Prevention and Management of Spinal Cord Ischemia After Aortic Surgery: An Umbrella Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Data Synthesis
2.7. Quality Appraisal
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Incidence and Risk Factors (Q1)
3.4. Pathogenesis (Q2)
3.5. Early Diagnosis (Q3)
3.6. Prevention (Q4)
3.7. Complications Associated with CSFD (Q5)
3.8. Treatment (Q6)
3.9. Prognosis (Q7)
3.10. Results of Quality Appraisal
4. Discussion
4.1. Overview of Our Findings
4.2. Prevention and Treatment of SCI in the Guidelines
4.3. Evidence Gaps and Future Perspectives
4.4. Ongoing Trials
4.5. Key Challenges in Studying Spinal Cord Injury
4.6. The Role of CSF Drainage
4.7. The Role of Neuromonitoring
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMT | Best medical treatment |
| CSF | Cerebrospinal fluid |
| CSFD | Cerebrospinal fluid drain |
| CT | Computerized tomography |
| CNIHE | Canadian National Institute of Health Economics |
| DTA | Descending thoracic aortic |
| F/B-EVAR | Fenestrated or branched endovascular repair |
| GRADE | Grading of Recommendations Assessment |
| LSA | Left subclavian artery |
| MA | Meta-analysis |
| MEPs | Motor evoked potentials |
| MICACE | Minimally invasive segmental spinal artery coil embolization |
| MRI | Magnetic resonance imaging |
| NIRS | Near-infrared spectroscopy |
| NOS | Newcastle Ottawa scale |
| OS | Open surgery |
| SCI | Spinal cord ischemia |
| SR | Systematic review |
| SSEPs | Somatosensory evoked potentials |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies—2 |
| TAAAs | Thoracoabdominal aortic aneurysms |
| TASP | Temporary aneurysm sac perfusion |
| TBAD | Type B aortic dissection |
| TCD | Transcranial Doppler |
| TEVAR | Thoracic endovascular aneurysm repair |
| US | Ultrasound |
| W/O | Without |
References
- Moulakakis, K.G.; Alexiou, V.G.; Karaolanis, G.; Sfyroeras, G.S.; Theocharopoulos, G.N.; Lazaris, A.M.; Kakisis, J.D.; Geroulakos, G. Spinal Cord Ischemia Following Elective Endovascular Repair of Infrarenal Aortic Aneurysms: A Systematic Review. Ann. Vasc. Surg. 2018, 52, 280–291. [Google Scholar] [CrossRef]
- Alzghari, T.; An, K.R.; Harik, L.; Rahouma, M.; Dimagli, A.; Perezgorvas-Olaria, R.; Demetres, M.; Cancelli, G.; Soletti, G., Jr.; Lau, C.; et al. Spinal Cord Injury After Open and Endovascular Repair of Descending Thoracic Aneurysm and Thoracoabdominal Aortic Aneurysm: An Updated Systematic Review and Meta-Analysis. Ann. Cardiothorac. Surg. 2023, 12, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Muston, B.T.; Bilbrough, J.; Bushati, Y.; Wilson-Smith, A.R.; Misfeld, M.; Yan, T. Open, Closed or a Bit of Both: A Systematic Review and Meta-Analysis of Staged Thoraco-Abdominal Aortic Aneurysm Repair. Ann. Cardiothorac. Surg. 2023, 12, 418–428. [Google Scholar] [CrossRef]
- Rocha, R.V.; Lindsay, T.F.; Friedrich, J.O.; Shan, S.; Sinha, S.; Yanagawa, B.; Al-Omran, M.; Forbes, T.L.; Ouzounian, M. Systematic Review of Contemporary Outcomes of Endovascular and Open Thoracoabdominal Aortic Aneurysm Repair. J. Vasc. Surg. 2020, 71, 1396–1412.e12. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Smalley, Z.; Nesvick, C.L.; Lee, S.L.; Michael, L.M. The Use of Lumbar Drains in Preventing Spinal Cord Injury Following Thoracoabdominal Aortic Aneurysm Repair: An Updated Systematic Review and Meta-Analysis. J. Neurosurg. Spine 2016, 25, 383–393. [Google Scholar] [CrossRef]
- Zheng, H.; Lin, D.; Cheng, Y.; Yan, C.; Yu, S.; Li, J.; Cheng, W. Systematic Review of the Effect of Cerebrospinal Fluid Drainage on Outcomes After Endovascular Type B Aortic Dissection Repair. J. Cardiothorac. Surg. 2024, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Frankort, J.; Mees, B.; Doukas, P.; Keszei, A.; Kontopodis, N.; Antoniou, G.A.; Jacobs, M.J.; Gombert, A. Systematic Review of the Effect of Cerebrospinal Fluid Drainage on Outcomes After Endovascular Descending Thoracic/Thoraco-Abdominal Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 501–512. [Google Scholar] [CrossRef]
- Cinà, C.S.; Abouzahr, L.; Arena, G.O.; Laganà, A.; Devereaux, P.J.; Farrokhyar, F. Cerebrospinal Fluid Drainage to Prevent Paraplegia during Thoracic and Thoracoabdominal Aortic Aneurysm Surgery: A Systematic Review and Meta-Analysis. J. Vasc. Surg. 2004, 40, 36–44. [Google Scholar] [CrossRef]
- Lella, S.K.; Waller, H.D.; Pendleton, A.; Latz, C.A.; Boitano, L.T.; Dua, A. A Systematic Review of Spinal Cord Ischemia Prevention and Management After Open and Endovascular Aortic Repair. J. Vasc. Surg. 2022, 75, 1091–1106. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Li, L.; Asemota, I.; Liu, B.; Gomez-Valencia, J.; Lin, L.; Arif, A.W.; Siddiqi, T.J.; Usman, M.S. AMSTAR 2 Appraisal of Systematic Reviews and Meta-Analyses in the Field of Heart Failure from High-Impact Journals. Syst. Rev. 2022, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Prasad, M. Introduction to the GRADE Tool for Rating Certainty in Evidence and Recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Wong, C.S.; Healy, D.; Canning, C.; Coffey, J.C.; Boyle, J.R.; Walsh, S.R. A Systematic Review of Spinal Cord Injury and Cerebrospinal Fluid Drainage After Thoracic Aortic Endografting. J. Vasc. Surg. 2012, 56, 1438–1447. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kawaguchi, M.; Noguchi, Y.; Yoshitani, K.; Kawamata, M.; Masui, K.; Nakayama, T.; Yamada, Y. Systematic Review of Motor Evoked Potentials Monitoring during Thoracic and Thoracoabdominal Aortic Aneurysm Open Repair Surgery: A Diagnostic Meta-Analysis. J. Anesth. 2016, 30, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Harky, A.; Fok, M.; Fraser, H.; Howard, C.; Rimmer, L.; Bashir, M. Could Cerebrospinal Fluid Biomarkers Offer Better Predictive Value for Spinal Cord Ischaemia Than Current Neuromonitoring Techniques During Thoracoabdominal Aortic Aneurysm Repair—A Systematic Review. Braz. J. Cardiovasc. Surg. 2019, 34, 464–471. [Google Scholar] [CrossRef]
- Malloy, P.C.; Raghavan, A.; Elder, T.; Wright, J.; Wright, C.H.; Burant, C.; Sajatovic, M.; Hoffer, A. Cerebrospinal Fluid Drainage During Endovascular Aortic Aneurysm Repair: A Systematic Review of the Literature and Treatment Recommendations. Vasc. Endovasc. Surg. 2020, 54, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Batubara, E.A.D.; Nugraha, R.A.; Amshar, M.; Siddiq, T.; Indriani, S.; Adiarto, S. Ischemic Complications Following Thoracic Endovascular Aortic Repair with and Without Revascularization of Left Subclavian Artery: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2022, 86, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Y.; Lin, S.; Xiao, J.; Ai, W.; Zhang, W.W. Systematic Review and Meta-Analysis of Association of Prophylactic Cerebrospinal Fluid Drainage in Preventing Spinal Cord Ischemia After Thoracic Endovascular Aortic Repair. J. Vasc. Surg. 2022, 75, 1478–1489.e5. [Google Scholar] [CrossRef]
- Pini, R.; Faggioli, G.; Paraskevas, K.I.; Alaidroos, M.; Palermo, S.; Gallitto, E.; Gargiulo, M. A Systematic Review and Meta-Analysis of the Occurrence of Spinal Cord Ischemia After Endovascular Repair of Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2022, 75, 1466–1477.e8. [Google Scholar] [CrossRef]
- Chen, C.-H.J.; Jiang, H.; Nguyen, V.D.D. Prophylactic Cerebrospinal Fluid Drainage and Spinal Cord Ischemia in Thoracic and Thoracoabdominal Endovascular Procedures: A Systematic Review and Meta-Analysis. Ann. Cardiothorac. Surg. 2023, 12, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Spinella, P.C.; Wade, C.E.; Blackbourne, L.H.; Borgman, M.A.; Zarzabal, L.A.; Du, F.; Perkins, J.G.; Maegele, M.; Schreiber, M.; Hess, J.R.; et al. The Association of Blood Component Use Ratios with the Survival of Massively Transfused Trauma Patients with and Without Severe Brain Injury. J. Trauma-Inj. Infect. Crit. Care 2011, 71, S343–S352. [Google Scholar] [CrossRef] [PubMed]
- Sef, D.; Thet, M.S.; Miskolczi, S.; Velissaris, T.; De Silva, R.; Luthra, S.; Turina, M.I. Perioperative Neuromonitoring during Thoracoabdominal Aortic Aneurysm Open Repair: A Systematic Review. Eur. J. Cardiothorac. Surg. 2023, 63, ezad221. [Google Scholar] [CrossRef] [PubMed]
- Leone, N.; D’Oria, M.; Mani, K.; Oderich, G.; Maleti, G.; Bartolotti, L.A.M.; Silingardi, R.; Lepidi, S.; Gennai, S. Systematic Review and Meta-Analysis of Cerebrospinal Fluid Drain-Related Mortality and Morbidity After Fenestrated-Branched Endovascular Aortic Repair. J. Vasc. Surg. 2024, 80, 586–594.e5. [Google Scholar] [CrossRef]
- Thet, M.S.; D’Oria, M.; Sef, D.; Klokocovnik, T.; Oo, A.Y.; Lepidi, S. Neuromonitoring during Endovascular Thoracoabdominal Aortic Aneurysm Repair: A Systematic Review. Ann. Vasc. Surg. 2024, 109, 206–215. [Google Scholar] [CrossRef]
- Soliman, M.A.; Ramadan, A.; Shah, A.S.; Corr, S.J.; Abdelazeem, B.; Rahimi, M. Postoperative Spinal Cord Ischemia Monitoring: A Review of Techniques Available After Endovascular Aortic Repair. Ann. Vasc. Surg. 2024, 106, 438–466. [Google Scholar] [CrossRef]
- Spratt, J.R.; Walker, K.L.; Neal, D.; Arnaoutakis, G.J.; Martin, T.D.; Back, M.R.; Zasimovich, Y.; Franklin, M.; Shahid, Z.; Upchurch, G.R.; et al. Rescue Therapy for Symptomatic Spinal Cord Ischemia After Thoracic Endovascular Aortic Repair. J. Thorac. Cardiovasc. Surg. 2024, 168, 15–25.e11. [Google Scholar] [CrossRef]
- Kwon, B.K.; Tetreault, L.A.; Martin, A.R.; Arnold, P.M.; Marco, R.A.W.; Newcombe, V.F.J.; Zipser, C.M.; McKenna, S.L.; Korupolu, R.; Neal, C.J.; et al. A Clinical Practice Guideline for the Management of Patients with Acute Spinal Cord Injury: Recommendations on Hemodynamic Management. Glob. Spine J. 2024, 14, 187S–211S. [Google Scholar] [CrossRef]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the Management of Peripheral Arterial and Aortic Diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Quddusi, A.; Skelly, A.C.; Brodt, E.D.; Moghaddamjou, A.; Malvea, A.; Hejrati, N.; Srikandarajah, N.; Alvi, M.A.; Stabler-Morris, S.; et al. Definition, Frequency and Risk Factors for Intra-Operative Spinal Cord Injury: A Knowledge Synthesis. Glob. Spine J. 2024, 14, 80S–104S. [Google Scholar] [CrossRef] [PubMed]
- Srikandarajah, N.; Hejrati, N.; Alvi, M.A.; Quddusi, A.; Tetreault, L.A.; Evaniew, N.; Skelly, A.C.; Douglas, S.; Rahimi-Movaghar, V.; Arnold, P.M.; et al. Prevention, Diagnosis, and Management of Intraoperative Spinal Cord Injury in the Setting of Spine Surgery: A Proposed Care Pathway. Glob. Spine J. 2024, 14, 166S–173S. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, F.; Tsilimparis, N.; Rohlffs, F.; Debus, E.S.; Larena-Avellaneda, A.; Wipper, S.; Kölbel, T. Staged Procedures for Prevention of Spinal Cord Ischemia in Endovascular Aortic Surgery. Gefasschirurgie 2018, 23, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sickels, A.D.; Novak, Z.; Schanzer, A.; Farber, M.A.; Sweet, M.P.; Oderich, G.S.; Schneider, D.B.; Eagleton, M.J.; Timaran, C.; Gasper, W.J.; et al. Evolving Practices of Spinal Drain Use for Branch/Fenestrated Endovascular Aortic Repair Patients in the United States Aortic Research Consortium. J. Vasc. Surg. 2025; ahead of print. [Google Scholar] [CrossRef]
- Rosvall, L.; Karelis, A.; Sonesson, B.; Dias, N.V. A Dedicated Preventive Protocol Sustainably Avoids Spinal Cord Ischemia After Endovascular Aortic Repair. Front. Cardiovasc. Med. 2024, 11, 1440674. [Google Scholar] [CrossRef]
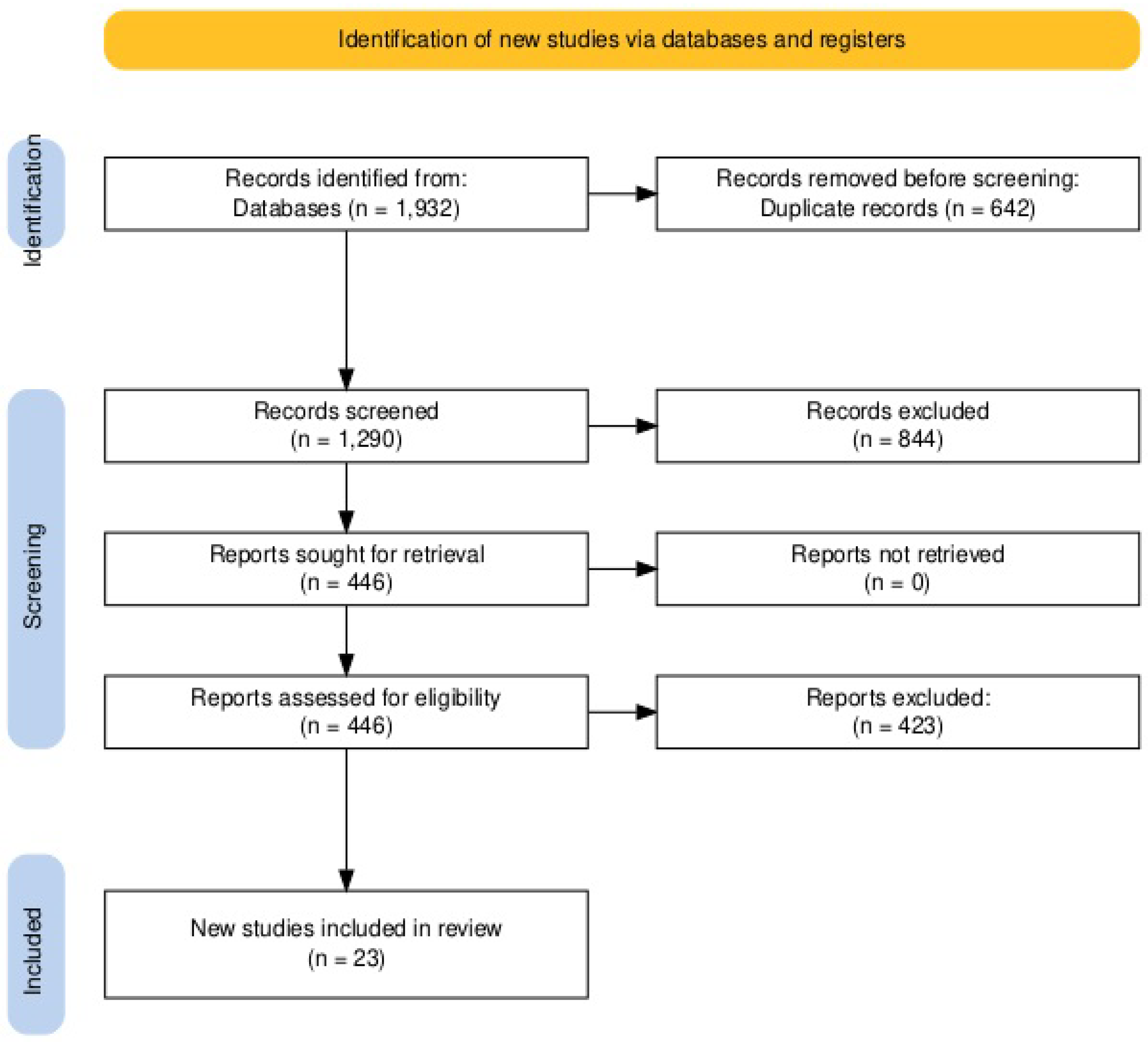
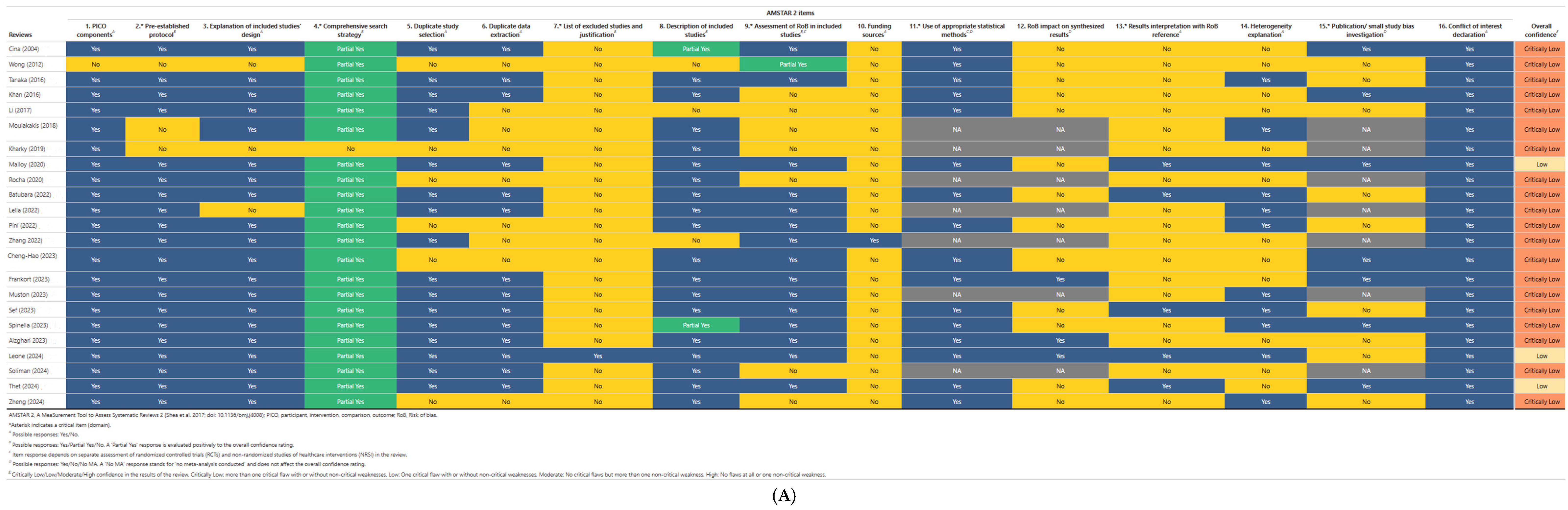
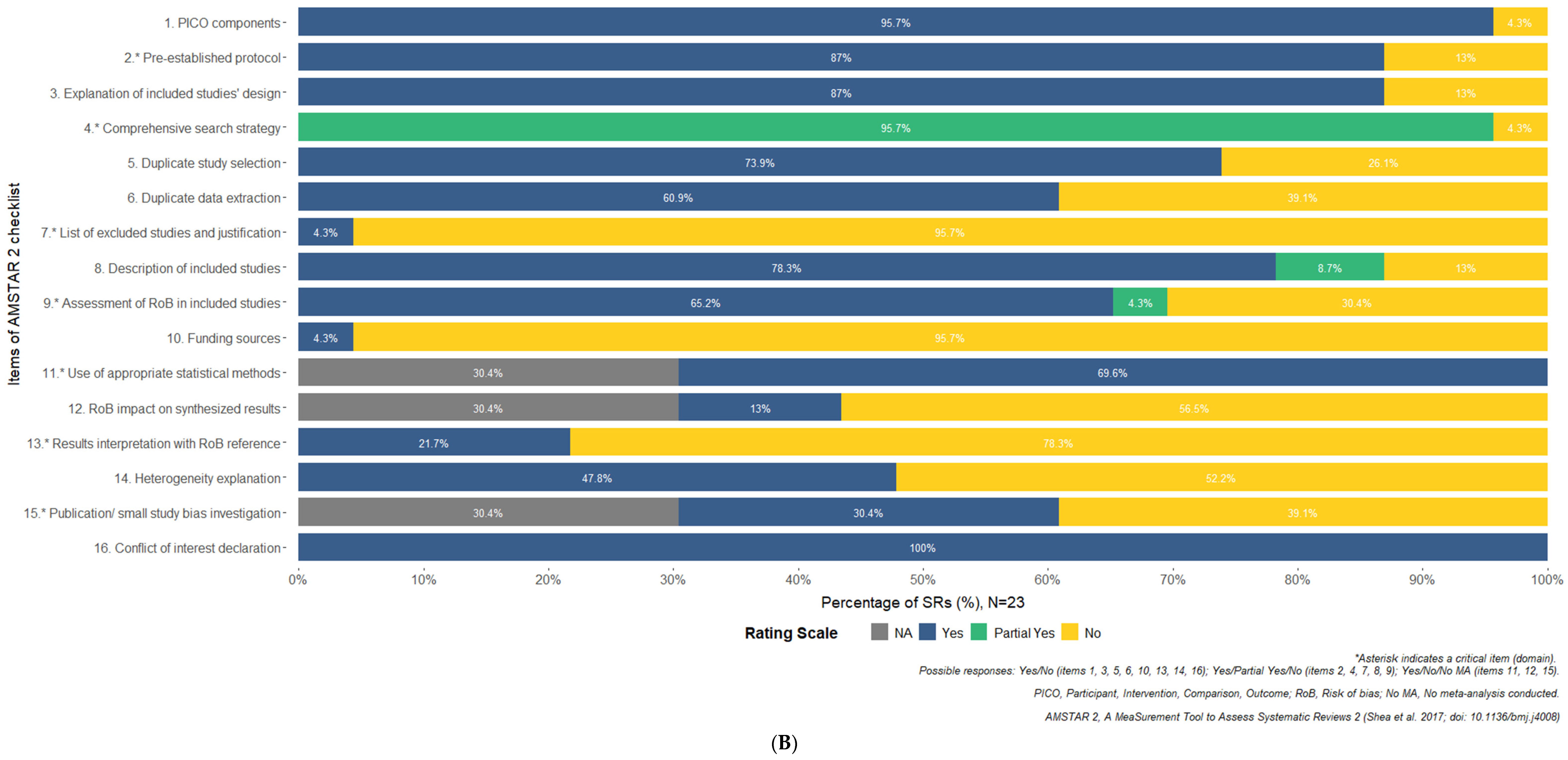
| Research Question | Patient | Intervention | Comparator | Outcome | Time |
|---|---|---|---|---|---|
| Q1 | Patients undergoing aortic surgery | Risk factors related to SCI following surgery | None or standard aortic surgery | Incidence and risk factors for SCI | ANY |
| Q2 | Patients experiencing SCI post-aortic surgery | Mechanisms leading to SCI | Standard aortic surgery patients without SC | Understanding of pathogenesis in SCI after surgery | ANY |
| Q3 | Patients at risk for SCI post-aortic surgery | Early diagnostic methods | Standard diagnostic methods or no early diagnostic approach | Diagnostic accuracy, timeliness, and effectiveness in detecting SC | Intraoperative and early postoperative periods |
| Q4 | Patients undergoing aortic surgery at risk for SCI | Preventive interventions | Standard care without specific preventive measures | Incidence of SCI, early detection, and neurological outcomes | Intraoperative period, early post surgery |
| Q5 | Patients undergoing aortic surgery at risk for SCI | Presence of complications linked to CSFD | No CSFD or standard care | Incidence of complications linked to CSFD | Early and late postoperative periods |
| Q6 | Patients with SCI post-aortic surgery | Treatment options | No treatment or standard management protocols | Recovery of neurological function and reduction in SCI severity | Immediate postoperative to long-term follow-up |
| Q7 | Patients with SCI following aortic surgery | Factors influencing prognosis | No SCI or standard aortic surgery without complication | Functional recovery, quality of life, mortality rates | Short-term to long-term follow-up |
| Authors | SD | Databases | Period | Patients | Intervention | Comparator | Outcomes | Moderators | Quality Assessment | Studies (Patients) | Study Question (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cina (2004) [8] | MA | Ovid software | Until June 2002 | Patients requiring TAAA repair | Prophylactic CSFD | No prophylactic CSFD | SCI and complications | Subgroup analysis for study design | Jadad three-item scale | 8 (1143) | Q1, Q4 |
| Wong (2012) [14] | MA | PubMed, the Cochrane Library, and Conference abstracts | 2000–2011 | Patients undergoing TEVAR | Prophylactic CSFD | No prophylactic CSFD | SCI | (-) | Downs and Black checklist | 46 (4936) | Q1, Q5 |
| Tanaka (2016) [15] | MA | MEDLINE, the Cochrane Central Register, EMBASE, CINAHAL, and the Japanese Central Review of Medicine | Until September 1st 2015 | Patients requiring TAAA repair | TAAA surgery with MEPs | (-) | Sensitivity and specificity for detecting SCI | Anesthesia, and surgery details as moderators for MEPs accuracy; subgroup for several cut-off values | QUADAS-2 | 19 (-) | Q3 |
| Khan (2016) [5] | MA | Clinicaltrials.gov, Cochrane Library, PubMed/MEDLINE, and Scopus | Until August 2015 | Patients requiring TAAA repair | CSFD | W/O or selective CSFD | SCI | Early vs. late SCI | Jadad and NOS | 10 (2013) | Q1, Q4, Q5 |
| Li (2017) [11] | MA | PubMed, EMBASE, Cochrane Library, Web of Science, and ScienceDirect | (-) | Patients with TBAD requiring surgery | TEVAR | OS, BMT | 30-day/in-hospital mortality and morbidity, including paraplegia | (-) | 16 (10,307) | Q1 | |
| Moulakakis (2018) [1] | SR | PubMed and Scopus | Until December 2017 | Patients with infrarenal AAA | Elective endovascular repair | (-) | SCI, including its incidence, risk factors, clinical presentation, and outcomes | Patient characteristics, procedural factors, and anatomical variations | 18 (25) | Q1, Q7 | |
| Harky (2019) [16] | SR | PubMed, MEDLINE, EMBASE, Google Scholar, SCOPUS, and Cochrane | (-) | Patients requiring TAAA repair | (-) | (-) | SCI | (-) | (-) | 15 (265) | Q3 |
| Rocha (2020) [4] | MA | MEDLINE and EMBASE | January 2006 to March 201 | Patients with thoracic aortic pathology | Open repair | Endovascular repair | In-hospital or 30-day mortality and morbidity, including SCI | Permanent vs. overall | Checklist | 71 (-) | Q1, Q2 |
| Malloy (2020) [17] | SR | PubMed/ MEDLINE, Scopus, Ovid, Cochrane, and EMBASE | 1 January 2016 to 17 December 2018 | Patients with aortic aneurysm requiring repair | TEVAR | (-) | SCI (overall vs. temporary) | Various CSF protocols | Joanna Briggs Institute checklist | 8 (859) | Q1, Q4, Q5 |
| Batubara (2022) [18] | MA | PubMed, Ovid Medline, and Cochrane | NR | Patients undergoing Thoracic EVAR | With LSAR | W/O LSAR | Ischemia, stroke, and SCI | (-) | NOS | 22 (11,386) | Q4 |
| Zhang (2022) [19] | MA | MEDLINE, EMBASE, and Cochrane | Until 1 April 2020 | Patients undergoing TEVAR | Prophylactic CSFD | W/O or selective prophylactic CSFD | SCI, CSFD-related complications | Pathology (aneurysm vs. dissection); hybrid procedures | Downs and Black score | 34 (3561) | Q1, Q4, Q5 |
| Pini (2022) [20] | MA | PubMed, EMBASE, and Cochrane Database | Until 1 February 2021 | Patients with TAAA | Endovascular repair | (-) | SCI | (1) TAAA extension; (2) overall vs. permanent SCI; (3) use of CSFD; (4) prophylactic vs. symptomatic; (5) staged vs. nonstaged approach | NOS | 27 (2333) | Q1, Q4, Q6 |
| Lella (2022) [9] | SR | Cochrane and PubMed | 2012 to 2021 | Patients with DTA and TAAA | Open or endovascular repair | (-) | SCI | Type and extent of aortic pathology, operative technique, SCI protection or mitigation strategies, and rates of overall and permanent SCI | NR | 41 (-) | Q1, Q4, Q6 |
| Cheng-Hao (2023) [21] | MA | Scopus, EMBASE, Medline, and Cochrane and Evidence-Based Medicine Reviews | Until September 2022 | Patients undergoing TEVAR | Routine CSFD | Selective CSFD and no CSFD | SCI (any vs. permanent vs. transient) rate, complication rates, and operative outcomes | Immediate vs. delayed; transient vs. permanent; aneurysm vs. dissection; emergency vs. elective | Modified Institute of Health Economics scale | 40 (4973) | Q1, Q4, Q5 |
| Frankort (2023) [7] | MA | MEDLINE, EMBASE, and CINAHL | Until November 2022 | Patients undergoing TEVAR | Prophylactic CSFD | W/O prophylactic CSFD | SCI (early onset of late), complications, and mortality | Risk of bias | NOS/GRADE | 28 (4814) | Q1, Q4, Q5, Q7 |
| Muston (2023) [3] | MA | EMBASE, PubMed, Scopus | Until 3 January 2023 | Open group | Endovascular (TEVAR and F/B EVAR), and hybrid groups | SCI (permanent, in-hospital) | (-) | CNIHE tool | 20 (924) | Q1, Q4 | |
| Spinella (2023) [22] | MA | PubMed/MEDLINE | Until November 2020 | Patients with TAAA | Type A: single step | Type B: staged with reperfusion branches; Type C: staged with positioning of the thoracic component | SCI (transient and permanent) | Age, extent of the aneurysm, the diameter of the aneurysm, and the use of CSFD | (-) | 53 (3095) | Q1, Q4 |
| Sef (2023) [23] | SR | PubMed, EMBASE via Ovid, Cochrane library, and Clinical Trials Gov | Until December 2022 | Patients undergoing open TAAA repair | Neuromonitoring methods MEPs, SSEPs, NIRS, and TCD | Mortality, SCI, and neurologic deficit | (-) | NOS | 27 (3130) | Q1. Q2, Q3 | |
| Alzghari (2024) [2] | MA | Ovid MEDLINE, Ovid EMBASE, and the Cochrane Library | Until September 2022 | Patients with DTA and TAAA | Open or endovascular repair | (-) | SCI, temporary SCI, operative mortality, long-term mortality, postoperative stroke, and CSFD-related complications | Subgroup analyses and multivariate analyses for a multitude of factors | NOS | 239 (61,962) | Q1, Q4, Q5, Q7 |
| Leone (2024) [24] | MA | MEDLINE, EMBASE, and Scopus | 2000–2023 | Patients undergoing F/B-EVAR | CSFD | (-) | CSFD-related mortality and morbidity | (-) | NOS | 6 (730) | Q4, Q5 |
| Zheng (2024) [6] | MA | PubMed, EMBASE, Web of Science, and Cochrane Library | Up to May 2023 | Patients undergoing TEVAR (elective or emergency) for TBAD | Routine prophylactic CSFD | Selective prophylactic CSFD, W/O prophylactic CSFD | Permanent SCI, temporary SCI, CSFD-related complications, and 30-day mortality | With or W/O CSFD | Downs and Black score | 34 (2749) | Q1, Q4, Q5 |
| Thet (2024) [25] | SR | PubMed, MEDLINE via Ovid, EMBASE, Scopus, and Cochrane CENTRAL | 1998–2024 | Patients with TAAA | TEVAR or F/B-EVAR | (-) | SCI and early mortality | Permanent vs. transient, neuromonitoring (MEPs, SSEPs, NIRS) | NOS | 11 (1069) | Q1, Q3, Q4 |
| Soliman (2024) [26] | SR | PubMed, Scopus, and Google Scholar | 1995–2022 | Experimental animals or patients undergoing TAAA repair | Detection and monitoring of SCI using various monitoring techniques | (-) | SCI, SC-blood flow | (-) | NR | 59 (-) | Q3 |
| Authors | Studies (Patients) | Overall | Transient | Permanent | Early | Late |
|---|---|---|---|---|---|---|
| Cina (2004) [8] | 8 (1143) | 33% | (-) | (-) | ||
| Wong (2012) [14] | 46 (4936) | 3.47% (95% CI, 1.98–5.37%) | ||||
| Tanaka (2016) [15] | 19 (-) | 0 to 16.7% | ||||
| Khan (2016) [5] | 10 (2013) | (-) | ||||
| Li (2017) [11] | 16 (10,307) | 0 to 50% | ||||
| Moulakakis (2018) [1] | 18 (25) | 1% (range: 0–8%) | ||||
| Harky (2019) [16] | 15 (265) | (-) | ||||
| Rocha (2020) [4] | 71 (-) | Endovascular: 13.5% (95% CI, 10.5–16.7%); Open: 7.4% (95% CI, 6.2–8.7%, p < 0.01) | Endovascular: 5.2% (95% CI, 3.8–6.7%); Open: 4.4% [95% CI, 3.3–5.6%, p = 0.39] | |||
| Malloy (2020) [17] | 8 (859) | 0–17% | 0–2% | |||
| Batubara (2022) [18] | 22(11,386) | 2.5% (n = 283, R = 11,065) | ||||
| Zhang (2022) [19] | 34 (3561) | Endovascular for aortic: 3.49% (95% CI, 0.23–6.76%); Endovascular for dissection: 3.20% (95% CI, 0.00–7.20%) | ||||
| Pini (2022) [20] | 27 (2333) | Endovascular: 11% (95%CI, 8–15%) | ||||
| Lella (2022) [9] | 41 (-) | Overall: 0–16%; Endovascular 0–35%; Open: 3.1–33.5% | Endovascular: 2–20.5%; Open: 1.11% | |||
| Cheng-Hao (2023) [21] | 40 (4973) | TEVAR: 3.5% (95% CI: 2.6–4.4%) | 1.3% (95% CI: 0.7–1.8%) | 1.9% (95% CI: 1.2–2.5% | ||
| Frankort (2023) [7] | 28 (4814) | Endovascular: 5%, (95% CI 0–14%) | ||||
| Muston (2023) [3] | 20 (924) | Overall: 5.4% (95%CI 5.1–5.8%); Open: 1.4% (95% CI, 1.3–1.5%); Hybrid: 3.2% (95% CI, 2.8–3.6%); Endovascular: 9.8% (95% CI, 9.2–10.4%) | ||||
| Sef (2023) [23] | 27 (3130) | 0% to 17.0% | 1.3% to 12.0% | |||
| Alzghari (2024) [2] | 239 (61,962) | Overall: 3.3% (95%CI 2.9–3.8%); Open 4.0% (95% CI, 3.3–4.8%); Endovascular: 2.9% (95% CI, 2.4–3.5%) | ||||
| Zheng (2024) [6] | 34 (2749) | 1.0% (95% CI, 0.00–1.0%) | 2.0% (95% CI, 1.0–2.0) | |||
| Thet (2024) [25] | 11 (1069) | 3.8 to 17.3% | 2.7 to 5.8% |
| Incidence | Technique | Range | Reference Studies |
|---|---|---|---|
| Overall | Open | 1.4–33.5% (prevalent value around 10%) | Rocha, 2019 [4]; Lella, 2022 [9]; Muston, 2023 [3] |
| Endovascular | 0–35% (prevalent value around 3.5%) | Wong, 2012 [14]; Rocha, 2019 [4]; Pini, 2022 [20]; Zhang, 2022 [19]; Lella, 2022 [9]; Cheng-Hao, 2023 [21]; Frankort, 2023 [7]; Muston, 2023 [3] | |
| Not defined | 3.8–33% | Cina, 2004 [8]; Lella, 2022 [9]; Muston, 2023 [3] | |
| Permanent | Open | 3.3–11% | Rocha, 2019 [4]; Alzghari, 2024 [2] |
| Endovascular | 2.9–6.7% | Rocha, 2019 [4]; Lella, 2022 [9]; Alzghari, 2024 [2] | |
| Not defined | 2–7–5.8% | Thet, 2024 [25] | |
| Late | Open | NR | NR |
| Endovascular | 1.9% (95% CI: 1.2–25%) | Cheng-Hao, 2023 [21] | |
| Not defined | 1.3–12% | Sef, 2023 [23] |
| Q | Question | Study Design | Risk of Bias | Inconsistency | Indirectness | Publication Bias | Magnitude of Effect | Dose Response | Testing Moderators | Overall Quality | Grade |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Incidence and risk factors | 4 | −1 | −1 | 0 | 0 | 0 | 0 | 1 | 3 | Moderate |
| Q2 | Pathogenesis | 2 | −1 | 0 | −1 | 0 | 0 | 0 | 0 | 0 | Very low |
| Q3 | Early detection | 4 | −1 | −1 | 0 | 0 | 0 | 0 | 0 | 2 | Low |
| Q4 | Prevention | 4 | −1 | −1 | 0 | 0 | 0 | 0 | 1 | 3 | Moderate |
| Q5 | Complications associated with CSFD | 4 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | Moderate |
| Q6 | Treatment | 2 | −1 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Q7 | Prognosis | 2 | −1 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Q | Question | Findings | Grade | Areas of Future Research |
|---|---|---|---|---|
| Q1 | Incidence and risk factors | Open: 10%; endovascular up to 3.5%; permanent: 3.5% | Moderate | High-quality studies are needed |
| Q2 | Pathogenesis | Disruption of the spinal cord’s blood supply at some point, leading to hypoxia, metabolic arrest, and ischemia | Very low | Pathogenetic mechanisms at the cellular and molecular level |
| Q3 | Early detection | Neuromonitoring could be an option | Low | Optimization of neuromonitoring intraoperative, and maybe early postoperatively |
| Q4 | Prevention | CSFD seems to work in open aortic repair, LSA F/B EVAR | Moderate | High-quality studies are needed |
| Q5 | Complications associated with CSFD | Morbidity between 10 and 23%, non-negligible mortality | Moderate | Identify high-risk patients |
| Q6 | Treatment | Selective CSFD, optimize perfusion and hemodynamic parameters | Very low | Study the role of several drugs, including steroids, calcium channel blockers, fasudil, and milrinone |
| Q7 | Prognosis | Mortality of up to 75% in the first year | Very low | Studies focusing on the morbidity and mortality associated with SCI after aortic repair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brotis, A.G.; Kalogeras, A.; Bareka, M.; Arnaoutoglou, E.; Spanos, K.; Matsagkas, M.; Fountas, K.N. Prevention and Management of Spinal Cord Ischemia After Aortic Surgery: An Umbrella Review. Brain Sci. 2025, 15, 409. https://doi.org/10.3390/brainsci15040409
Brotis AG, Kalogeras A, Bareka M, Arnaoutoglou E, Spanos K, Matsagkas M, Fountas KN. Prevention and Management of Spinal Cord Ischemia After Aortic Surgery: An Umbrella Review. Brain Sciences. 2025; 15(4):409. https://doi.org/10.3390/brainsci15040409
Chicago/Turabian StyleBrotis, Alexandros G., Adamantios Kalogeras, Metaxia Bareka, Eleni Arnaoutoglou, Kostas Spanos, Miltiadis Matsagkas, and Kostas N. Fountas. 2025. "Prevention and Management of Spinal Cord Ischemia After Aortic Surgery: An Umbrella Review" Brain Sciences 15, no. 4: 409. https://doi.org/10.3390/brainsci15040409
APA StyleBrotis, A. G., Kalogeras, A., Bareka, M., Arnaoutoglou, E., Spanos, K., Matsagkas, M., & Fountas, K. N. (2025). Prevention and Management of Spinal Cord Ischemia After Aortic Surgery: An Umbrella Review. Brain Sciences, 15(4), 409. https://doi.org/10.3390/brainsci15040409










