In Vitro Enzymatic and Kinetic Studies, and In Silico Drug-Receptor Interactions, and Drug-Like Profiling of the 5-Styrylbenzamide Derivatives as Potential Cholinesterase and β-Secretase Inhibitors with Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Materials
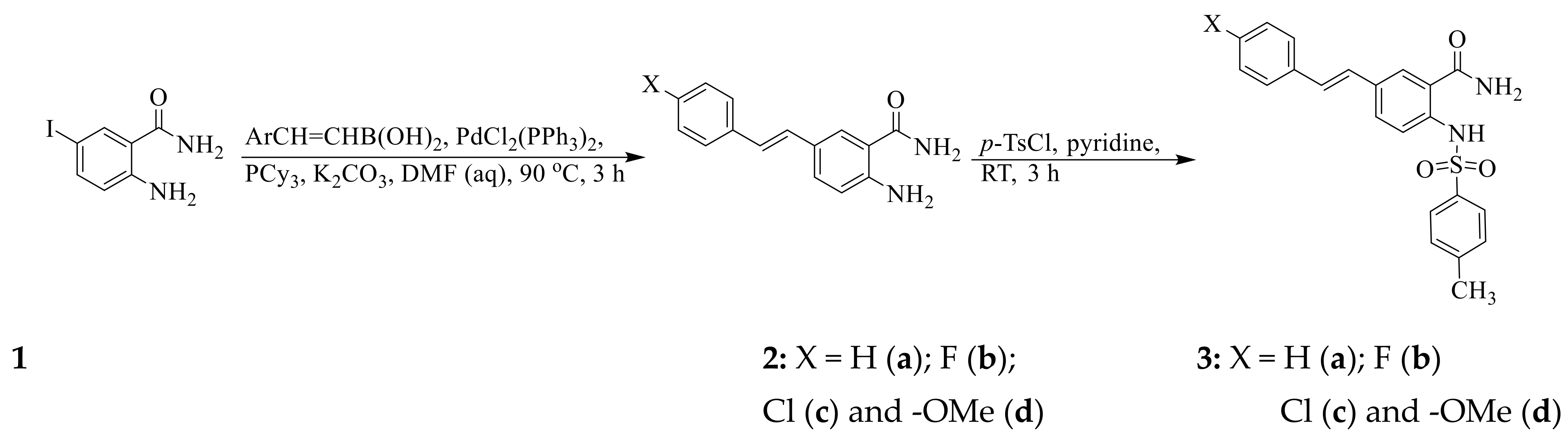
2.2. Typical Procedure for the Synthesis of the 5-Styrylbenzamides 2a–d
2.3. Typical Procedure for the Synthesis of the (E) 5-styryl-2-(p-tolylsulfonyl)benzamide 3a–d
2.4. Cholinesterase Inhibition Assays of 2a–d and 3a–d
2.4.1. AChE Inhibition Assay of 2a–d and 3a–d
2.4.2. BChE Inhibition Assay of 2a–d and 3a–d
2.5. β-Secretase (BACE-1) Inhibition Assay of 2a–d and 3a–d
2.6. Antioxidant Activity of 2a–d and 3a–d
2.6.1. Determination of the Reducing Activity of the Stable, Radical DPPH by 2a–d and 3a–d
2.6.2. Nitric Oxide Radical Scavenging Activity of Compounds 2a–d and 3a–d
2.7. Enzyme Kinetic Studies of 2a and 3b against AChE, BChE, and β-Secretase
2.7.1. Enzyme Kinetic Studies of 2a and 3b against AChE and BChE
2.7.2. Enzyme Kinetic Studies of 2a and 3b on β-Secretase
2.8. Metal Binding Studies of 2a and 3b
2.9. Molecular Modelling Studies
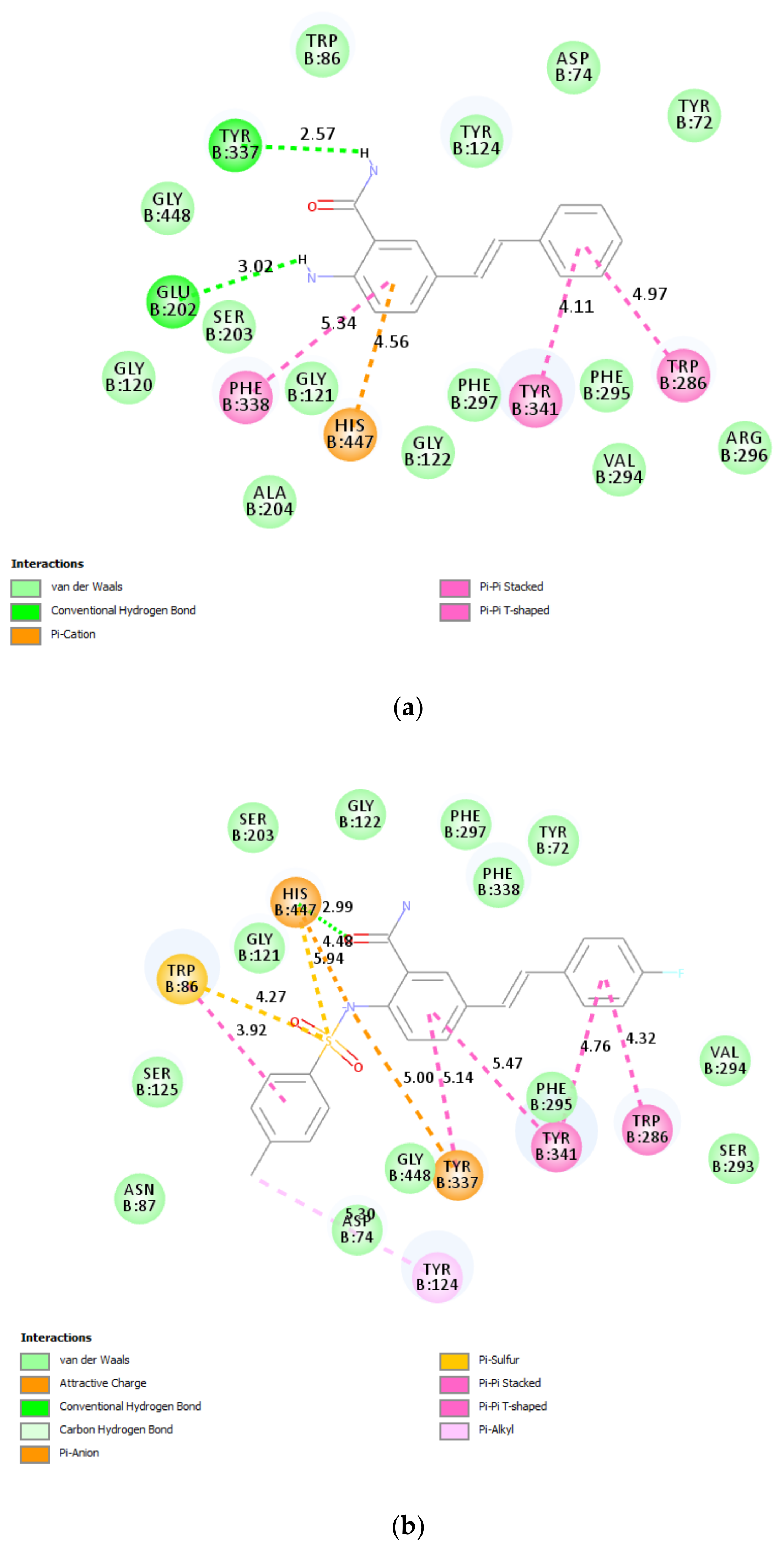
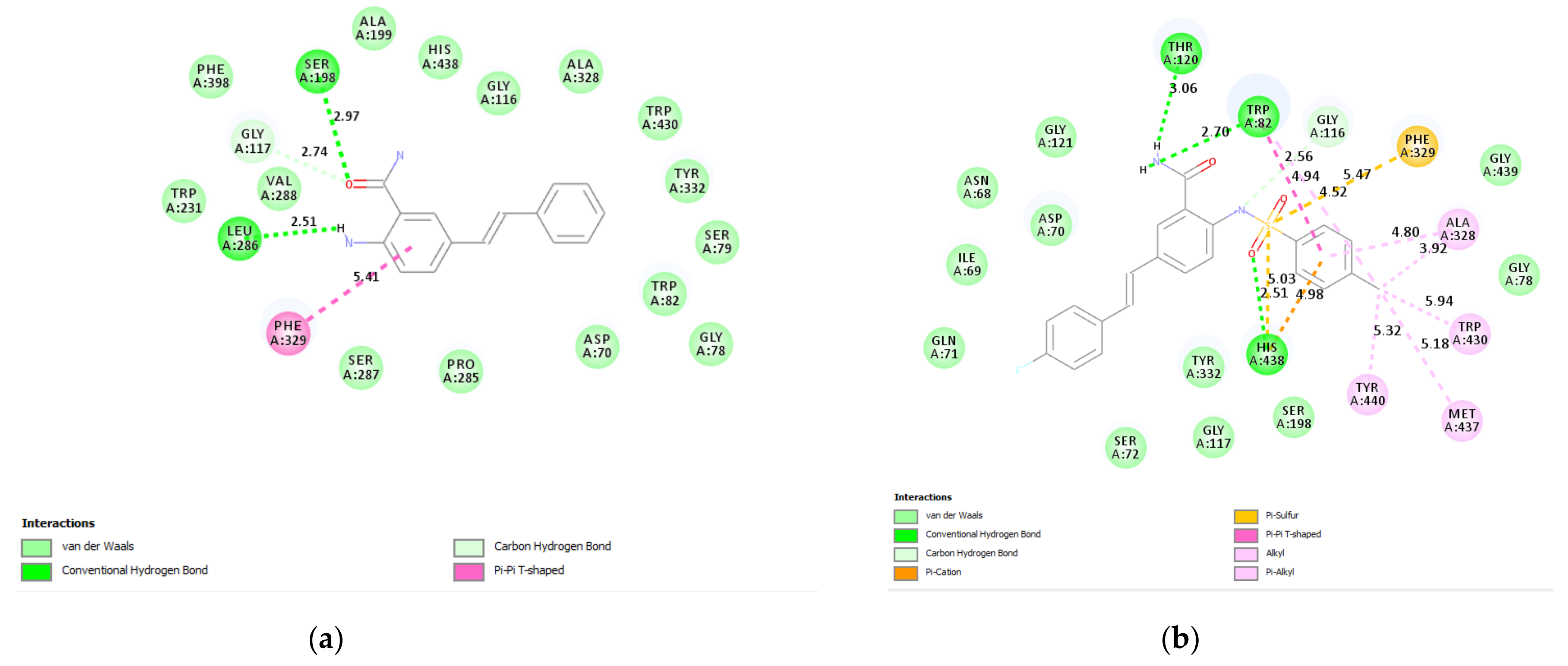
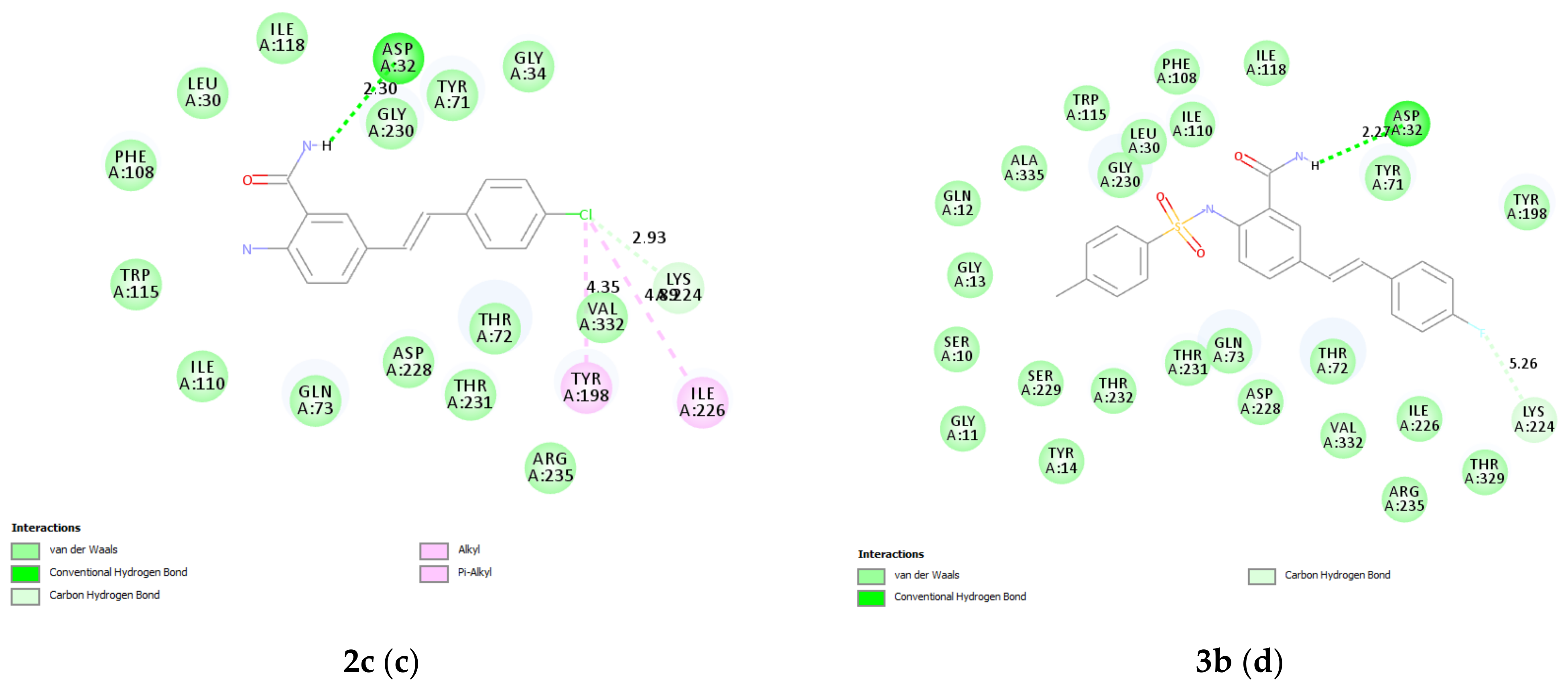
2.9.1. Molecular Docking of 2a, 2c, and 3b
2.9.2. Predication of Physicochemical Parameters for 2a and 3b
2.10. Evaluation of Cytotoxicity (MTT assay) of 2a and 3b on the Vero and A549 Cells
2.11. Measurement of Reactive Oxygen Species (ROS) in Cells
3. Results and Discussion
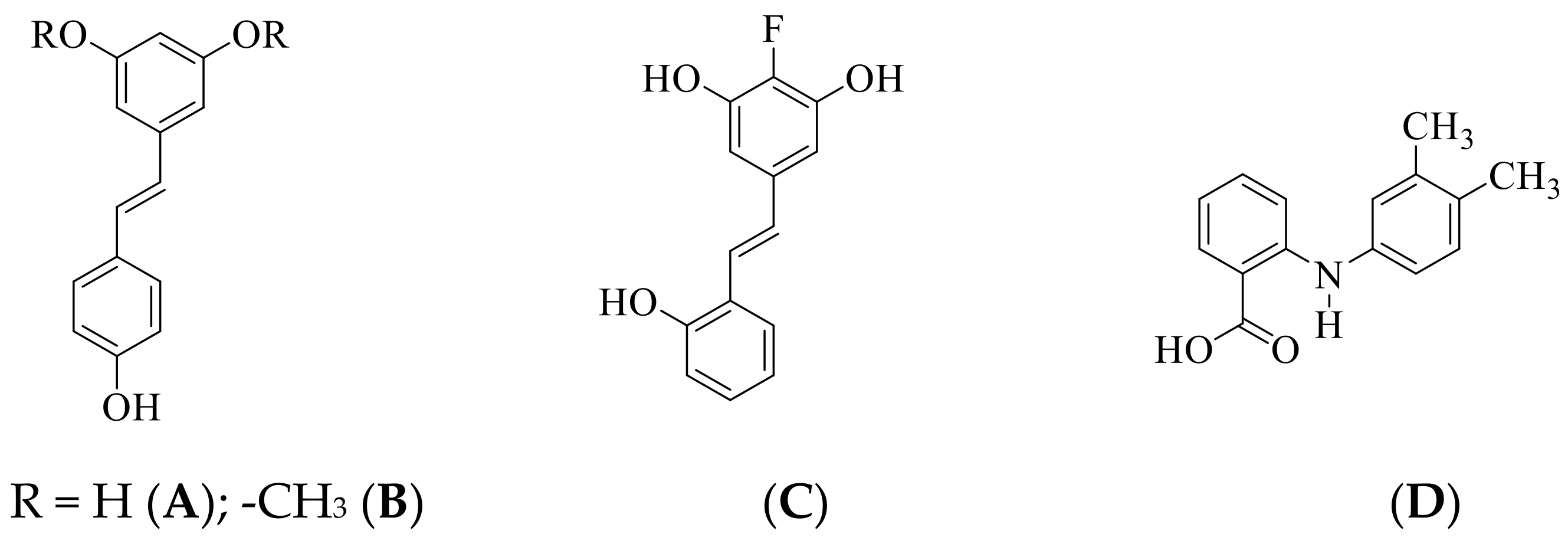
3.1. Chemical Synthesis
3.2. Biology
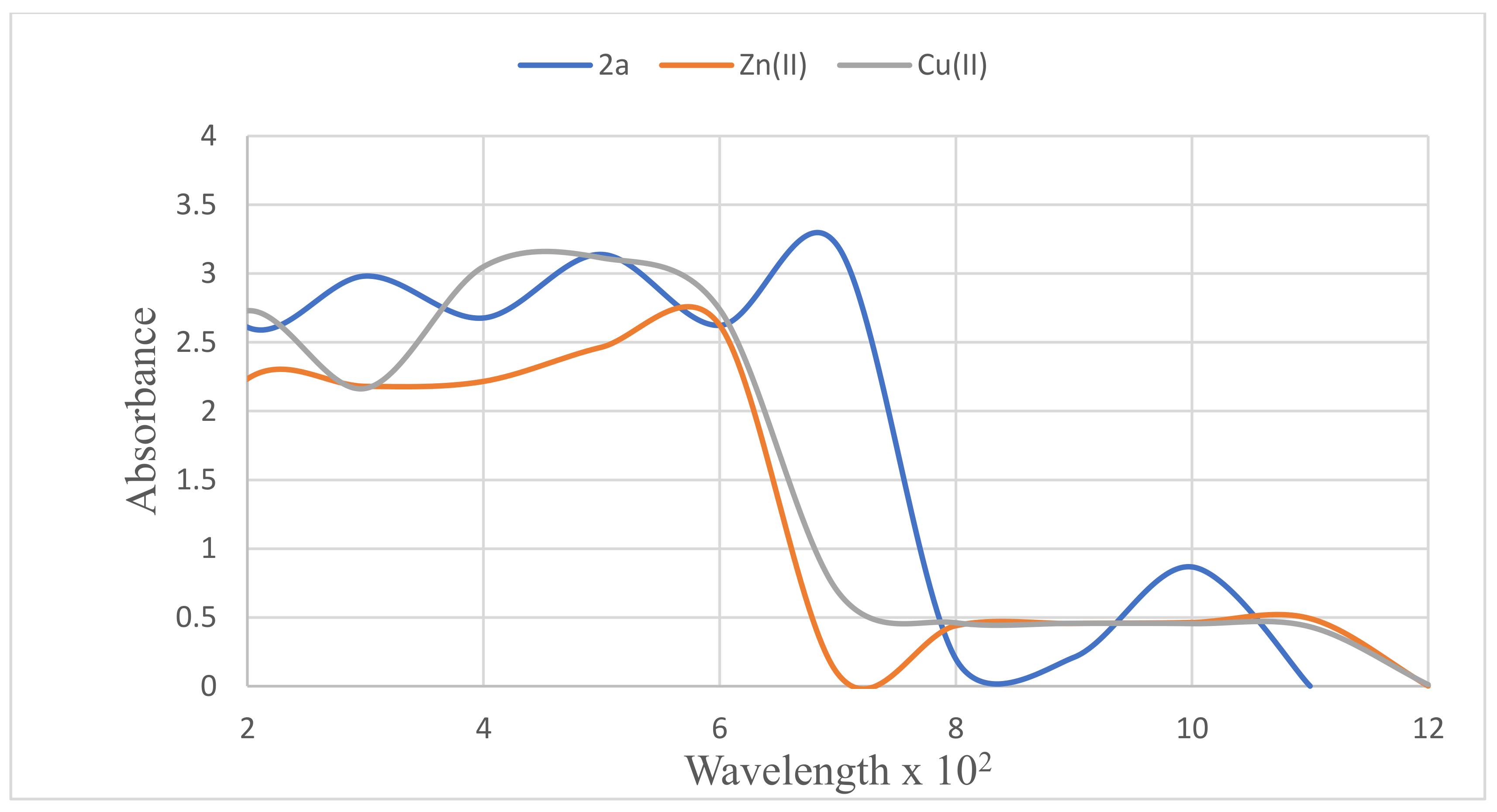
3.3. Metal Chelation Study of Compound 2a
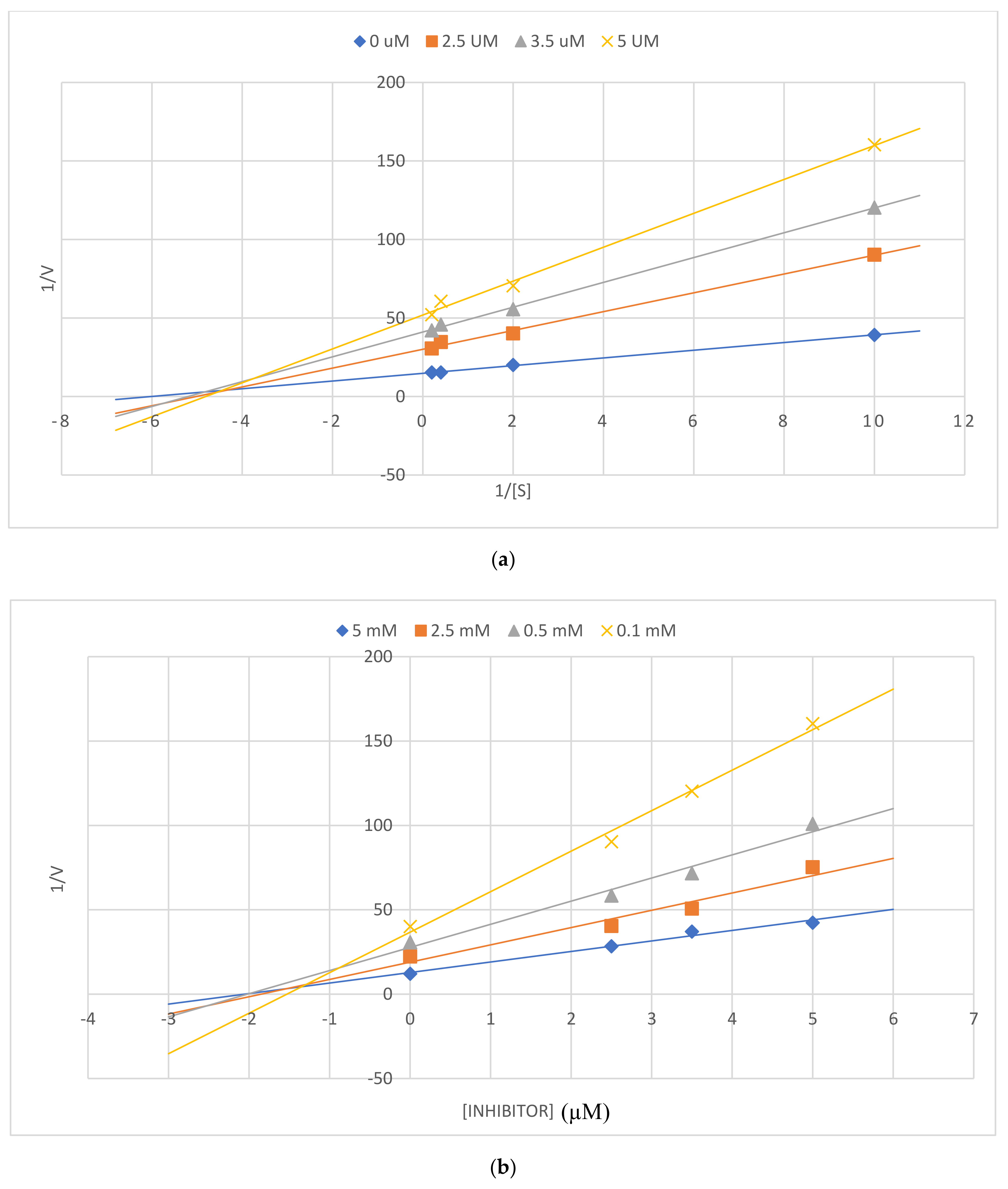
3.4. Kinetic Studies of 2a on AChE, BChE, and β-Secretase
3.5. Kinetic Study of 2a on β-Secretase
3.6. Computational Studies
3.6.1. Docking of 2a and 3b into Cholinesterase Enzyme (AChE and BChE) Binding Sites
3.6.2. β-Secretase 2a, 2c, and 3b
3.7. Prediction of ADME Descriptors for 2a and 3b
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhang, H. Reconsideration of anticholinesterase therapeutic strategies against Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; Patel, N.R.; Kanhed, A.M.; Telic, D.M.; Patel, K.B.; Joshia, P.D.; Patel, S.P.; Gandhi, P.M.; Chaudhary, B.N.; Prajapati, N.K.; et al. Novel carbazole-stilbene hybrids as multifunctional anti-Alzheimer agents. Bioorg. Chem. 2020, 101, 103977. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Song, Q.; Cao, Z.; Yu, G.; Liu, Z.; Tan, Z.; Den, Y. Design, synthesis and evaluation of flurbiprofen-clioquinol hybrids as multitarget-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2020, 289, 115374. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [CrossRef]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Patterson, C. The state of the art of dementia research: New frontiers. In World Alzheimer Report 2018; Alzheimer’s Disease international (ADI): London, UK, 2018; pp. 32–36. [Google Scholar]
- Chen, S.-Y.; Chen, Y.; Li, Y.-P.; Chen, S.-H.; Tan, J.-H.; Ou, T.-M.; Gu, L.-Q.; Huang, Z.-S. Design, synthesis, and biological evaluation of curcumin analogues as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2011, 19, 5596–5604. [Google Scholar] [CrossRef]
- Bajda, M.; Guzior, N.; Ignasik, M.; Malawska, B. Multi-target-directed ligands in Alzheimer’s disease treatment. Curr. Med. Chem. 2011, 18, 4949–4975. [Google Scholar] [CrossRef]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer’s disease. Curr. Med. Chem. 2015, 22, 373–404. [Google Scholar]
- Li, Y.R.; Li, S.; Lin, C.-C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 1, 69–82. [Google Scholar] [CrossRef]
- Mattio, L.M.; Marengo, M.; Parravicini, C.; Eberini, I.; Dallavalle, S.; Bonomi, F.; Iametti, S.; Pinto, A. Inhibition of Pancreatic α-amylase by Resveratrol derivatives: Biological activity and molecular modelling evidence for cooperativity between viniferin enantiomers. Molecules 2019, 24, 3225. [Google Scholar] [CrossRef]
- Zhang, A.J.; Rimando, A.M.; Mizuno, C.S.; Mathews, S.T. α-glucosidase inhibitory effect of resveratrol and piceatannol. J. Nutr. Biochem. 2017, 47, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Albert, S.; Kluge, R.; Strohl, D. Resveratrol derived butyrylcholinesterase inhibitors. Arch. Pharm. Chem. Life Sci. 2013, 346, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.X.; Larsen, S.W.; Pye, C.; Glabreath, M.; Isovitsch, R.; Fradenger, E.A. The functional group on (E)-4, 4′–disubstituted stilbenes influences toxicity and antioxidant activity in differentiated PC-12 cells. Bioorg. Med. Chem. Lett. 2013, 23, 6355–6359. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Kim, H.S.; Woo, R.S.; Park, C.H.; Shin, K.Y.; Lee, J.P.; Chang, K.A.; Kim, S.; Suh, Y.H. Mefenamic acid shows neuroprotective effects and improves cognitive impairment in in vitro and in vivo Alzheimer’s disease models. Mol. Pharmacol. 2006, 69, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Sultana, N.; Tariq, M.I.; Parvez, M. Synthesis, molecular packing and anti-cholinesterase activity of some new C-2 N-substituted anthranilamide derivatives. Z. Kristallogr. Cryst. Mater. 2019, 234, 605–611. [Google Scholar] [CrossRef]
- Banks, W.A. Drug delivery to the brain in Alzheimer’s disease: Consideration of the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 629–639. [Google Scholar] [CrossRef]
- Barraza, S.J.; Delekta, P.C.; Sindac, J.A.; Dobry, C.J.; Xiang, J.; Keep, R.F.; Miller, D.J.; Larsen, S.D. Discovery of anthranilamides as a novel class of inhibitors of neurotropic alphavirus replication. Bioorg. Med. Chem. 2015, 23, 1569–1587. [Google Scholar] [CrossRef]
- Sunitha, K.; Hemshekhar, M.; Thushara, R.M.; Santhosh, M.S.; Yariswamy, M.; Kemparaju, K.; Girish, K.S. N-Acetylcysteine amide: A derivative to fulfil the promises of N-Acetylcysteine. Free Radic. Res. 2013, 47, 357–367. [Google Scholar] [CrossRef]
- Xie, R.; Li, Y.; Tang, P.; Yuan, Q. Design, synthesis and biological evaluation of novel 2-aminobenzamides containing dithiocarbamate moiety as histone deacetylase inhibitors and potent antitumor agents. Eur. J. Med. Chem. 2018, 143, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abbasi, M.A.; Siddiqui, S.Z.; Shahzadi, S.; Raza, H.; Hussain, G.; Shah, S.A.A.; Ashraf, M.; Shahid, M.; Seo, S.Y.; et al. Designing of promising medicinal scaffolds for Alzheimer’s disease through enzyme inhibition, lead optimization, molecular docking and dynamic simulation approaches. Bioorg. Chem. 2019, 91, 103138. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Lee, Y.-R.; Mastuki, S.T.; Leong, S.W.; Ibrahim, W.N.W.; Latif, M.A.M.; Ramli, A.N.M.; Aluwi, M.F.F.M.; Faudzia, S.M.M.; Kim, G.-H. Development of diarylpentadienone analogues as alpha-glucosidase inhibitor: Synthesis, in vitro biological and in vivo toxicity evaluations, and molecular docking analysis. Bioorg. Chem. 2020, 104, 104277. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.; Guan, W.; Huang, L.; Xu, X.; Zhang, C.; Chen, X.; Wu, Y.; Zeng, G.; Zhong, N. Antitumor effect of para-toluenesulfonamide against lung cancer xenograft in a mouse model. J. Thorac. Dis. 2013, 5, 472–483. [Google Scholar]
- Mphahlele, M.J.; Paumo, H.K.; Rhyman, L.; Ramasami, P. Synthesis and photophysical properties of polycarbo-substituted quinazolines derived from the 2-aryl-4-chloro-6-iodoquinazolines. Molecules 2015, 20, 14656–14683. [Google Scholar] [CrossRef]
- Agbo, E.N.; Gildenhuys, S.; Choong, Y.S.; Mphahlele, M.J.; More, G.K. Synthesis of Furocoumarin–stilbene hybrids as potential multifunctional drugs against multiple biochemical targets associated with Alzheimer’s disease. Bioorg. Chem. 2020, 101, 103997. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Francis, P.L.; Rhodes, P.; Moncada, S. A rapid and simple method for the measurement of nitrite and nitrate in plasma by high performance capillary electrophoresis. Biochem. Biophys. Res. Commun. 1994, 200, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- James, J.; Fiji, N.; Roy, D.; Andrew, M.G.D.; Shihabudeen, M.S.; Chattopadhyay, D.; Thirumurugan, K. A rapid method to assess reactive oxygen species in yeast using H2DCF-DA. Anal. Methods 2015, 7, 8572–8575. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Maluleka, M.M.; Rhyman, L.; Ramasami, P.; Mampa, R.M. Spectroscopic, DFT, and XRD studies of hydrogen bonds in N-unsubstituted 2-aminobenzamides. Crystals 2017, 22, 83. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Onwu, E.E.; Maluleka, M.M. Spectroscopic, X-ray diffraction and density functional theory study of intra- and intermolecular hydrogen bonds in ortho-(4-tolylsulfonamido)benzamides. Molecules 2021, 26, 926. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G. Advances in the treatment of Alzheimer’s disease: Benefits of dual cholinesterase inhibition. Eur. Neurol. 2002, 47, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Kiewert, C.; Duysen, E.G.; Lockridge, O.; Greig, N.H.; Klein, J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J. Neurochem. 2007, 100, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, H.; Li, Q.; Chen, Y.; Li, Q.; Zhou, Y.; Feng, F.; Liu, W.; Guo, Q.; Sun, H. Expansion of the scaffold diversity for the development of highly selective butyrylcholinesterase (BChE) inhibitors: Discovery of new hits through the pharmacophore model generation, virtual screening and molecular dynamics simulation. Bioorg. Chem. 2019, 85, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mohan, C.G. Dual binding site and selective acetylcholinesterase inhibitors derived from integrated pharmacophore models and sequential virtual screening. BioMed Res. Int. 2014, 2014, 291214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Xu, Z.; Li, H.; Liu, H.; Zhu, W. Halogen bonding for rational drug design and new drug discovery. Expert Opin. Drug Discov. 2012, 7, 375–383. [Google Scholar] [CrossRef]
- Reid, G.A.; Darvesh, S. Butyrylcholinesterase-knockout reduces brain deposition of fibrillar β-amyloid in an Alzheimer mouse model. Neuroscience 2015, 298, 424–435. [Google Scholar] [CrossRef]
- Asai, M.; Hattori, C.; Iwata, N.; Saido, T.C.; Sasagawa, N.; Szabo, B.; Hashimoto, Y.; Maruyama, K.; Tanuma, S.; Kiso, Y. The novel β-Secretase inhibitor KMI-429 reduces amyloid-b peptide reduction in amyloid precursor protein transgenic and wild- type mice. J. Neurochem. 2006, 96, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraja, J.; Esakkidurai, T.; Subbaraj, P.; Shobana, S. Mixed ligand complex formation of 2-aminobenzamide with Cu(II) in the presence of some amino acids: Synthesis, structural, biological, pH-metric, spectrophotometric and thermodynamic studies. Spectrochim. Acta A 2013, 114, 607–621. [Google Scholar] [CrossRef]
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Djemil, A.; Mancini, F.; Andrisano, V.; Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharmacol. Res. Perspect. 2014, 2, e00023. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Fedorko, J.M.; Vinayaka, C.R.; Mehekar, R.; Radić, Z.; Taylor, P.; Lockridge, O.; Doctor, B.P. Aromatic amino-acid residues at the active and peripheral aninonic sites control the binding of E2020 (Arcept®) to cholinesterase. Eur. J. Biochem. 2003, 270, 4447–4458. [Google Scholar] [CrossRef]
- Brus, A.; Kosak, U.; Turk, S.; Pislar, A.; Coquelle, N.; Kos, J.; Stoja, J.; Colletier, J.P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.A.; Sousa, E. Bace-1 and γ-secretase as therapeutic targets for Alzheimer’s disease. Pharmaceuticals 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Butini, S.; Brogi, S.; Novellino, E.; Campiani, G.; Ghosh, A.K.; Brindisi, M.; Gemma, S. The structural evolution of β-secretase inhibitors: A focus on the development of small-molecule inhibitors. Curr. Top. Med. Chem. 2013, 13, 1787–1807. [Google Scholar] [CrossRef]
- Edfelt, F.N.B.; Folmer, R.H.A.; Breeze, A.L. Fragment screening to predict druggability (ligandability) and lead discovery success. Drug Discov. Today 2011, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.P.; Murcko, A.A.; Murcko, M.A. Recognizing molecules with drug-like properties. Curr. Opin. Chem. Biol. 1999, 3, 384–387. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Zhao, Y.; Abraham, M.H.; Lee, J.; Hersey, A. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2018, 19, 1446–1457. [Google Scholar] [CrossRef]




| Compound | [IC50 (SD) μM] | ||||
|---|---|---|---|---|---|
| AChE | BChE | β-Secretase | DPPH | NO | |
| 2a | 2.3 ± 0.11 | 4.7 ± 0.32 | 15.8 ± 0.26 | 5.8 ± 0.32 | 10.3 ± 0.12 |
| 2b | 5.4 ± 0.12 | 19.7 ± 0.25 | 13.9 ± 0.25 | 12.9 ± 0.22 | 15.8 ± 0.30 |
| 2c | 8.6 ± 0.42 | 18.9 ± 0.24 | 6.7 ± 0.18 | 11.3 ± 0.11 | 9.3 ± 0.13 |
| 2d | 7.8 ± 0.30 | 14.6 ± 0.47 | 17.2 ± 0.31 | 20.8 ± 0.24 | 11.2 ± 0.22 |
| 3a | 9.5 ± 0.53 | 10.8 ± 0.40 | 16.5 ± 0.31 | 19.4 ± 0.40 | 13.1 ± 0.43 |
| 3b | 4.3 ± 0.48 | 8.1 ± 0.52 | 10.1 ± 0.18 | 25.6 ± 0.30 | 7.6 ± 0.26 |
| 3c | 7.8 ± 0.21 | 6.9 ± 0.10 | 15.0 ± 0.16 | 22.4 ± 0.51 | 8.6 ± 0.30 |
| 3d | 11.3 ± 0.23 | 13.3 ± 0.26 | 19.3 ± 0.24 | 9.1 ± 0.26 | 12.3 ± 0.31 |
| Donepezil | 1.24 ± 0.15 | 2.98 ± 0.18 | - | - | - |
| Quercetin | - | - | 10.4 ± 0.32 | - | - |
| Ascorbic acid | - | - | - | 4.18 ± 0.13 | 6.23 ± 0.13 |
| Property | 2a | 3b |
|---|---|---|
| miLogP | 2.73 | 4.62 |
| Topological polar surface area (Å) | 69.12 | 89.26 |
| Absorption (%) | 85.15 | 78.21 |
| Number of atom | 18 | 29 |
| Molecular weight | 238.29 | 410.47 |
| Molecular volume | 224.43 | 349.88 |
| Hydrogen bond acceptor | 3 | 5 |
| Hydrogen bond donor | 4 | 3 |
| Rotatable bonds | 3 | 6 |
| Lipinski’s violation | 0 | 0 |
| Blood-brain barrier (BBB) | Yes | No |
| IC50 (μM) | ||
|---|---|---|
| Compound | Vero Cells | A549 Cells |
| 2a | 163.00 ± 0.55 | 35.40 ± 0.13 |
| 3b | 191.40 ± 0.60 | 55.00 ± 0.11 |
| Doxorubicin | 0.66 ± 0.12 | 1.14 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mphahlele, M.J.; Agbo, E.N.; More, G.K.; Gildenhuys, S. In Vitro Enzymatic and Kinetic Studies, and In Silico Drug-Receptor Interactions, and Drug-Like Profiling of the 5-Styrylbenzamide Derivatives as Potential Cholinesterase and β-Secretase Inhibitors with Antioxidant Properties. Antioxidants 2021, 10, 647. https://doi.org/10.3390/antiox10050647
Mphahlele MJ, Agbo EN, More GK, Gildenhuys S. In Vitro Enzymatic and Kinetic Studies, and In Silico Drug-Receptor Interactions, and Drug-Like Profiling of the 5-Styrylbenzamide Derivatives as Potential Cholinesterase and β-Secretase Inhibitors with Antioxidant Properties. Antioxidants. 2021; 10(5):647. https://doi.org/10.3390/antiox10050647
Chicago/Turabian StyleMphahlele, Malose J., Emmanuel N. Agbo, Garland K. More, and Samantha Gildenhuys. 2021. "In Vitro Enzymatic and Kinetic Studies, and In Silico Drug-Receptor Interactions, and Drug-Like Profiling of the 5-Styrylbenzamide Derivatives as Potential Cholinesterase and β-Secretase Inhibitors with Antioxidant Properties" Antioxidants 10, no. 5: 647. https://doi.org/10.3390/antiox10050647
APA StyleMphahlele, M. J., Agbo, E. N., More, G. K., & Gildenhuys, S. (2021). In Vitro Enzymatic and Kinetic Studies, and In Silico Drug-Receptor Interactions, and Drug-Like Profiling of the 5-Styrylbenzamide Derivatives as Potential Cholinesterase and β-Secretase Inhibitors with Antioxidant Properties. Antioxidants, 10(5), 647. https://doi.org/10.3390/antiox10050647







