and Entropy. IRBM 2021, 43, 434–446. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, X.; Li, H.; Zhang, T.; Gu, L.; Tao, Q. An asynchronous artifact-enhanced electroencephalogram based control paradigm assisted by slight facial expression. Front. Neurosci. 2022, 16, 892794. [Google Scholar] [CrossRef] [PubMed]
- King, J.-T.; John, A.R.; Wang, Y.-K.; Shih, C.-K.; Zhang, D.; Huang, K.-C.; Lin, C.-T. Brain Connectivity Changes During Bimanual and Rotated Motor Imagery. IEEE J. Transl. Eng. Health Med. 2022, 10, 2100408. [Google Scholar] [CrossRef]
- Jin, J.; Sun, H.; Daly, I.; Li, S.; Liu, C.; Wang, X.; Cichocki, A. A Novel Classification Framework Using the Graph Representations of Electroencephalogram for Motor Imagery Based Brain-Computer Interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 30, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, S.; Ma, Z.; Lei, W.; Chen, C. Regularized RKHS-Based Subspace Learning for Motor Imagery Classification. Entropy 2022, 24, 195. [Google Scholar] [CrossRef]
- Gao, C.; Sun, J. Motor Imagery Recognition Method Based on Multisource Transfer Learning and Multiclassifier Fusion. Wirel. Commun. Mob. Comput. 2022, 2022, 3893866. [Google Scholar] [CrossRef]
- Bang, J.-S.; Lee, M.-H.; Fazli, S.; Guan, C.; Lee, S.-W. Spatio-Spectral Feature Representation for Motor Imagery Classification Using Convolutional Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2021, 33, 3038–3049. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, G.; Du, C.; Liang, R.; Han, C.; Zheng, X.; Zhang, S.; Wang, J.; Tian, P.; Jia, Y. Enhancement of capability for motor imagery using vestibular imbalance stimulation during brain computer interface. J. Neural Eng. 2021, 18, 056064. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xi, X.; Wang, T.; Wang, J.; Kong, W.; Zhao, Y.-B.; Zhang, Q. Effects of transcranial direct current stimulation on brain network connectivity and complexity in motor imagery. Neurosci. Lett. 2021, 757, 135968. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Y.; Li, Z.; Huang, S.; Zhang, D. Neural Decoding of Chinese Sign Language With Machine Learning for Brain–Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2721–2732. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mosavi, M.R. Comparison of two methods of removing EOG artifacts for use in a motor imagery-based brain computer interface. Evol. Syst. 2019, 12, 527–540. [Google Scholar] [CrossRef]
- Marcos-Martínez, D.; Martínez-Cagigal, V.; Santamaría-Vázquez, E.; Pérez-Velasco, S.; Hornero, R. Neurofeedback Training Based on Motor Imagery Strategies Increases EEG Complexity in Elderly Population. Entropy 2021, 23, 1574. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Youn, J.; Kim, S.-H.; Kim, J. Effects of Frontal Theta Rhythms in a Prior Resting State on the Subsequent Motor Imagery Brain-Computer Interface Performance. Front. Neurosci. 2021, 15, 663101. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Ghorbanzadeh, P.; Rastegarnia, A. Probability mapping based artifact detection and removal from single-channel EEG signals for brain-computer interface applications. J. Neurosci. Methods 2021, 360, 109249. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, N.; Choi, K.-S. A Generalizable and Discriminative Learning Method for Deep EEG-Based Motor Imagery Classification. Front. Neurosci. 2021, 15, 760979. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xie, J.; Pan, C.; Wu, X.; Hu, D. Multi-feature fusion method based on WOSF and MSE for four-class MI EEG identification. Biomed. Signal Process. Control 2021, 69, 102907. [Google Scholar] [CrossRef]
- Panche, I.D.L.P.; Álvarez-Meza, A.; Gómez, P.M.H.; Cárdenas-Peña, D.; Patiño, J.I.R.; Orozco-Gutiérrez, Á. Kernel-Based Phase Transfer Entropy with Enhanced Feature Relevance Analysis for Brain Computer Interfaces. Appl. Sci. 2021, 11, 6689. [Google Scholar] [CrossRef]
- Xu, C.; Sun, C.; Jiang, G.; Chen, X.; He, Q.; Xie, P. Two-level multi-domain feature extraction on sparse representation for motor imagery classification. Biomed. Signal Process. Control 2020, 62, 102160. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Yang, Z.; Hu, X.; Zhang, C. A method from offline analysis to online training for the brain-computer interface based on motor imagery and speech imagery. Biomed. Signal Process. Control 2020, 62, 102100. [Google Scholar] [CrossRef]
- Velasquez-Martinez, L.; Caicedo-Acosta, J.; Castellanos-Dominguez, G. Entropy-Based Estimation of Event-Related De/Synchronization in Motor Imagery Using Vector-Quantized Patterns. Entropy 2020, 22, 703. [Google Scholar] [CrossRef]
- Trendafilov, D.; Schmitz, G.; Hwang, T.-H.; Effenberg, A.O.; Polani, D. Tilting Together: An Information-Theoretic Characterization of Behavioral Roles in Rhythmic Dyadic Interaction. Front. Hum. Neurosci. 2020, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Ren, L.; Cui, W. Feature Extraction of Brain–Computer Interface Electroencephalogram Based on Motor Imagery. IEEE Sens. J. 2019, 20, 11787–11794. [Google Scholar] [CrossRef]
- Li, M.; Wang, R.; Xu, D. An Improved Composite Multiscale Fuzzy Entropy for Feature Extraction of MI-EEG. Entropy 2020, 22, 1356. [Google Scholar] [CrossRef]
- Kwon, M.; Cho, H.; Won, K.; Ahn, M.; Jun, S.C. Use of Both Eyes-Open and Eyes-Closed Resting States May Yield a More Robust Predictor of Motor Imagery BCI Performance. Electronics 2020, 9, 690. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Z.L.; Gu, Z.; Li, Y. Deep Temporal-Spatial Feature Learning for Motor Imagery-Based Brain–Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Zeming, F.; Rehman, A.U.; Ullah, I.; Li, G.; Xiao, G. Motor Imagery EEG Signals Decoding by Multivariate Empirical Wavelet Transform-Based Framework for Robust Brain–Computer Interfaces. IEEE Access 2019, 7, 171431–171451. [Google Scholar] [CrossRef]
- Ingram, T.G.; Solomon, J.P.; Westwood, D.A.; Boe, S.G. Movement related sensory feedback is not necessary for learning to execute a motor skill. Behav. Brain Res. 2018, 359, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Panche, I.D.L.P.; Alvarez-Meza, A.M.; Orozco-Gutierrez, A. A Data-Driven Measure of Effective Connectivity Based on Renyi’s α-Entropy. Front. Neurosci. 2019, 13, 1277. [Google Scholar] [CrossRef]
- Chen, B.; Ma, R.; Yu, S.; Du, S.; Qin, J. Granger Causality Analysis Based on Quantized Minimum Error Entropy Criterion. IEEE Signal Process. Lett. 2019, 26, 347–351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).