Comparison of Enriched Acoustic Environment and White Noise as Sound Stimuli for Tinnitus Treatment: A 4-Month Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
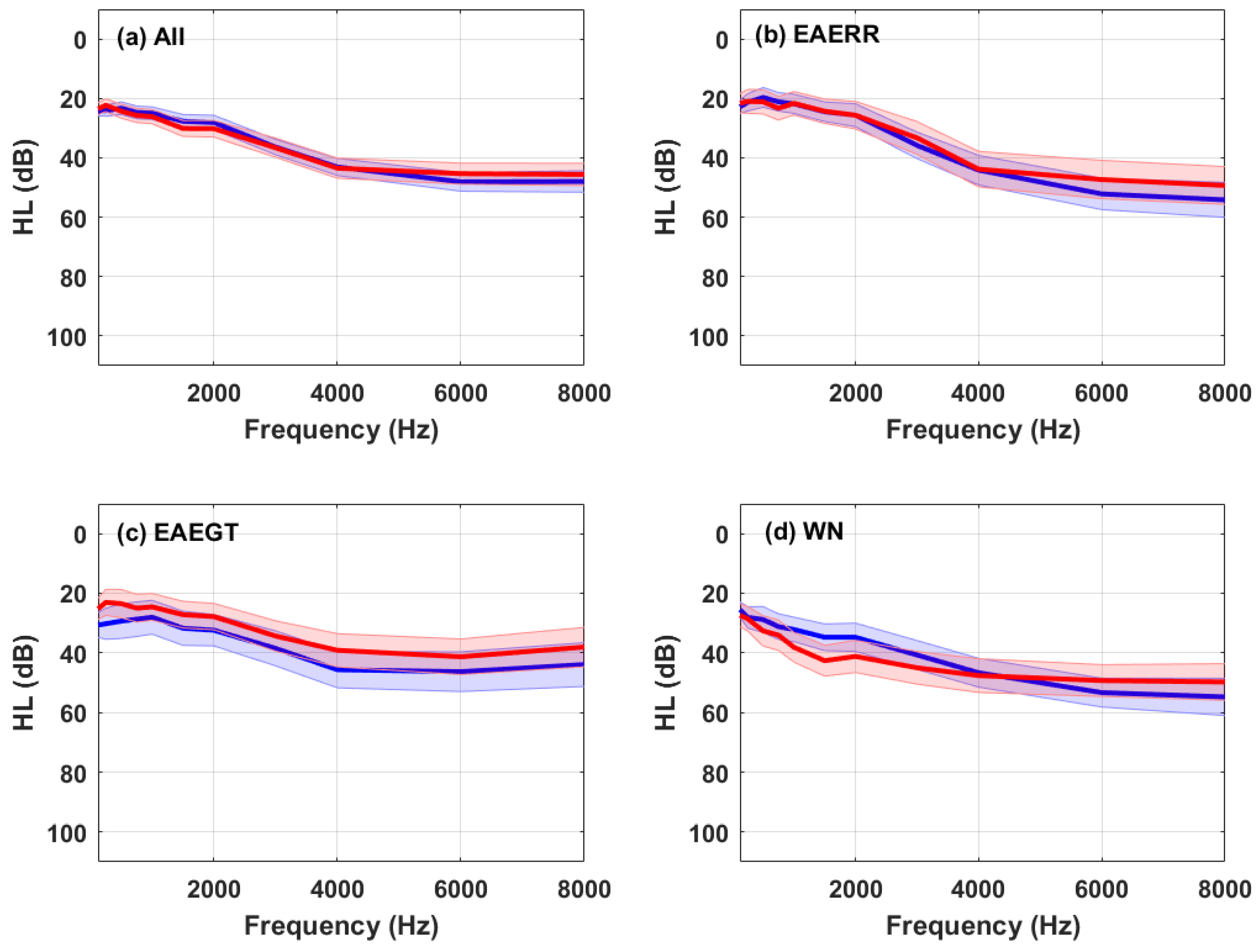
2.2. Hearing Levels
2.3. Tinnitus Characteristics
2.4. Counseling
2.5. Sound Therapy
2.6. Tinnitus Severity
2.7. Emotional State
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jastreboff, P.J.; Hazell, J.W.P. Tinnitus Retraining Therapy: Implementing the Neurophysiological Model; Cambridge University Press: Cambridge, UK, 2004; ISBN 978-0-521-08837-4. [Google Scholar]
- Jarach, C.M.; Lugo, A.; Scala, M.; Van Den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-Analysis. JAMA Neurol. 2022, 79, 888. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Schechter, M.A.; Zaugg, T.L.; Griest, S.; Jastreboff, P.J.; Vernon, J.A.; Kaelin, C.; Meikle, M.B.; Lyons, K.S.; Stewart, B.J. Clinical Trial to Compare Tinnitus Masking and Tinnitus Retraining Therapy. Acta Otolaryngol. Suppl. 2006, 17, 64–69. [Google Scholar] [CrossRef]
- Hoare, D.J.; Kowalkowski, V.L.; Kang, S.; Hall, D.A. Systematic Review and Meta-Analyses of Randomized Controlled Trials Examining Tinnitus Management. Laryngoscope 2011, 121, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Searchfield, G.D.; Durai, M.; Linford, T. A State-of-the-Art Review: Personalization of Tinnitus Sound Therapy. Front. Psychol. 2017, 8, 1599. [Google Scholar] [CrossRef]
- Durai, M.; Searchfield, G.D. A Mixed-Methods Trial of Broad Band Noise and Nature Sounds for Tinnitus Therapy: Group and Individual Responses Modeled under the Adaptation Level Theory of Tinnitus. Front. Aging Neurosci. 2017, 9, 44. [Google Scholar] [CrossRef]
- Hobson, J.; Chisholm, E.; El Refaie, A. Sound Therapy (Masking) in the Management of Tinnitus in Adults. Cochrane Database Syst. Rev. 2010, 12, CD006371. [Google Scholar] [CrossRef]
- Henry, J.A.; Griest, S.; Thielman, E.; McMillan, G.; Kaelin, C.; Carlson, K.F. Tinnitus Functional Index: Development, Validation, Outcomes Research, and Clinical Application. Hear. Res. 2016, 334, 58–64. [Google Scholar] [CrossRef]
- Kim, B.J.; Chung, S.-W.; Jung, J.Y.; Suh, M.-W. Effect of Different Sounds on the Treatment Outcome of Tinnitus Retraining Therapy. Clin. Exp. Otorhinolaryngol. 2014, 7, 87–93. [Google Scholar] [CrossRef]
- Barozzi, S.; Del Bo, L.; Crocetti, A.; Dyrlund, O.; Passoni, S.; Zolin, A.; Panicucci, E.; Mancuso, A.; Kaur, M.; Searchfield, G.D. A Comparison of Nature and Technical Sounds for Tinnitus Therapy. Acta Acust. United Acust. 2016, 102, 540–546. [Google Scholar] [CrossRef]
- Davis, P.B.; Wilde, R.A.; Steed, L.G.; Hanley, P.J. Treatment of Tinnitus with a Customized Acoustic Neural Stimulus: A Controlled Clinical Study. Ear Nose Throat J. 2008, 87, 330–339. [Google Scholar] [CrossRef]
- Henry, J.A.; Rheinsburg, B.; Zaugg, T. Comparison of Custom Sounds for Achieving Tinnitus Relief. J. Am. Acad. Audiol. 2004, 15, 585–598. [Google Scholar] [CrossRef]
- Kidd, G.; Mason, C.R.; Arbogast, T.L. Similarity, Uncertainty, and Masking in the Identification of Nonspeech Auditory Patterns. J. Acoust. Soc. Am. 2002, 111, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Cobo, P.; Cuesta, M.; De La Colina, C. Customised Enriched Acoustic Environment for Sound Therapy of Tinnitus. Acta Acust. 2021, 5, 34. [Google Scholar] [CrossRef]
- Cuesta, M.; Garzón, C.; Cobo, P. Efficacy of Sound Therapy for Tinnitus Using an Enriched Acoustic Environment with Hearing-Loss Matched Broadband Noise. Brain Sci. 2022, 12, 82. [Google Scholar] [CrossRef]
- Fernández, M.; Cuesta, M.; Sanz, R.; Cobo, P. Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes. Audiol. Res. 2022, 13, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Cobo, P. Enriched Acoustic Environment as a Customized Treatment for Tinnitus: A Non-Controlled Longitudinal Study. J. Otol. 2024, 19, 63–71. [Google Scholar] [CrossRef]
- Schaette, R.; Kempter, R. Development of Tinnitus-Related Neuronal Hyperactivity through Homeostatic Plasticity after Hearing Loss: A Computational Model. Eur. J. Neurosci. 2006, 23, 3124–3138. [Google Scholar] [CrossRef]
- Noreña, A.J.; Eggermont, J.J. Enriched Acoustic Environment after Noise Trauma Reduces Hearing Loss and Prevents Cortical Map Reorganization. J. Neurosci. 2005, 25, 699–705. [Google Scholar] [CrossRef]
- Fernández-Ledesma, M.; Sanz-Fernández, R.; Ruiz, M.C.; Parra, P.C. Asociación entre el estado emocional y el grado de severidad del acúfeno en una cohorte de 53 participantes: Estado emocional y severidad del acúfeno. Auditio 2024, 8, e105. [Google Scholar] [CrossRef]
- Sendesen, E.; Turkyilmaz, D. Investigation of the Effectiveness of Sound Enrichment in the Treatment of Tinnitus Due to Hearing Loss. Brain Behav. 2024, 14, e3520. [Google Scholar] [CrossRef]
- Herráiz, C.; Hernández Calvín, F.J.; Plaza, G.; Tapia, M.C.; De los Santos, G. Evaluación de la incapacidad en los pacientes con acúfenos (Evaluation of handicap in tinnitus patients). Acta Otorrinolaringol. Esp. 2001, 52, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Reixach, M.M.; Sampedro, J.J.N.; Minguez, M.S.G.; Rey-Martínez, J.; Altuna, X. Translation into Spanish and Validation of the Tinnitus Functional Index (TFI). Acta Otorrinolaringol. Esp. 2023, 74, 305–314. [Google Scholar] [CrossRef]
- Langguth, B.; De Ridder, D. Minimal Clinically Important Difference of Tinnitus Outcome Measurement Instruments—A Scoping Review. J. Clin. Med. 2023, 12, 7117. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, P.J. 25 Years of Tinnitus Retraining Therapy. HNO 2015, 63, 307–311. [Google Scholar] [CrossRef]
- Fackrell, K.; Hall, D.A.; Barry, J.; Hoare, D.J. Integrating Distribution-Based and Anchor-Based Techniques to Identify Minimal Important Change for the Tinnitus Functional Index (TFI) Questionnaire. Brain Sci. 2022, 12, 726. [Google Scholar] [CrossRef]
- Zeman, F.; Koller, M.; Figueiredo, R.; Aazevedo, A.; Rates, M.; Coelho, C.; Kleinjung, T.; De, R.D.; Langguth, B.; Landgrebe, M. Tinnitus Handicap Inventory for Evaluating Treatment Effects: Which Changes Are Clinically Relevant? Otolaryngol. Head Neck Surg. 2011, 145, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- López-Roig, S. Ansiedad y depresión. Validación de la escala HADS en pacientes oncológicos. Rev. Psicol. Salud 2000, 12, 127–155. [Google Scholar] [CrossRef]
- Puhan, M.A.; Frey, M.; Büchi, S.; Schünemann, H.J. The Minimal Important Difference of the Hospital Anxiety and Depression Scale in Patients with Chronic Obstructive Pulmonary Disease. Health Qual. Life Outcomes 2008, 6, 46. [Google Scholar] [CrossRef]
- de Filippis, R.; Mercurio, M.; Segura-Garcia, C.; De Fazio, P.; Gasparini, G.; Galasso, O. Defining the Minimum Clinically Important Difference (MCID) in the Hospital Anxiety and Depression Scale (HADS) in Patients Undergoing Total Hip and Knee Arthroplasty. Orthop. Traumatol. Surg. Res. 2023, 110, 103689. [Google Scholar] [CrossRef]
- Schlee, W.; Langguth, B.; De Ridder, D.; Vanneste, S.; Kleinjung, T.; Møller, A.R. (Eds.) Textbook of Tinnitus; Springer International Publishing: Cham, Switzerland, 2024; ISBN 978-3-031-35646-9. [Google Scholar]
- Roberts, L.E.; Husain, F.T.; Eggermont, J.J. Role of Attention in the Generation and Modulation of Tinnitus. Neurosci. Biobehav. Rev. 2013, 37, 1754–1773. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M.M.; Stegeman, I.; Ho-Kang-You, K.E.; Stokroos, R.J.; Smit, A.L. The Effect of Mindfulness-Based Interventions on Tinnitus Distress. A Systematic Review. Front. Neurol. 2019, 10, 1135. [Google Scholar] [CrossRef]
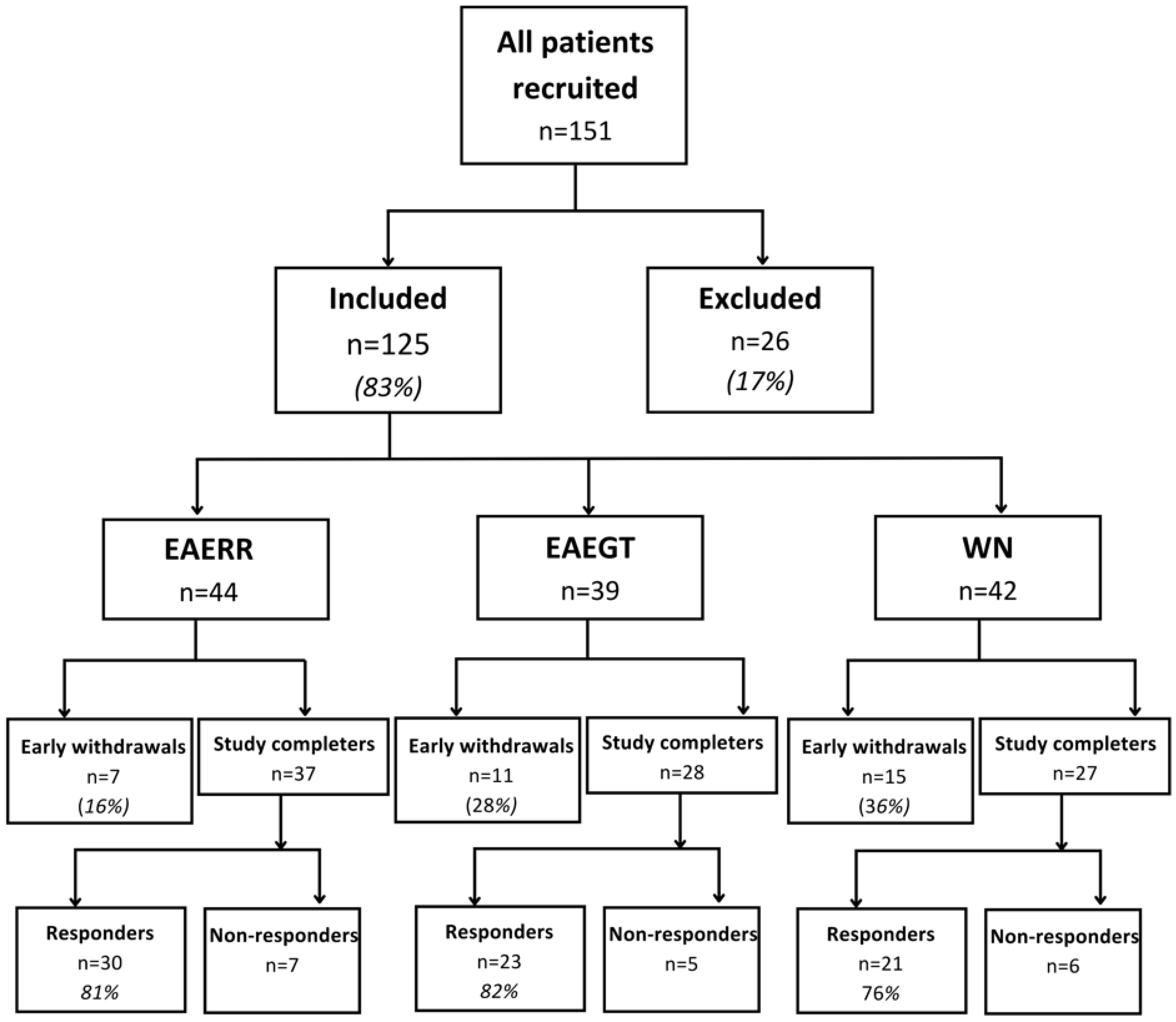

| All (n = 74) | EAERR (n = 30) | EAEGT (n = 23) | WN (n = 21) | |
|---|---|---|---|---|
| Gender | Male: 48 (62%) | Male: 20 (67%) | Male: 13 (57%) | Male: 15 (71%) |
| Fem: 26 (38%) | Fem: 10 (33%) | Fem: 10 (43%) | Fem: 6 (29%) | |
| Age (years) | M: 52.9 SD: 11.8 | M: 53.3 SD: 12.2 | M: 51.9 SD. 11.7 | M: 53.3 SD: 11.0 |
| Tinnitus duration (years) | M: 7.8 SD: 10.8 | M: 8.2 SD: 11.5 | M: 9.1 SD: 10.7 | M: 5.8 SD: 10.2 |
| Tinnitus pitch (Hz) | M: 4342 SD: 2563 | M: 5242 SD: 2888 | M: 3344 SD: 2113 | M: 4149 SD: 2140 |
| THIbaseline | M: 53.5 SD: 22.2 | M: 50.85 SD: 23.2 | M: 52.9 SD: 21.0 | M: 58.8 SD: 22.6 |
| All (n = 18) | EAERR (n = 7) | EAEGT (n = 5) | WN (n = 6) | |
|---|---|---|---|---|
| Gender | Male: 16 (89%) | Male: 7 (100%) | Male: 3 (60%) | Male: 6 (100%) |
| Fem: 2 (11%) | Fem: 0 (0%) | Fem: 2 (40%) | Fem: 0 (0%) | |
| Age (years) | M: 58.1 SD: 13.3 | M: 57.3 SD: 10.4 | M: 58.4 SD. 15.7 | M: 58.8 SD: 13.1 |
| Tinnitus duration (years) | M: 8.7 SD: 10.6 | M: 3.0 SD: 0.9 | M: 6.7 SD: 6.8 | M: 17.0 SD: 12.9 |
| Tinnitus pitch (Hz) | M: 5762.6 SD: 3414.2 | M: 5702.7 SD: 2453.6 | M: 5947.0 SD: 5345.0 | M: 5678.7 SD: 1468.8 |
| THIbaseline | M: 53.7 SD: 23.2 | M: 52.3 SD: 24.2 | M: 58.8 SD: 23.4 | M: 51.0 SD: 18.7 |
| Stimuli | THI | TFI | HADA | HADD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THI0 | THI4 | ΔTHI | TFI0 | TFI4 | ΔTFI | HADSA0 | HADSA4 | ΔHADSA | HADSD0 | HADSD4 | ΔHADSD | |
| EAERR | 50.8 | 24.7 | 26.1 (***) | 51.8 | 25.5 | 26.2 (***) | 11.4 | 5.9 | 5.5 (**) | 6.6 | 4.1 | 2.5 (*) |
| EAEGT | 52.9 | 25.9 | 27.0 (***) | 55.2 | 27.7 | 27.4 (***) | 10.6 | 5.7 | 4.9 (***) | 6.9 | 3.5 | 3.4 (***) |
| WN | 58.0 | 29.1 | 28.9 (***) | 60.2 | 31.1 | 29.2 (***) | 10.1 | 6.5 | 3.6 (**) | 6.7 | 4.3 | 2.4 |
| Stimuli | THI | TFI | HADA | HADD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THI0 | THI4 | ΔTHI | TFI0 | TFI4 | ΔTFI | HADSA0 | HADSA4 | ΔHADSA | HADSD0 | HADSD4 | ΔHADSD | |
| EAERR | 52.2 | 51.4 | 0.9 | 57.3 | 56.4 | 0.9 | 10.9 | 9.0 | 1.9 | 6.9 | 4.9 | 2.0 |
| EAEGT | 58.8 | 64.0 | −5.2 | 61.4 | 63.9 | −2.6 | 10.8 | 11.2 | −0.4 | 8.0 | 9.6 | −1.6 |
| WN | 51.0 | 63.0 | −12. | 58.3 | 73.5 | −15.3 | 7.8 | 9.3 | −1.5 | 5.5 | 7.0 | −1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ledesma, M.; Sanz-Fernández, R.; Cuesta, M.; Cobo, P. Comparison of Enriched Acoustic Environment and White Noise as Sound Stimuli for Tinnitus Treatment: A 4-Month Feasibility Study. Brain Sci. 2025, 15, 342. https://doi.org/10.3390/brainsci15040342
Fernández-Ledesma M, Sanz-Fernández R, Cuesta M, Cobo P. Comparison of Enriched Acoustic Environment and White Noise as Sound Stimuli for Tinnitus Treatment: A 4-Month Feasibility Study. Brain Sciences. 2025; 15(4):342. https://doi.org/10.3390/brainsci15040342
Chicago/Turabian StyleFernández-Ledesma, Marta, Ricardo Sanz-Fernández, María Cuesta, and Pedro Cobo. 2025. "Comparison of Enriched Acoustic Environment and White Noise as Sound Stimuli for Tinnitus Treatment: A 4-Month Feasibility Study" Brain Sciences 15, no. 4: 342. https://doi.org/10.3390/brainsci15040342
APA StyleFernández-Ledesma, M., Sanz-Fernández, R., Cuesta, M., & Cobo, P. (2025). Comparison of Enriched Acoustic Environment and White Noise as Sound Stimuli for Tinnitus Treatment: A 4-Month Feasibility Study. Brain Sciences, 15(4), 342. https://doi.org/10.3390/brainsci15040342








