Research on Adaptive Discriminating Method of Brain–Computer Interface for Motor Imagination
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Data Collection and Processing
2.2.1. Experimental Data Collection
2.2.2. Data Processing
2.3. MI-BCI Adaptability
2.4. Feature Extraction and Classification
2.4.1. Power Spectral Density
2.4.2. Wavelet Transform and Common Spatial Pattern
2.4.3. Riemannian Manifold
2.4.4. Filter Bank Common Spatial Pattern
2.4.5. Classification
2.5. Brain Functional Network Construction
2.5.1. Functional Connection
2.5.2. Network Properties
3. Results
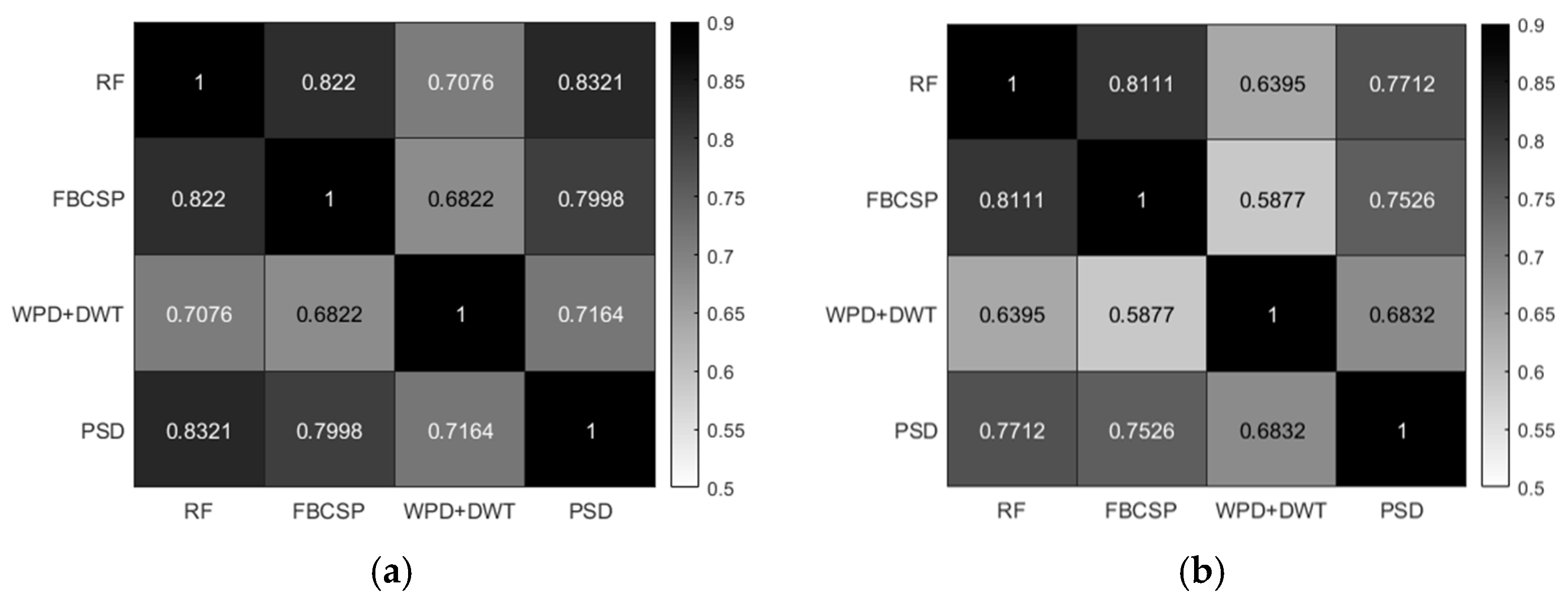
3.1. MI-BCI Adaptability Results
3.2. Brain Network Visualization Results
3.3. Correlation Analysis Results Between Brain Networks and MI-BCI Adaptability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces, a review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef]
- Di Mambro, A. Beyond Brainwaves: Exploring Emotions, Identity, and Motor Imagery Through EEG-Based BCI. 2025. Available online: https://iris.uniroma1.it/handle/11573/1732465 (accessed on 10 March 2025).
- Xu, S.; Liu, Y.; Lee, H.; Li, W. Neural interfaces: Bridging the brain to the world beyond healthcare. Exploration 2024, 4, 20230146. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H.; Geyer, S.; Naito, E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J. Neurophysiol. 2003, 90, 3304–3316. [Google Scholar] [CrossRef]
- McFarland, D.J.; Wolpaw, J.R. Brain-computer interfaces for communication and control. Commun. ACM 2011, 54, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R. Brain-computer interfaces (BCIs) for communication and control. In Proceedings of the 9th International ACM SIGACCESS Conference on Computers and Accessibility, Tempe, AZ, USA, 15–17 October 2007; pp. 1–2. [Google Scholar]
- Pineda, J.A.; Giromini, L.; Soghoyan, G.; Bohm, M.; Maryanovsky, D.; Zennaro, A. Effects of repetitive transcranial magnetic stimulation (rTMS) on attribution of movement to ambiguous stimuli and EEG mu suppression. Brain Res. 2018, 1680, 69–76. [Google Scholar]
- Di Giacomo, J.; Gongora, M.; Silva, F.; Nicoliche, E.; Bittencourt, J.; Marinho, V.; Gupta, D.; Orsini, M.; Teixeira, S.; Cagy, M.; et al. Repetitive transcranial magnetic stimulation changes cognitive/motor tasks performance: An absolute alpha and beta power study. Neurosci. Lett. 2021, 753, 135866. [Google Scholar] [CrossRef] [PubMed]
- Cattai, T.; Colonnese, S.; Corsi, M.-C.; Bassett, D.S.; Scarano, G.; Fallani, F.D.V. Phase/amplitude synchronization of brain signals during motor imagery BCI tasks. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1168–1177. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Spatiotemporal ERD/ERS patterns during voluntary movement and motor imagery. Suppl. Clin. Neurophysiol. 2000, 53, 196–198. [Google Scholar]
- Hammer, E.M.; Kaufmann, T.; Kleih, S.C.; Blankertz, B.; Kübler, A. Visuo-motor coordination ability predicts performance with brain-computer interfaces controlled by modulation of sensorimotor rhythms (SMR). Front. Hum. Neurosci. 2014, 8, 574. [Google Scholar] [CrossRef]
- Phunruangsakao, C.; Achanccaray, D.; Bhattacharyya, S.; Izumi, S.-I.; Hayashibe, M. Effects of visual-electrotactile stimulation feedback on brain functional connectivity during motor imagery practice. Sci. Rep. 2023, 13, 17752. [Google Scholar] [CrossRef]
- Confalonieri, L.; Pagnoni, G.; Barsalou, L.; Rajendra, J.; Eickhoff, S.; Butler, A. Brain activation in primary motor and somatosensory cortices during motor imagery correlates with motor imagery ability in stroke patients. ISRN Neuro 2012, 2012, 613595. [Google Scholar] [CrossRef] [PubMed]
- Padfield, N.; Zabalza, J.; Zhao, H.; Masero, V.; Ren, J. EEG-based brain-computer interfaces using motor-imagery: Techniques and challenges. Sensors 2019, 19, 1423. [Google Scholar] [CrossRef]
- Singh, A.; Hussain, A.A.; Lal, S.; Guesgen, H.W. A comprehensive review on critical issues and possible solutions of motor imagery based electroencephalography brain-computer interface. Sensors 2021, 21, 2173. [Google Scholar] [CrossRef]
- Hamedi, M.; Salleh, S.-H.; Noor, A.M. Electroencephalographic motor imagery brain connectivity analysis for BCI: A review. Neural Comput. 2016, 28, 999–1041. [Google Scholar] [CrossRef] [PubMed]
- Munzert, J.; Lorey, B.; Zentgraf, K. Cognitive motor processes: The role of motor imagery in the study of motor representations. Brain Res. Rev. 2009, 60, 306–326. [Google Scholar] [CrossRef]
- Bastos, A.M.; Schoffelen, J.-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Feng, Z.; Qian, L.; Hu, H.; Sun, Y. Functional connectivity for motor imaginary recognition in brain-computer interface. In Proceedings of the 2020 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Toronto, ON, Canada, 11–14 October 2020; pp. 3678–3682. [Google Scholar]
- Xu, L.; Zhang, H.; Hui, M.; Long, Z.; Jin, Z.; Liu, Y.; Yao, L. Motor execution and motor imagery: A comparison of functional connectivity patterns based on graph theory. Neuroscience 2014, 261, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Oikonomou, V.P.; Georgiadis, K.; Liaros, G.; Nikolopoulos, S.; Kompatsiaris, I. A comparison study on EEG signal processing techniques using motor imagery EEG data. In Proceedings of the 2017 IEEE 30th International Symposium on Computer-Based Medical Systems (CBMS), Thessaloniki, Greece, 22–24 June 2017; pp. 781–786. [Google Scholar]
- Alessio, S.M.; Alessio, S.M. Discrete wavelet transform (DWT). In Digital Signal Processing and Spectral Analysis for Scientists: Concepts and Applications; Springer: Cham, Switzerland, 2016; pp. 645–714. [Google Scholar]
- Jiang, A.; Shang, J.; Liu, X.; Tang, Y.; Kwan, H.K.; Zhu, Y. Efficient CSP algorithm with spatio-temporal filtering for motor imagery classification. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1006–1016. [Google Scholar] [CrossRef]
- Liu, W.; Guo, C.; Gao, C. A cross-session motor imagery classification method based on Riemannian geometry and deep domain adaptation. Expert Syst. Appl. 2024, 237, 121612. [Google Scholar] [CrossRef]
- Chin, Z.Y.; Ang, K.K.; Wang, C.; Guan, C.; Zhang, H. Multi-class filter bank common spatial pattern for four-class motor imagery BCI. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 571–574. [Google Scholar]
- Chatterjee, R.; Bandyopadhyay, T. EEG based motor imagery classification using SVM and MLP. In Proceedings of the 2016 2nd International Conference on Computational Intelligence and Networks (CINE), Bhubaneswar, India, 11 January 2016; pp. 84–89. [Google Scholar]
- Jia, H.; Wang, S.; Zheng, D.; Qu, X.; Fan, S. Comparative study of motor imagery classification based on BP-NN and SVM. J. Eng. 2019, 2019, 8646–8649. [Google Scholar] [CrossRef]
- Kim, H.; Yoshimura, N.; Koike, Y. Classification of movement intention using independent components of premovement EEG. Front. Hum. Neurosci. 2019, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Vinck, M.; Oostenveld, R.; Van Wingerden, M.; Battaglia, F.; Pennartz, C.M.A. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. NeuroImage 2011, 55, 1548–1565. [Google Scholar] [CrossRef]
- Hardmeier, M.; Hatz, F.; Bousleiman, H.; Schindler, C.; Stam, C.J.; Fuhr, P. Reproducibility of functional connectivity and graph measures based on the phase lag index (PLI) and weighted phase lag index (wPLI) derived from high resolution EEG. PLoS ONE 2014, 9, e108648. [Google Scholar] [CrossRef]
- Ismail, L.E.; Karwowski, W. A graph theory-based modeling of functional brain connectivity based on EEG: A systematic review in the context of neuroergonomics. IEEE Access 2020, 8, 155103–155135. [Google Scholar] [CrossRef]
- Sedgwick, P. Pearson’s correlation coefficient. BMJ 2012, 345, e4483. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, T.; Li, F.; Li, M.; Liu, D.; Zhang, R.; He, H.; Li, P.; Gong, J.; Luo, C.; et al. Structural and functional correlates of motor imagery BCI performance: Insights from the patterns of fronto-parietal attention network. NeuroImage 2016, 134, 475–485. [Google Scholar] [CrossRef]
- Giannopulu, I.; Mizutani, H. Neural kinesthetic contribution to motor imagery of body parts: Tongue, hands, and feet. Front. Hum. Neurosci. 2021, 15, 602723. [Google Scholar] [CrossRef]
- Papaxanthis, C.; Schieppati, M.; Gentili, R.; Pozzo, T. Imagined and actual arm movements have similar durations when performed under different conditions of direction and mass. Exp. Brain Res. 2002, 143, 447–452. [Google Scholar] [CrossRef]




| Method | Calculation Formula | Remark |
|---|---|---|
| Node degree | represents the total number of nodes, represents the degree of node , represents the number of edges between the neighbors of node , represents the shortest path length between node and node , represents the number of shortest paths from node to node that pass through node . | |
| Clustering coefficient | ||
| Characteristic path length | ||
| Local efficiency | ||
| Betweenness centrality |
| RF | FBCSP | WPD + DWT | PSD | |||||
|---|---|---|---|---|---|---|---|---|
| p | r | p | r | p | r | p | r | |
| CL | 0.050 * | 0.278 * | 0.060 | 0.267 | 0.019 * | 0.330 * | 0.143 | 0.210 |
| KL | 0.371 | 0.129 | 0.691 | 0.058 | 0.060 | 0.268 | 0.339 | 0.138 |
| BCL | 0.983 | 0.003 | 0.236 | −0.171 | 0.700 | −0.056 | 0.953 | 0.008 |
| EL | 0.037 * | 0.297 * | 0.046 * | 0.283 * | 0.009 * | 0.364 * | 0.075 | 0.254 |
| LL | 0.461 | 0.107 | 0.799 | 0.037 | 0.228 | 0.174 | 0.405 | 0.120 |
| CD | 0.049 * | −0.280 * | 0.042 * | −0.288 * | 0.021 * | −0.326 * | 0.025 * | −0.316 * |
| KD | 0.630 | −0.070 | 0.756 | −0.045 | 0.304 | −0.148 | 0.272 | −0.158 |
| BCD | 0.646 | −0.066 | 0.941 | 0.011 | 0.535 | −0.090 | 0.216 | −0.178 |
| ED | 0.077 | −0.253 | 0.071 | −0.258 | 0.032 * | −0.304 * | 0.032 * | −0.304 * |
| LD | 0.608 | −0.074 | 0.618 | −0.072 | 0.291 | −0.152 | 0.208 | −0.181 |
| RF | FBCSP | WPD + DWT | PSD | |||||
|---|---|---|---|---|---|---|---|---|
| p | r | p | r | p | r | p | r | |
| CR | 0.198 | 0.185 | 0.177 | 0.194 | 0.066 | 0.262 | 0.312 | 0.146 |
| KR | 0.061 | 0.267 | 0.077 | 0.252 | 0.033 * | 0.303 * | 0.024 * | 0.320 * |
| BCR | 0.079 | 0.251 | 0.278 | 0.156 | 0.535 | 0.090 | 0.075 | 0.254 |
| ER | 0.194 | 0.187 | 0.137 | 0.213 | 0.059 | 0.269 | 0.218 | 0.177 |
| LR | 0.056 | 0.272 | 0.018 * | 0.332 * | 0.037 * | 0.296 * | 0.018 * | 0.332 * |
| CD | 0.125 | 0.220 | 0.387 | 0.125 | 0.155 | 0.204 | 0.527 | 0.092 |
| KD | 0.088 | 0.244 | 0.274 | 0.158 | 0.096 | 0.238 | 0.104 | 0.233 |
| BCD | 0.033 * | 0.302 * | 0.085 | 0.246 | 0.186 | 0.190 | 0.047 * | 0.282 * |
| ED | 0.153 | 0.205 | 0.398 | 0.122 | 0.117 | 0.224 | 0.450 | 0.109 |
| LD | 0.073 | 0.256 | 0.148 | 0.208 | 0.064 | 0.264 | 0.081 | 0.249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Liu, H.; Duan, F.; Che, Y.; Yan, Z. Research on Adaptive Discriminating Method of Brain–Computer Interface for Motor Imagination. Brain Sci. 2025, 15, 412. https://doi.org/10.3390/brainsci15040412
Gong J, Liu H, Duan F, Che Y, Yan Z. Research on Adaptive Discriminating Method of Brain–Computer Interface for Motor Imagination. Brain Sciences. 2025; 15(4):412. https://doi.org/10.3390/brainsci15040412
Chicago/Turabian StyleGong, Jifeng, Huitong Liu, Fang Duan, Yan Che, and Zheng Yan. 2025. "Research on Adaptive Discriminating Method of Brain–Computer Interface for Motor Imagination" Brain Sciences 15, no. 4: 412. https://doi.org/10.3390/brainsci15040412
APA StyleGong, J., Liu, H., Duan, F., Che, Y., & Yan, Z. (2025). Research on Adaptive Discriminating Method of Brain–Computer Interface for Motor Imagination. Brain Sciences, 15(4), 412. https://doi.org/10.3390/brainsci15040412





