Effects of Different Individuals and Verbal Tones on Neural Networks in the Brain of Children with Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
- Severe body movements that could interfere with EEG recording;
- Hearing impairment that could affect the perception of verbal stimuli;
- Medical conditions contraindicating the use of EEG equipment.
2.2. Ethical Considerations
2.3. Stimuli
- 20 sentences of speaking based on asking;
- 20 sentences based on informing;
- 40 sentences based on a mixed approach that combined asking and informing.
- Asking sentences were delivered in a friendly manner;
- Informing sentences were delivered in a non-friendly manner;
- The mixed approach incorporated tones appropriate for both asking and informing.
2.4. Procedure
- Control condition: 1 min EEG recording in silence, followed by 1 min recording with white noise;
- 5 min break;
- Mother’s speaking conditions: asking, informing, and mixed, with 5 min breaks between each condition;
- 10 min break;
- Repeat of control condition;
- PT’s speaking conditions: asking, informing, and mixed, with 5 min breaks between each condition.
2.5. EEG Recording and Analysis
2.6. Statistical Analysis
3. Results
3.1. Participant Baseline Characteristics
3.2. EEG Analysis
3.2.1. Participant A
3.2.2. Participant B
3.2.3. Participant C
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matteucci, G.; Guyoton, M.; Mayrhofer, J.M.; Auffret, M.; Foustoukos, G.; Petersen, C.C.H.; El-Boustani, S. Cortical sensory processing across motivational states during goal-directed behavior. Neuron 2022, 110, 4176–4193.e10. [Google Scholar] [CrossRef]
- Kouneiher, F.; Charron, S.; Koechlin, E. Motivation and cognitive control in the human prefrontal cortex. Nat. Neurosci. 2009, 12, 939–945. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, H.; Fu, V. Intrinsic motivation. Pract. Neurol. 2023, 23, 489–492. [Google Scholar] [CrossRef]
- Tatla, S.K.; Sauve, K.; Virji-Babul, N.; Holsti, L.; Butler, C.; Van Der Loos, H.F.M. Evidence for outcomes of motivational rehabilitation interventions for children and adolescents with cerebral palsy: An American academy for cerebral palsy and developmental medicine systematic review. Dev. Med. Child Neurol. 2013, 55, 593–601. [Google Scholar] [CrossRef] [PubMed]
- LePine, J.A.; LePine, M.A.; Jackson, C.L. Challenge and Hindrance Stress: Relationships with Exhaustion, Motivation to Learn, and Learning Performance. J. Appl. Psychol. 2004, 89, 883–891. [Google Scholar] [CrossRef]
- Ryan, R.M.; Mims, V.; Koestner, R. Relation of reward contingency and interpersonal context to intrinsic motivation: A review and test using cognitive evaluation theory. J. Personal. Soc. Psychol. 1983, 45, 736–750. [Google Scholar] [CrossRef]
- Halamish, V.; Madmon, I.; Moed, A. Motivation to learn. Exp. Psychol. 2019, 66, 319–330. [Google Scholar] [CrossRef]
- Núñez, L.; Midgley, N.; Capella, C.; Alamo, N.; Mortimer, R.; Krause, M. The therapeutic relationship in child psychotherapy: Integrating the perspectives of children, parents and therapists. Psychother. Res. 2021, 31, 988–1000. [Google Scholar] [CrossRef]
- van Egmond, M.C.; Hanke, K.; Omarshah, T.T.; Navarrete Berges, A.; Zango, V.; Sieu, C. Self-esteem, motivation and school attendance among sub-Saharan African girls: A self-determination theory perspective. Int. J. Psychol. 2020, 55, 842–850. [Google Scholar] [CrossRef]
- Bratton, S.C.; Ray, D.; Rhine, T.; Jones, L. The Efficacy of Play Therapy with Children: A Meta-Analytic Review of Treatment Outcomes. Prof. Psychol. Res. Pract. 2005, 36, 376–390. [Google Scholar] [CrossRef]
- Franchak, J.M. The ecology of infants’ perceptual-motor exploration. Curr. Opin. Psychol. 2020, 32, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.C.; Meyer, D.K.; Schweinle, A. The Importance of emotion in theories of motivation: Empirical, methodological, and theoretical considerations from a goal theory perspective. Int. J. Educ. Res. 2003, 39, 375–393. [Google Scholar] [CrossRef]
- Vogl, J.L.; Dunne, E.C.; Liu, C.; Bradley, A.; Rwei, A.; Lonergan, E.K.; Hopkins, B.S.; Kwak, S.S.; Simon, C.D.; Rand, C.M.; et al. Kangaroo Father Care: A pilot feasibility study of physiologic, biologic, and psychosocial measures to capture the effects of father-infant and mother-infant skin-to-skin contact in the Neonatal Intensive Care Unit. Dev. Psychobiol. 2021, 63, 1521–1533. [Google Scholar] [CrossRef]
- King, G.; Williams, L.; Hahn Goldberg, S. Family-oriented services in pediatric rehabilitation: A scoping review and framework to promote parent and family wellness. Child Care Health Dev. 2017, 43, 334–347. [Google Scholar] [CrossRef]
- Khetani, M.A.; Albrecht, E.C.; Jarvis, J.M.; Pogorzelski, D.; Cheng, E.; Choong, K. Determinants of change in home participation among critically illchildren. Dev. Med. Child Neurol. 2018, 60, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Sundara, M.; Zhou, Z.L.; Breiss, C.; Katsuda, H.; Steffman, J. Infants’ developing sensitivity to native language phonotactics: A meta-analysis. Cognition 2022, 221, 104993. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroglu, C.; Karakaş, S.; Schürmann, M. Gamma, alpha, Delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. 2001, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Woertz, M.; Müller, G.; Wriessnegger, S.; Pfurtscheller, K. Contrasting behavior of beta event-related synchronization and somatosensory evoked potential after median nerve stimulation during finger manipulation in man. Neurosci. Lett. 2002, 323, 113–116. [Google Scholar] [CrossRef]
- Cho, S.S.; Pellecchia, G.; Ko, J.H.; Ray, N.; Obeso, I.; Houle, S.; Strafella, A.P. Effect of continuous theta burst stimulation of the right dorsolateral prefrontal cortex on cerebral blood flow changes during decision making. Brain Stimul. 2012, 5, 116–123. [Google Scholar] [CrossRef]
- Strauß, A.; Wöstmann, M.; Obleser, J. Cortical alpha oscillations as a tool for auditory selective inhibition. Front. Hum. Neurosci. 2014, 8, 350. [Google Scholar] [CrossRef]
- Babu Henry Samuel, I.; Wang, C.; Hu, Z.; Ding, M. The frequency of alpha oscillations: Task-dependent modulation and its functional significance. Neuroimage 2018, 183, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Clements, G.M.; Bowie, D.C.; Gyurkovics, M.; Low, K.A.; Fabiani, M.; Gratton, G. Spontaneous alpha and theta oscillations are related to complementary aspects of cognitive control in younger and older adults. Front. Hum. Neurosci. 2021, 15, 621620. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.-T.; Ranganath, C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage 2014, 85, 721–729. [Google Scholar] [CrossRef]
- Romeo, Z.; Spironelli, C. Theta oscillations underlie the interplay between emotional processing and empathy. Heliyon 2024, 10, e34581. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Herrojo Ruiz, M.H.; Kilavik, B.E.; Lundqvist, M.; Starr, P.A.; Aron, A.R. Beta oscillations in working memory, executive control of movement and thought, and sensorimotor function. J. Neurosci. 2019, 39, 8231–8238. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Caplan, J.B.; Madsen, J.R.; Schulze-Bonhage, A.; Aschenbrenner-Scheibe, R.; Newman, E.L.; Kahana, M.J. Human theta oscillations related to sensorimotor integration and spatial learning. J. Neurosci. 2003, 23, 4726–4736. [Google Scholar] [CrossRef]
- Chikazoe, J.; Konishi, S.; Asari, T.; Jimura, K.; Miyashita, Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. J. Cogn. Neurosci. 2007, 19, 69–80. [Google Scholar] [CrossRef]
- Knyazev, G.G. EEG correlates of self-referential processing. Front. Hum. Neurosci. 2013, 7, 264. [Google Scholar] [CrossRef]
- Compton, R.J.; Arnstein, D.; Freedman, G.; Dainer-Best, J.D.; Liss, A. Cognitive control in the intertrial interval: Evidence from EEG alpha power. Psychophysiology 2011, 48, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.K.; Wilson, T.W. Cortical theta–gamma coupling governs the adaptive control of motor commands. Brain Commun. 2022, 4, fcac249. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Joos, K.; Ost, J.; De Ridder, D.D. Influencing connectivity and cross-frequency coupling by real-time source localized neurofeedback of the posterior cingulate cortex reduces tinnitus related distress. Neurobiol. Stress. 2018, 8, 211–224. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2012; Volume 5. [Google Scholar]
- Jensen, O.; Tesche, C.D. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002, 15, 1395–1399. [Google Scholar] [CrossRef]
- Sigala, R.; Haufe, S.; Roy, D.; Dinse, H.R.; Ritter, P. The role of alpha-rhythm states in perceptual learning: Insights from experiments and computational models. Front. Comput. Neurosci. 2014, 8, 36. [Google Scholar] [CrossRef]
- Berger, A.M.; Davelaar, E.J. Frontal alpha oscillations and attentional control: A virtual reality neurofeedback study. Neuroscience 2018, 378, 189–197. [Google Scholar] [CrossRef]
- Nigbur, R.; Ivanova, G.; Stürmer, B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011, 122, 2185–2194. [Google Scholar] [CrossRef]
- Brickwedde, M.; Krüger, M.C.; Dinse, H.R. Somatosensory alpha oscillations gate perceptual learning efficiency. Nat. Commun. 2019, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Doya, K. Reinforcement learning: Computational theory and biological mechanisms. HFSP J. 2007, 1, 30–40. [Google Scholar] [CrossRef]
- Hwang, K.; Ghuman, A.S.; Manoach, D.S.; Jones, S.R.; Luna, B. Frontal preparatory neural oscillations associated with cognitive control: A developmental study comparing young adults and adolescents. Neuroimage 2016, 136, 139–148. [Google Scholar] [CrossRef]
- Stoll, F.M.; Wilson, C.R.E.; Faraut, M.C.M.; Vezoli, J.; Knoblauch, K.; Procyk, E. The Effects of Cognitive Control and Time on Frontal Beta Oscillations. Cereb. Cortex 2016, 26, 1715–1732. [Google Scholar] [CrossRef] [PubMed]
- Piai, V.; Roelofs, A.; Rommers, J.; Maris, E. Beta oscillations reflect memory and motor aspects of spoken word production. Hum. Brain Mapp. 2015, 36, 2767–2780. [Google Scholar] [CrossRef]
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Mishkin, M. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 2011, 12, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Tanji, J.; Hoshi, E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol. Rev. 2008, 88, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Babiloni, C.; Ferretti, A.; Perrucci, M.G.; Romani, G.L.; Rossini, P.M.; Tartaro, A.; Del Gratta, C. Human secondary somatosensory cortex is involved in the processing of somatosensory rare stimuli: An fMRI study. Neuroimage 2008, 40, 1765–1771. [Google Scholar] [CrossRef]
- Beer, J.S.; John, O.P.; Scabini, D.; Knight, R.T. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. J. Cogn. Neurosci. 2006, 18, 871–879. [Google Scholar] [CrossRef]
- Moore, R.C.; Dev, S.I.; Jeste, D.V.; Dziobek, I.; Eyler, L.T. Distinct neural correlates of emotional and cognitive empathy in older adults. Psychiatry Res. 2015, 232, 42–50. [Google Scholar] [CrossRef]
- Pu, Y.; Cheyne, D.; Sun, Y.; Johnson, B.W. Theta oscillations support the interface between language and memory. Neuroimage 2020, 215, 116782. [Google Scholar] [CrossRef]
- Nelson, B.D.; Sarapas, C.; Robison-Andrew, E.J.R.; Altman, S.E.; Campbell, M.L.; Shankman, S.A. Frontal brain asymmetry in depression with comorbid anxiety: A neuropsychological investigation. J. Abnorm. Psychol. 2012, 121, 579–591. [Google Scholar] [CrossRef]
- Mesa-Gresa, P.; Gil-Gómez, J.-A.; Lozano-Quilis, J.A.; Schoeps, K.; Montoya-Castilla, I. Electrophysiological correlates of the emotional response on brain activity in adolescents. Biomed. Signal Process. Control 2024, 89, 105754. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroglu, C.; Karakaş, S.; Schürmann, M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci. Lett. 1999, 259, 165–168. [Google Scholar] [CrossRef]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef]
- Aftanas, L.I.; Golocheikine, S.A. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neurosci. Lett. 2001, 310, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W.; Freunberger, R.; Pecherstorfer, T.; Hanslmayr, S.; Doppelmayr, M. Relevance of EEG alpha and theta oscillations during task switching. Exp. Brain Res. 2006, 170, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Russegger, H.; Pachinger, T.; Schwaiger, J. Induced alpha band power changes in the human EEG and attention. Neurosci. Lett. 1998, 244, 73–76. [Google Scholar] [CrossRef]
- Toscani, M.; Marzi, T.; Righi, S.; Viggiano, M.P.; Baldassi, S. Alpha waves: A neural signature of visual suppression. Exp. Brain Res. 2010, 207, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Stancák, A., Jr.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Bressler, S.L.; Ding, M. Response preparation and inhibition: The role of the cortical sensorimotor beta rhythm. Neuroscience 2008, 156, 238–246. [Google Scholar] [CrossRef]
- Gruzelier, J.H. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014, 44, 124–141. [Google Scholar] [CrossRef]
- Benca, R.M.; Obermeyer, W.H.; Larson, C.L.; Yun, B.; Dolski, I.; Kleist, K.D.; Weber, S.M.; Davidson, R.J. EEG alpha power and alpha power asymmetry in sleep and wakefulness. Psychophysiology 1999, 36, 430–436. [Google Scholar] [CrossRef]
- Smith, E.E.; Zambrano-Vazquez, L.; Allen, J.J.B. Patterns of alpha asymmetry in those with elevated worry, trait anxiety, and obsessive-compulsive symptoms: A test of the worry and avoidance models of alpha asymmetry. Neuropsychologia 2016, 85, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Cincotti, F.; Mattia, D.; Marciani, M.G.; Baccalà, L.A.; de Vico Fallani, F.; Salinari, S.; Ursino, M.; Zavaglia, M.; Babiloni, F. Assessing cortical functional connectivity by partial directed coherence: Simulations and application to real data. IEEE Trans. Biomed. Eng. 2006, 53, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Okibayashi, M.; Danjo, N.; Ishikawa, A.; Inoue, Y.; et al. Neurofeedback using real-time near-infrared spectroscopy enhances motor imagery related cortical activation. PLoS ONE 2012, 7, e32234. [Google Scholar] [CrossRef]
- Strait, M.; Scheutz, M. What we can and cannot (yet) do with functional near infrared spectroscopy. Front. Neurosci. 2014, 8, 117. [Google Scholar] [CrossRef]




| Participant Characteristics | |||
|---|---|---|---|
| A | B | C | |
| Age (years) | 3 | 7 | 9 |
| Gender (boy/girl) | boy | girl | girl |
| Disease | cerebral palsy (CP) | ||
| Classified | severe psychosomatic disorder | ||
| Verbal Communication | vocalizing but struggling to produce meaningful words | ||
| Motor Level | difficulty turning over; unable to move voluntarily | difficulty turning over; unable to move voluntarily | able to crawl a few meters |
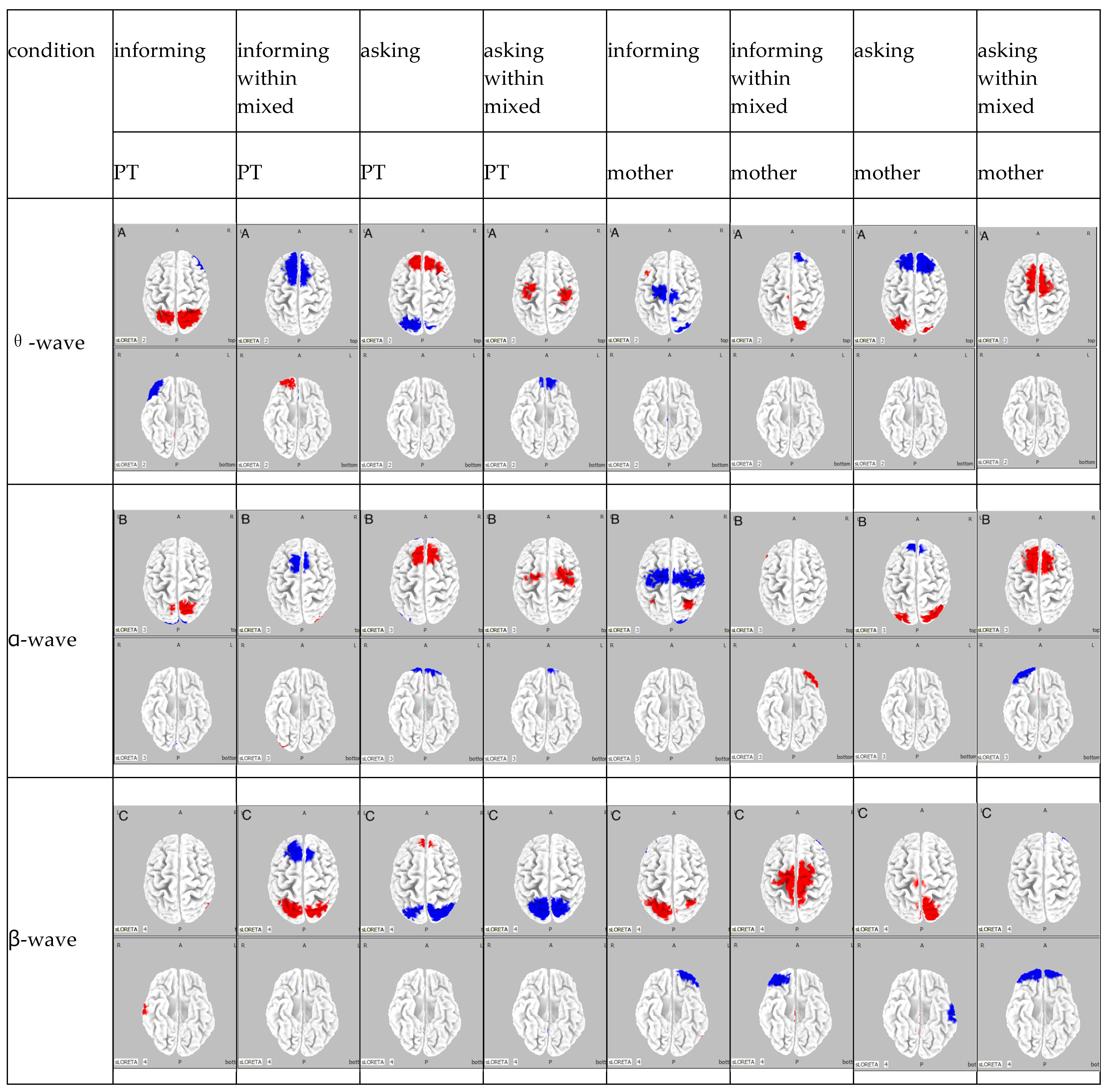
| Participant | Condition | Activity (Power Value) | θ-Wave | α-Wave | β-Wave | |
|---|---|---|---|---|---|---|
| A | PT’s | informing condition | amplification (max) | somatosensory association areas (1.64 × 100) | somatosensory association areas (1.54 × 100) | right superior temporal gyrus (1.27 × 100) |
| reduction (minimum) | right inferior frontal gyrus (−1.75 × 100) | visual association area (−1.45 × 100) | ||||
| asking condition | amplification (max) | frontal eye fields (1.68 × 100) | right visual association area (7.79 × 100) | frontal eye fields (1.02 × 100) | ||
| reduction (minimum) | somatosensory association areas (1.67 × 100) | frontal poles (−8.78 × 100) | somatosensory association areas (−1.40 × 100) | |||
| informing within mixed condition | amplification (max) | right frontal pole (8.63 × 10−1) | right visual association area (7.26 × 10−1) | somatosensory association areas (1.33 × 100) | ||
| reduction (minimum) | somatosensory association areas (−8.54 × 10−1) | supplementary motor areas (−6.7 × 10−1) | frontal eye fields (−1.29 × 100) | |||
| asking within mixed condition | amplification (max) | supplementary motor areas (1.32 × 100) | supplementary motor areas (1.06 × 100) | |||
| reduction (minimum) | orbitofrontal cortex (−1.39 × 100) | orbitofrontal cortex (−1.03 × 100) | somatosensory association areas (−1.54 × 100) | |||
| mother’s | informing condition | amplification (max) | frontal eye fields (7.76 × 10−1) | somatosensory association areas (7.78 × 10−1) | somatosensory association areas (−1.32 × 100) | |
| reduction (minimum) | visual association areas (−9.43 × 100) | supplementary motor areas (−7.93 × 100) | left frontal pole (−1.46 × 100) | |||
| asking condition | amplification (max) | somatosensory association areas (1.08 × 100) | visual association areas (1.15 × 100) | somatosensory association areas (1.45 × 100) | ||
| reduction (minimum) | frontal eye fields (−1.38 × 100) | frontal eye fields (−9.59 × 100) | Inferior temporal gyrus (−1.40 × 100) | |||
| informing within mixed condition | amplification (max) | right somatosensory association area (1.33 × 100) | right visual association area (1.51 × 100) | supplementary motor areas (1.66 × 100) | ||
| reduction (minimum) | right dorsolateral prefrontal cortex (−1.50 × 100) | left orbitofrontal cortex (−8.17 × 100) | right orbitofrontal cortex (−1.36 × 100) | |||
| asking within mixed condition | amplification (max) | supplementary motor areas (2.10 × 100) | supplementary motor areas (1.40 × 100) | |||
| reduction (minimum) | frontal poles (−1.25 × 100) | orbitofrontal cortex (−1.98 × 100) | ||||
| B | PT’s | informing condition | amplification (max) | dorsal posterior cingulate cortex (2.1 × 100) | ||
| reduction (minimum) | dorsolateral prefrontal cortex (−3.64 × 100) | supplementary motor areas (−2.46 × 100) | frontal poles (−3.13 × 100) | |||
| asking condition | amplification (max) | orbitofrontal cortices (2.01 × 100) | somatosensory association areas (1.56 × 100) | frontal poles (1.65 × 100) | ||
| reduction (minimum) | supplementary motor areas (−1.93 × 100) | supplementary motor areas (−1.69 × 100) | supplementary motor areas (−1.57 × 100) | |||
| informing within mixed condition | amplification (max) | somatosensory association (2.18 × 100) | right frontal pole (1.36 × 100) | frontal poles (3.16 × 100) | ||
| reduction (minimum) | orbitofrontal cortex (−2.67 × 100) | visual association areas (−2.45 × 100) | somatosensory association areas (−3.30 × 100) | |||
| asking within mixed condition | amplification (max) | frontal pole (8.07 × 10−1) | right frontal pole (2.09 × 100) | somatosensory association areas (1.26 × 100) | ||
| reduction (minimum) | dorsolateral prefrontal cortex (−8.12 × 10−1) | frontal pole (−2.39 × 100) | ||||
| mother’s | informing condition | amplification (max) | left inferior frontal gyrus (1.88 × 100) | left visual association area (1.25 × 100) | right middle temporal gyrus (1.97 × 100) | |
| reduction (minimum) | somatosensory association areas (−2.73 × 100) | left orbitofrontal cortex (−1.42 × 100) | left somatosensory association area (−2.53 × 100) | |||
| asking condition | amplification (max) | right middle temporal gyrus (2.29 × 100) | right dorsolateral prefrontal cortex (1.52 × 100) | left somatosensory association area (2.58 × 100) | ||
| reduction (minimum) | right visual association areas (−1.32 × 100) | left frontal pole (−2.82 × 100) | ||||
| informing within mixed condition | amplification (max) | left frontal eye fields (2.16 × 100) | ||||
| reduction (minimum) | left frontal pole (−4.23 × 100) right fusiform gyrus (−3.97 × 100) | visual association areas (−2.22 × 100) | visual association areas (−3.04 × 100) | |||
| asking within mixed condition | amplification (max) | left frontal pole (1.21 × 100) | somatosensory areas (2.98 × 100) | middle temporal gyri (1.66 × 100) | ||
| reduction (minimum) | frontal poles (−2.50 × 100) | right frontal pole (−1.89 × 100) | ||||
| C | PT’s | informing condition | amplification (max) | left frontal pole (2.13 × 100) | right dorsolateral prefrontal cortex (1.79 × 100) | right dorsolateral prefrontal cortex (1.28 × 100) |
| reduction (minimum) | left visual association area (−2.11 × 100) | right visual association area (−1.42 × 100) | left somatosensory association area (−1.25 × 100) | |||
| asking condition | amplification (max) | somatosensory association areas (1.37 × 100) | somatosensory association areas (1.33 × 100) | |||
| reduction (minimum) | left orbitofrontal cortex(−1.41 × 100) | orbitofrontal cortex (−2.52 × 100) | middle temporal gyrus (−1.38 × 100) | |||
| informing within mixed condition | amplification (max) | superior temporal gyrus (7.87 × 10−1) | right somatosensory association area (1.35 × 100) | |||
| reduction (minimum) | somatosensory association areas (−7.29 × 10−1) | left frontal pole (−2.36 × 100) | orbitofrontal cortices (−2.01 × 100) | |||
| asking within mixed condition | amplification (max) | ventral prefrontal cortex (1.84) | ||||
| reduction (minimum) | orbitofrontal cortices (−1.1 × 100) | right frontal pole (−9.80 × 10−1) | ||||
| mother’s | informing condition | amplification (max) | orbitofrontal cortices (1.91 × 100) | ventral prefrontal cortex (1.32 × 100) | ||
| reduction (minimum) | somatosensory association area (−1.88 × 100) | |||||
| asking condition | amplification (max) | right frontal pole (1.71 × 100) | left somatosensory association area (8.68 × 10−1) | right dorsolateral prefrontal cortex (1.29 × 100) | ||
| reduction (minimum) | supplementary motor areas (−1.01 × 100) | |||||
| informing within mixed condition | amplification (max) | right inferior temporal gyrus (5.82 × 10−1) | frontal poles (1.40 × 100) | right frontal pole (8.96 × 10−1) | ||
| reduction (minimum) | left visual association area (−6.37 × 10−1) | right visual association area (−1.04 × 100) | right angular gyrus (−1.55 × 100) | |||
| asking within mixed condition | amplification (max) | somatosensory association area (1.27 × 100) | frontal eye fields (1.44 × 100) | |||
| reduction (minimum) | left frontal pole (−1.16 × 100) | frontal poles (−7.69 × 10−1) | left orbitofrontal cortex (−1.55 × 100) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, R.; Ito, H.; Kitai, K.; Okuyama, K.; Katayama, O.; Morita, K.; Murata, S.; Kodama, T. Effects of Different Individuals and Verbal Tones on Neural Networks in the Brain of Children with Cerebral Palsy. Brain Sci. 2025, 15, 397. https://doi.org/10.3390/brainsci15040397
Yamauchi R, Ito H, Kitai K, Okuyama K, Katayama O, Morita K, Murata S, Kodama T. Effects of Different Individuals and Verbal Tones on Neural Networks in the Brain of Children with Cerebral Palsy. Brain Sciences. 2025; 15(4):397. https://doi.org/10.3390/brainsci15040397
Chicago/Turabian StyleYamauchi, Ryosuke, Hiroki Ito, Ken Kitai, Kohei Okuyama, Osamu Katayama, Kiichiro Morita, Shin Murata, and Takayuki Kodama. 2025. "Effects of Different Individuals and Verbal Tones on Neural Networks in the Brain of Children with Cerebral Palsy" Brain Sciences 15, no. 4: 397. https://doi.org/10.3390/brainsci15040397
APA StyleYamauchi, R., Ito, H., Kitai, K., Okuyama, K., Katayama, O., Morita, K., Murata, S., & Kodama, T. (2025). Effects of Different Individuals and Verbal Tones on Neural Networks in the Brain of Children with Cerebral Palsy. Brain Sciences, 15(4), 397. https://doi.org/10.3390/brainsci15040397







