γ-Aminobutyric Acid Transporter Mutation GAT1 (S295L) Substantially Impairs Neurogenesis in Dentate Gyrus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. mRNA Expression Analysis
| Bdnf | rt-f: | 5′-TCATACTTCGGTTGCATGAAGG-3′; |
| Bdnf | rt-r: | 5′-AGACCTCTCGAACCTGCCC-3′; |
| Gabra1 | rt-f: | 5′-AAAAGTCGGGGTCTCTCTGAC-3′; |
| Gabra1 | rt-r: | 5′-CAGTCGGTCCAAAATTCTTGTGA-3′; |
| Gabra2 | rt-f: | 5′-GGACCCAGTCAGGTTGGTG-3′; |
| Gabra2 | rt-r: | 5′-TCCTGGTCTAAGCCGATTATCAT-3′; |
| Gabrg2 | rt-f: | 5′-AGAAAAACCCTCTTCTTCGGATG-3′; |
| Gabrg2 | rt-r: | 5′-GTGGCATTGTTCATTTGAATGGT-3′; |
| Hprt | rt-f: | 5′-CTTTGCTGACCTGCTGGATT-3′; |
| Hprt | rt-r: | 5′-TATGTCCCCCGTTGACTGAT-3′; |
| Slc6a1 | rt-f: | 5′-GAAAGCTGTCTGATTCTGAGGTG-3′; |
| Slc6a1 | rt-r: | 5′-AGCAAACGATGATGGAGTCCC-3′. |
2.3. Western Blotting
2.4. Mouse Immunofluorescence
2.5. Mouse Immunohistochemistry
2.6. BrdU Labeling Experiment
2.7. Elisa
2.8. Confocal Microscopy and Image Acquisition
2.9. Sholl Analysis and Three-Dimensional (3D) Reconstruction of DCX+ Immature Neurons
2.10. RNA-Seq and Data Analyses
2.11. Statistical Analysis
3. Results
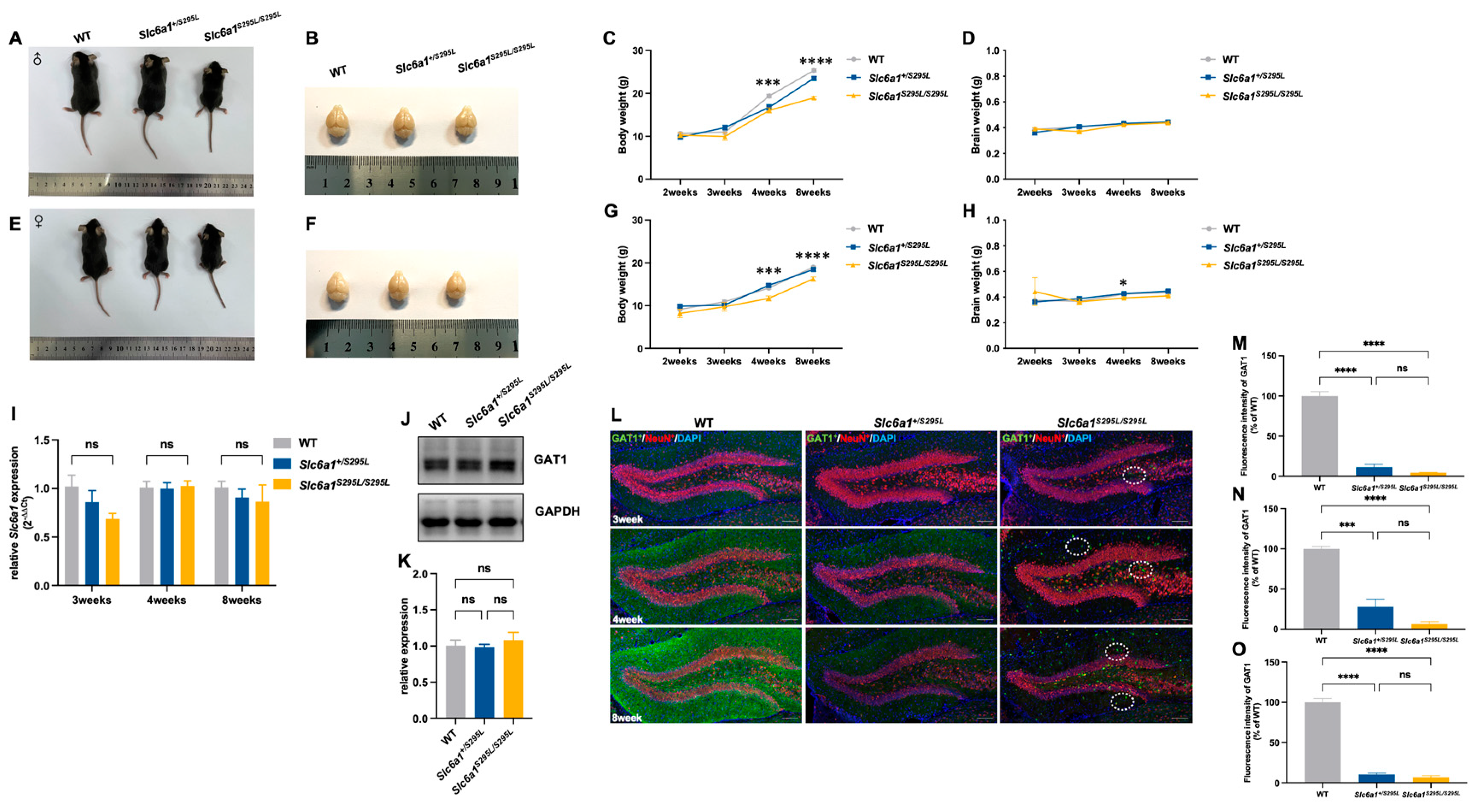
3.1. Slc6a1S295L/S295L Mice Exhibited Weight Loss and Impaired GAT1 Expression
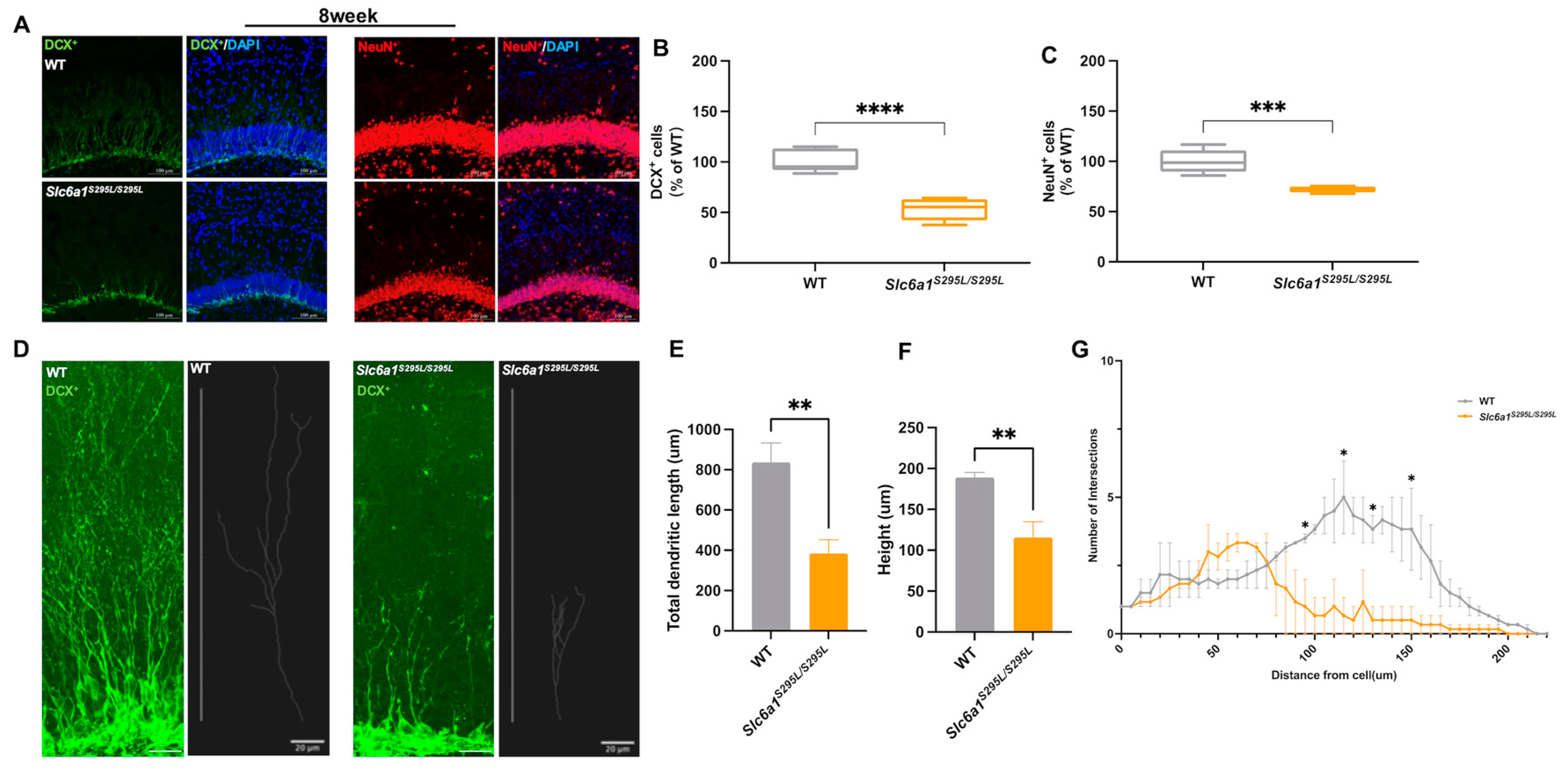
3.2. GAT1 (S295L) Impaired Neurogenesis in the Hippocampus of Adult Slc6a1 Mutant Mice
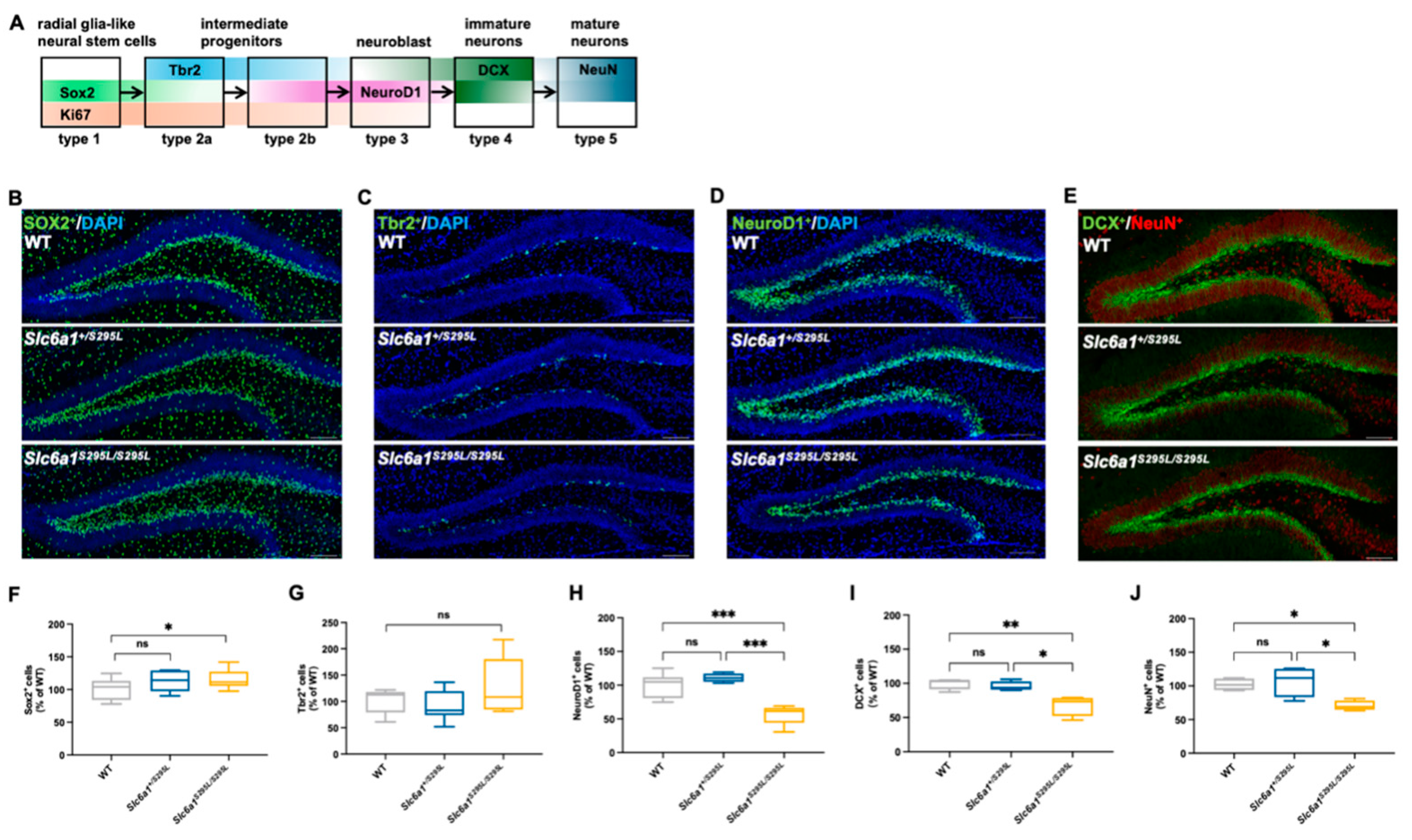
3.3. GAT1 (S295L) Impaired the Neuronal Fate Choices in Neurogenesis in the DG of Mutant Mice
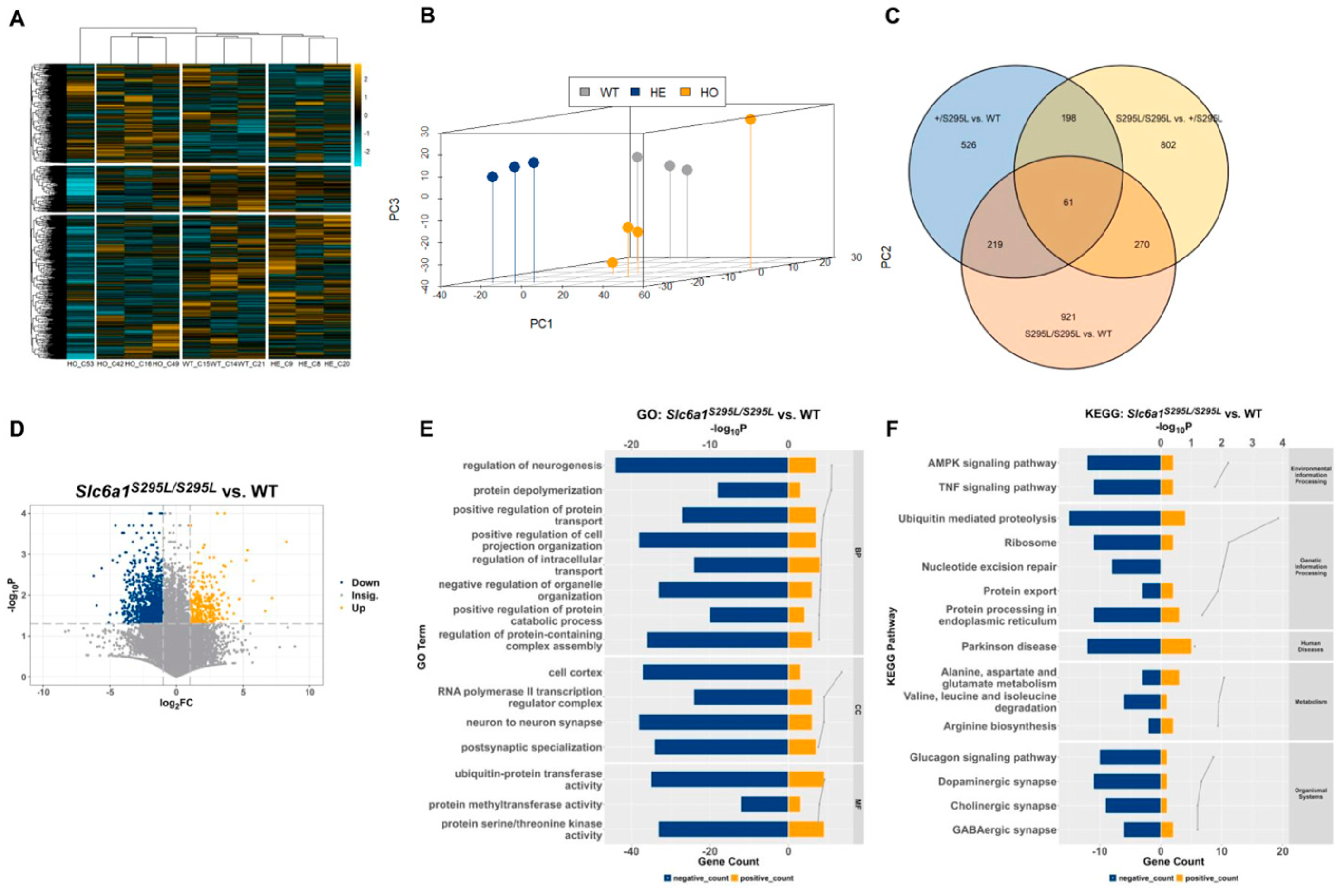
3.4. Transcriptome Analysis Provided Further Evidence of Role of Slc6a1 (S295L) Mutation in Neurodevelopmental Disorders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GABA | γ-Aminobutyric acid |
| GAT1 | GABA transporter 1 |
| DG | Dentate gyrus |
| NSCs | Neural stem cells |
| Sox2 | SRY-box transcription factor 2 |
| Tbr2 | T-box brain protein 2 |
| NeuroD1 | Neurogenic differentiation 1 |
| DCX | Doublecortin |
| NeuN | Neuronal nuclear protein |
| WT | Wild type |
| BDNF | Brain-derived neurotrophic factor |
References
- Boyle, C.A.; Boulet, S.; Schieve, L.A.; Cohen, R.A.; Blumberg, S.J.; Yeargin-Allsopp, M.; Visser, S.; Kogan, M.D. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011, 127, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Zafar, S.; Jabeen, I. Structure, Function, and Modulation of γ-Aminobutyric Acid Transporter 1 (GAT1) in Neurological Disorders: A Pharmacoinformatic Prospective. Front. Chem. 2018, 6, 397. [Google Scholar] [CrossRef]
- Stefanski, A.; Pérez-Palma, E.; Brünger, T.; Montanucci, L.; Gati, C.; Klöckner, C.; Johannesen, K.M.; Goodspeed, K.; Macnee, M.; Deng, A.T.; et al. SLC6A1 variant pathogenicity, molecular function and phenotype: A genetic and clinical analysis. Brain 2023, 146, 5198–5208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Poliquin, S.; Mermer, F.; Eissman, J.; Delpire, E.; Wang, J.; Shen, W.; Cai, K.; Li, B.M.; Li, Z.Y.; et al. Endoplasmic reticulum retention and degradation of a mutation in SLC6A1 associated with epilepsy and autism. Mol. Brain 2020, 13, 76. [Google Scholar] [CrossRef]
- Poliquin, S.; Hughes, I.; Shen, W.; Mermer, F.; Wang, J.; Mack, T.; Xu, D.; Kang, J.Q. Genetic mosaicism, intrafamilial phenotypic heterogeneity, and molecular defects of a novel missense SLC6A1 mutation associated with epilepsy and ADHD. Exp. Neurol. 2021, 342, 113723. [Google Scholar] [CrossRef]
- Wang, J.; Luttrell, J., IV; Zhang, N.; Khan, S.; Shi, N.; Wang, M.X.; Kang, J.-Q.; Wang, Z.; Xu, D. Exploring Human Diseases and Biological Mechanisms by Protein Structure Prediction and Modeling. Adv. Exp. Med. Biol. 2016, 939, 39–61. [Google Scholar] [CrossRef]
- Wang, J.; Shen, D.; Xia, G.; Shen, W.; Macdonald, R.; Xu, D.; Kang, J.-Q. Differential protein structural disturbances and suppression of assembly partners produced by nonsense GABRG2 epilepsy mutations: Implications for disease phenotypic heterogeneity. Sci. Rep. 2016, 6, 35294. [Google Scholar] [CrossRef]
- Mermer, F.; Poliquin, S.; Rigsby, K.; Rastogi, A.; Shen, W.; Romero-Morales, A.I.; Nwosu, G.I.; McGrath, P.; Demerast, S.; Aoto, J.; et al. Common molecular mechanisms of SLC6A1 variant-mediated neurodevelopmental disorders in astrocytes and neurons. Brain 2021, 144, 2499–2512. [Google Scholar] [CrossRef]
- Shen, W.; Nwosu, G.; Honer, M.; Clasadonte, J.; Schmalzbauer, S.; Biven, M.; Langer, K.; Flamm, C.; Poliquin, S.; Mermer, F.; et al. γ-Aminobutyric acid transporter and GABA(A) receptor mechanisms in Slc6a1(+/A288V) and Slc6a1(+/S295L) mice associated with developmental and epileptic encephalopathies. Brain Commun. 2024, 6, fcae110. [Google Scholar] [CrossRef] [PubMed]
- Accogli, A.; Addour-Boudrahem, N.; Srour, M. Neurogenesis, neuronal migration, and axon guidance. Handb. Clin. Neurol. 2020, 173, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhong, C.; Bonaguidi, M.A.; Sun, G.J.; Hsu, D.; Gu, Y.; Meletis, K.; Huang, Z.J.; Ge, S.; Enikolopov, G.; et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012, 489, 150–154. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Mercurio, S.; Serra, L.; Nicolis, S.K. More than just Stem Cells: Functional Roles of the Transcription Factor Sox2 in Differentiated Glia and Neurons. Int. J. Mol. Sci. 2019, 20, 4540. [Google Scholar] [CrossRef] [PubMed]
- Englund, C.; Fink, A.; Lau, C.; Pham, D.; Daza, R.A.M.; Bulfone, A.; Kowalczyk, T.; Hevner, R.F. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 247–251. [Google Scholar] [CrossRef]
- Hodge, R.D.; Kowalczyk, T.D.; Wolf, S.A.; Encinas, J.M.; Rippey, C.; Enikolopov, G.; Kempermann, G.; Hevner, R.F. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 3707–3717. [Google Scholar] [CrossRef]
- Amador-Arjona, A.; Cimadamore, F.; Huang, C.-T.; Wright, R.; Lewis, S.; Gage, F.H.; Terskikh, A.V. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E1936–E1945. [Google Scholar] [CrossRef]
- Pérez-Domínguez, M.; Tovar-Y-Romo, L.B.; Zepeda, A. Neuroinflammation and physical exercise as modulators of adult hippocampal neural precursor cell behavior. Rev. Neurosci. 2018, 29, 1–20. [Google Scholar] [CrossRef]
- Ge, S.; Goh, E.L.K.; Sailor, K.A.; Kitabatake, Y.; Ming, G.L.; Song, H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006, 439, 589–593. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Khazipov, R.; Leinekugel, X.; Caillard, O.; Gaiarsa, J.L. GABAA, NMDA and AMPA receptors: A developmentally regulated “ménage à trois”. Trends Neurosci. 1997, 20, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Kriegstein, A.R. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J. Neurosci. 2008, 28, 5547–5558. [Google Scholar] [CrossRef]
- Cancedda, L.; Fiumelli, H.; Chen, K.; Poo, M.M. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J. Neurosci. 2007, 27, 5224–5235. [Google Scholar] [CrossRef]
- Tozuka, Y.; Fukuda, S.; Namba, T.; Seki, T.; Hisatsune, T. GABAergic Excitation Promotes Neuronal Differentiation in Adult Hippocampal Progenitor Cells. Neuron 2005, 47, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Longair, M.H.; Baker, D.A.; Armstrong, J.D. Simple Neurite Tracer: Open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 2011, 27, 2453–2454. [Google Scholar] [CrossRef]
- Nicola, Z.; Fabel, K.; Kempermann, G. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 2015, 9, 53. [Google Scholar] [CrossRef]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef]
- Lindquist, B.E.; Voskobiynyk, Y.; Goodspeed, K.; Paz, J.T. Patient-derived SLC6A1 variant S295L results in an epileptic phenotype similar to haploinsufficient mice. Epilepsia 2023, 64, e214–e221. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Rioux, M.; Shaffo, F.; Hu, Y.; Yu, Z.; Xing, C.; Gray, S.J. AAV9/SLC6A1 gene therapy rescues abnormal EEG patterns and cognitive behavioral deficiencies in Slc6a1-/-mice. J. Clin. Investig. 2024, 135, 1558–8238. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Fiorentino, H.; Kuczewski, N.; Diabira, D.; Ferrand, N.; Pangalos, M.N.; Porcher, C.; Gaiarsa, J.-L. GABA(B) receptor activation triggers BDNF release and promotes the maturation of GABAergic synapses. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 11650–11661. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Yang, Y.; Liu, Y.; Ni, B.; Zhuang, H.; Chen, K.; Shi, J.; Zhu, C.; Wang, H.; Fei, J. γ-Aminobutyric Acid Transporter Mutation GAT1 (S295L) Substantially Impairs Neurogenesis in Dentate Gyrus. Brain Sci. 2025, 15, 393. https://doi.org/10.3390/brainsci15040393
Liu W, Yang Y, Liu Y, Ni B, Zhuang H, Chen K, Shi J, Zhu C, Wang H, Fei J. γ-Aminobutyric Acid Transporter Mutation GAT1 (S295L) Substantially Impairs Neurogenesis in Dentate Gyrus. Brain Sciences. 2025; 15(4):393. https://doi.org/10.3390/brainsci15040393
Chicago/Turabian StyleLiu, Weitong, Yantian Yang, Yichen Liu, Bingyan Ni, Hua Zhuang, Kexin Chen, Jiahao Shi, Chenxin Zhu, Haoyue Wang, and Jian Fei. 2025. "γ-Aminobutyric Acid Transporter Mutation GAT1 (S295L) Substantially Impairs Neurogenesis in Dentate Gyrus" Brain Sciences 15, no. 4: 393. https://doi.org/10.3390/brainsci15040393
APA StyleLiu, W., Yang, Y., Liu, Y., Ni, B., Zhuang, H., Chen, K., Shi, J., Zhu, C., Wang, H., & Fei, J. (2025). γ-Aminobutyric Acid Transporter Mutation GAT1 (S295L) Substantially Impairs Neurogenesis in Dentate Gyrus. Brain Sciences, 15(4), 393. https://doi.org/10.3390/brainsci15040393





