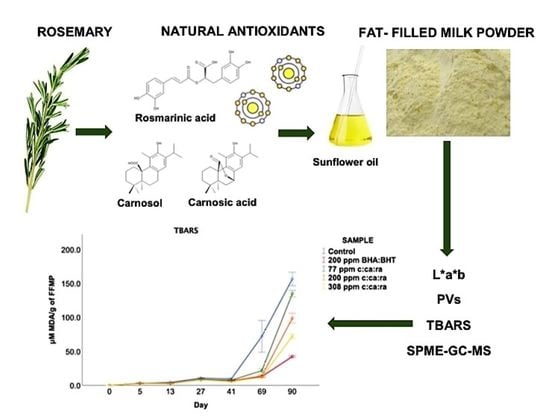
3.2. L*a*b Colour Determination of FFMPs before and after Accelerated Storage
Colour is an essential indicator of food quality and is critically important for consumer acceptance. The colour FFMPs (L*a*b) after accelerated storage were not highly different from that of samples tested after production and prior to storage (
Table 2). The maintenance of colour stability during storage could be due to the relatively low RH (23%) used during this study. Initiation of browning (Maillard) reactions takes place when water activity (aw) was above 0.2 (20% RH), whereas at 0.6 aw (RH 60%), the reaction rates reached their peak [
23]. Therefore, the rate of this type of reaction was considerably low at the RH used in this study (23%). At the same time, oxidation reactions were reduced as aw was decreased in a food product. However, when aw was below 0.4 (RH 40%), the reaction rates begin to increase again, and, therefore, at an RH of 23%, it can be substantially high [
23]. As it is illustrated in
Table 2, the L* values of the different FFMPs prior to accelerated storage were not significantly (
p > 0.05) different. The same trend (
p > 0.05) was also observed for their a* and b* values on the same sampling day. Indeed, L* values were generally stable throughout accelerated oxidation. However, statistically significant differences (
p < 0.05) were observed between the samples after 41 and 90 days of accelerated oxidation storage. After 41 days, a significantly lower (
p < 0.05) lightness (96.7) was observed for the samples supplemented with 308 ppm of c: ca: ra in comparison to the samples with 200 ppm of BHA: BHT (97.5). However, none of these samples was significantly (
p > 0.05) different from the control and the FFMPs with lower levels of phenolic compounds. After 90 days of storage, FFMPs with 200 and 308 ppm of BHA: BHT had significantly (
p < 0.05) lower L* values compared to BHA: BHT (97.4), which were equal to 96.5 and 96.6, respectively.
Furthermore, there was a statistically significant (
p < 0.05) effect of the different concentrations of c: ca: ra on the green to red colour coordinate a* values (5, 13, 27, 41 and 69 days of accelerated storage), as well as blue-to-yellow coordinate b* values (5, 13, 27, 41 and 90 days of accelerated storage) between the different FFMPs as shown in
Table 2. For example, whilst prior storage, both a* and b* values were not statistically (
p > 0.05) different, in all the evaluated FFMPs 69 days of storage, a* values of FFMPs with 308 ppm of c: ca: ra had a significantly (
p < 0.05) higher redness (−2.8) compared to those with 200 ppm of BHA: BHT (−2.7) as well as the control samples (−2.5). However, with respect to the FFMPs tested after 90 days of accelerated storage, b* values also exhibited significant effects among samples as a significantly higher yellow colour was observed in control samples (10.2) and those with 77 ppm of c: ca: ra (10.4) in comparison to the samples with 200 ppm of BHA: BHT (9.3). The latter, however, did not differ significantly (
p > 0.05) from those with 200 ppm (9.7) and 308 ppm (9.7) of c: ca: ra. In a previous study by Nielsen, Stapclteldt, and Skibsted [
24], whole milk powders stored under accelerated conditions at 50 °C and RH 31% for 49 days also turned progressively darker and more yellow pigmentation, with increasing storage time [
24]. The authors inferred that even if the rate of Maillard reactions, which form brown pigments throughout heat treatment, storage and exposure of milk powders to high temperatures [
21] were reduced with the use of a low RH, they may still have slightly contributed to the colour differences of the different FFMPs after accelerated storage, and particularly after 90 days.
3.3. PVs of FFMPs before and after Accelerated Storage
The measurement of PVs constitutes a common method for the determination of primary oxidation products such as peroxides (LOO) and hydroperoxides (LOOH) in the initial stages of lipid oxidation for assessing the oxidative rancidity of fats and oils [
25]. Cesa et al. [
26] showed that the use of 3: 1 of
n-hexane: PrOH, followed by iodometric titration yield the most representative results in the estimation of PVs in infant formulas when compared to other fat extraction protocols [
26]. Hence, this protocol was also employed in our study, and the subsequent iodometric titration finally yielded PVs in the same range as those previously reported [
11]. The influence of antioxidants during accelerated storage on PVs of FFMPs is shown in
Figure 1 and
Table A1 (
Appendix A). PVs were determined only for the samples after 69 and 90 days of accelerated storage, as both TBARS values and volatile oxidation compounds were negligible before, as shown in the subsequent sections. Therefore, as only these two sample sets exhibited substantial oxidation rates, they were the only powders considered satisfactory for highlighting the clear relationships between the different types of FFMPs.
Results indicated that PVs increased with storage time, exhibiting higher values for all the samples after 90 days of accelerated storage in comparison to 69 days. In addition, control samples had the highest PVs, after 69 and 90 days of accelerated storage. In the study of Zhang et al. [
11], where the oxidation rate of sunflower oil samples was assessed using PVs in the presence or absence of antioxidants at 60 °C for 21 days, control samples reached a maximum PV value equal to 272 meq/kg. Even though different conditions and methodology were applied in the present study, our control PVs were in the same range after 90 days of accelerated storage at 40 °C, with a PV equal to 285.4 meq/kg. On day 69, the PVs of control samples were 222.22 meq/kg, followed by the rest of the samples in the following descending order: 77 ppm of c: ca: ra (161.33 meq/kg) > 200 ppm of c: ca: ra (104.89 meq/kg) > 308 ppm of c: ca: ra (92.44 meq/kg) > 200 ppm BHA: BHT (88.00 meq/kg). Statistical analysis revealed that control samples did not differ significantly (
p > 0.05) from those containing 77 and 200 ppm of c: ca: ra. The lack of significance could be attributable to the fact that one of the three evaluated replicates in the case of control samples was highly skewing the data. However, this result is not surprising while assessing real-food samples and not controlled model systems. Particularly, in the case of control samples, where no antioxidants had been previously added, the oxidation rate on the surface of the FFMP can be substantially higher than that of the inner parts during storage. Hence, even if proper mixing was conducted prior to sampling, a higher level of oxidation was measured for this replicate, leading to this effect. However, a higher concentration of these phenolic compounds (308 ppm) seemed to effectively retard lipid deterioration of FFMPs as they exhibited a non-significant (
p > 0.05) difference from those containing 200 ppm of BHA and BHT.
After 90 days of accelerated storage, samples containing 200 ppm of BHA: BHT gave rise to the lowest values (158.8 meq/kg) compared to all the evaluated FFMPs, similarly to the samples received after 69 days. However, in that case, PV levels were significantly lower (p < 0.05) compared to 308 ppm of c: ca: ra, which exhibited the second-lowest PVs after 90 days of accelerated storage (219.9 meq/kg). Nonetheless, 308 ppm of c: ca: ra resulted in significantly (p < 0.05) lower PVs than control samples. On the other hand, 77 ppm (272.4 meq/kg) and 200 ppm of c: ca: ra (264 meq/kg) did not result in a significant (p > 0.05) difference in PVs in comparison to the control samples.
PVs followed similar trends to the results of HS-SPME-GC-MS analysis and TBARS assessment as presented in
Section 3.4 and
Section 3.5. However, as is apparent from examination of
Figure 1, even if the ranking of the different samples was similar, differences in PVs were not as apparent after 90 days of storage as those estimated after 69 days. This could be potentially attributed to the fact that most antioxidants remain effective during a certain period of time, whereas after that period, lower effectiveness was observed till the point that they entirely lose their efficacy [
27]. As it could be concluded, the effectiveness of the employed phenolic compounds (c: ca: ra) to inhibit lipid peroxidation, exhibited a concentration-related effect, with the highest concentration (308 ppm) resulting in the lowest PVs compared to the second-highest (200 ppm) and finally the lowest (77 ppm). A similar effect was observed in the previously stated study of Zheng et al. [
11], where the higher the concentration of carnosol was, the better the antioxidant potential in the sunflower oil samples [
11]. However, even if we received reproducible results, the classic iodometric method was not without weaknesses. Due to the fact that PVs determination is highly empirical, variations in the procedure may generally affect the results [
16].
3.4. TBARS of FFMPs before and after Accelerated Storage
TBARS were defined as the quantity of malondialdehyde (MDA) (in mg) present in 1 kg of sample and were used as indicators of secondary lipid oxidation [
11]. As illustrated in
Figure 2 and
Table A2 (
Appendix A), TBARS values for samples with added antioxidants were clearly different after 69 and 90 days of accelerated storage and indeed were substantially lower after 41 days as compared to untreated control samples. However, even if a number of significant differences were observed after 13 and 27 days in the different FFMPs, clear trends that enabled their detailed comparison were only observed in the highly oxidized samples after 69 and 90 days of accelerated storage. TBARS of the FFMPs were strongly correlated (
rs ≥ 0.650,
p < 0.01) with levels of the individual volatile compound, as shown in
Table A3 (
Appendix A).
Both TBARs and oxidatively derived volatiles have been routinely used to assess secondary oxidation in lipids, and, therefore, this correlation was not surprising and has been reported by other authors [
28]. After 69 days of accelerated storage, control FFMPs gave rise to the highest TBARS values equal to 72.14 μM MDA/g FFMP, followed by those with 77 ppm of c: ca: ra (21.76 μM MDA/g FFMP). FFMPs with 200 ppm of added c: ca: r resulted in the third-highest content of MDA, exhibiting a value of 13.95 μM MDA/g FFMP. Finally, the addition of 308 ppm c: ca: ra led to a lowest TBARS value that was equal to 12.16 μM MDA/g FFMP; however, not significantly (
p > 0.05) lower in comparison to that of 200 ppm of BHA: BHT (13.07 μM MDA/g FFMP). However, again as a result of the high degree of variability, only FFMPs supplemented either with 308 ppm c: ca: ra or 200 ppm BHA: BHT had significantly (
p < 0.05) lower values than the control FFMPs. After 90 days of accelerated storage, an apparent effect was observed, indicating that 200 ppm of BHA: BHT led to the lowest (42.42 μM MDA/g FFMP) oxidative deterioration of the samples. Samples with the highest concentration of phenolic compounds (308 ppm) had the second-lowest oxidation rate (72.27 μM MDA/g FFMP), followed by those with the same concentration as BHA: BHT (200 ppm) with values equal to 98.81 μM MDA/g FFMP. With respect to the samples with the lowest concentration (77 ppm) of phenolic compounds, higher TBARS values of 134.94 μM MDA/g of FFMP were observed. Control samples exhibited significantly higher (
p < 0.05) oxidation (156.55 μM MDA/g FFMP) compared to all the samples that were supplemented with natural or synthetic antioxidants, whereas their MDA concentration was more than two times higher in comparison to the control samples tested after 69 days of accelerated storage. However, except for reducing the shelf-life and nutritional value of food formulations, secondary oxidation products are toxic at high levels [
29].
The effect of rosemary and its major compounds on reducing the formation of MDA has been previously documented. Zhang et al. [
11] stated that carnosic acid was capable of reducing the formation of MDA in sunflower oil when used at a range of concentrations. In the same study, when the oxidation rate of sunflower oil samples was assessed with TBARS during the presence or absence of antioxidants at 60 °C for 21 days, control samples reached a maximum TBARS value equal to 216 μΜ MDA/g [
11]. Despite the fact the aforementioned authors’ employed different conditions and methodology than those applied in our study, our control TBARS were in the same range after 90 days of accelerated storage at 40 °C, with a previously stated TBARS value (156.55 μM MDA/g).
All the samples were also significantly (p < 0.05) different to each other after 90 days of accelerated storage. However, even if 308 ppm of c: ca: ra yielded significantly (p < 0.05) higher TBARS values than the samples containing 200 ppm of BHA and BHT, this concentration still resulted in a greater than 50% reduction in MDA compared to the control samples. ring with
Redondo-Cuevas et al. [
30] also concluded that potent antioxidant components present in rosemary, including carnosol, carnosic acid and rosmarinic acid were probably responsible for lower MDA formation in rapeseed oil stored after addition of varying concentrations of rosemary powder (0.25–2%
w/w) under accelerated conditions (120 °C for 1.5 and 3 h) as compared to the control samples. According to Senanayake [
31], synthetic phenolic based antioxidants, such as BHA and BHT, which are comprised of one aromatic one hydroxyl (OH) group, only have the capacity to donate one hydrogen (H
2). However, two OH groups were present in the aromatic rings (C11 and C12 positions) of both carnosol and carnosic acid. Hence, due to their structural superiority, these two compounds should have an intrinsically greater ability to scavenge free radicals and thus, retard lipid oxidation than BHA and BHT [
31].
3.5. Volatile Secondary Oxidation Products in FFMP’s
Odour is an essential index for assessing the quality of edible oils and products containing them [
32]. The primary UFA oxidation products are LOOH [
33], which arise from a reaction between an unsaturated lipid and O
2 and rapidly decompose into volatile and non-volatile constituents, including aldehydes (i.e., hexanal, hexenal, pentanal), ketones and hydrocarbons (i.e., pentane) that can negatively influence the aroma of a product [
33]. In addition, the presence of aldehydes can also result in toxicological effects [
29]. SPME is a rapid, sensitive, solventless, and cost-efficient method for the preparation of samples for GC analysis and is commonly applied for the determination of volatile compounds in fresh and oxidized oils [
32]. In this study, HS-SPME-GC-MS analysis was conducted to determine the levels of 13 volatile compounds, known to arise from the oxidation of UFA, namely 1-pentanol, 2-heptanone, 2-pentanone, 3-octen-2-one, 2,4-decadienal, hexanal, heptanal, octanal, pentanal, 2-nonenal (E), acetone, 2-nonanone and undecanal, before and after accelerated storage of the FFMPs.
The formation of the key volatile oxidation compounds in FFMPs was affected by the absence or presence of different antioxidants. As it was initially revealed after assessing the volatile composition among the different samples, acetone, 2-nonanone and undecanal were absent in all cases, irrespective of the sampling day. HS-SPME-GC-MS revealed clear differences between the results obtained before and after accelerated storage, as illustrated in
Figure 3a–j and
Table A4,
Table A5,
Table A6,
Table A7,
Table A8,
Table A9 and
Table A10. For all treatments, there was, as expected, a gradual increase in lipid oxidation derived in volatiles as the samples progressed through accelerated storage. The highest concentration compound measured in most batches was hexanal, which ranged from 730 to 92,143 μg/kg, and was approximately 2 to 10 times higher than any other quantified compound. This was expected given the fact that C18:2 is the most abundant UFA in sunflower oil, hexanal is the main volatile secondary oxidation product resulting from its oxidation [
2]. This effect was observed after all the sampling times (0 to 69 days), except after 90 days of accelerated storage, after which the samples had higher levels of pentanal, ranging from 19,117 to 101,202 μg/kg as opposed to a range of 21,672 to 92,143 μg/kg for hexanal.
Levels of pentanal, hexanal and heptanal are good indicators of lipid oxidation in FFMPs [
21], whereas hexanal has been reported as the major compound arising from lipid oxidation of milk powders [
34]. As it was previously stated, samples prior to and during accelerated storage till sampling day 41 had considerably lower concentrations of all the different volatile compounds when compared to the samples tested after 69 and 90 days of accelerated storage (40 °C, RH 23%). Furthermore, 4 out of 10 quantified compounds, namely 1-pentanol, 2-pentanone, 2,4-decadienal and 2-nonenal (E), were absent prior to 69 days of storage. Therefore, the main effects of the absence or presence of the different antioxidant compounds was most striking in the last two sampling days. As illustrated in
Figure 3i and
Table A9 (
Appendix A), pentanal content was 14,164 μg/kg for the control samples, followed by the samples containing 77 ppm c: ca: ra (4713 μg/kg), those with 200 ppm c: ca: ra (3616 μg/kg) and 308 ppm c: ca: ra (2646 μg/kg) and finally those with 200 ppm of BHA: BHT (1331 μg/kg). In all cases, levels of pentanal and hexanal were significantly (
p < 0.05) reduced in powders supplemented with antioxidants in comparison to the control samples. Indeed, after 90 days of accelerated storage (
Figure 3i),
Table A10 of
Appendix A), the highest pentanal concentration was again observed in the control samples and was equal to 101,202 μg/kg, equating to approximately 10 times higher levels compared to the control samples after 69 days of accelerated storage. Similarly, the lowest concentration was found in the samples containing 200 ppm of BHA: BHT (19,117 μg/kg). The second-lowest concentration (36,138 μg/kg) of pentanal was present in the samples containing the highest concentration of phenolic compounds (308 ppm), followed by those containing the same concentration (200 ppm) as BHA: BHT (62,673 μg/kg), and finally the samples containing a lower (77 ppm) concentration (84,638 μg/kg) of phenolics in comparison to BHA: BHT. However, the high variability of pentanal in the control samples after 90 days of accelerated storage led to a lack of statistically significant differences (
p > 0.05) after comparison with the supplemented with antioxidants samples. However, a clear trend was observed with respect to their efficiency and the potential of all the different antioxidant combinations in reducing pentanal levels. Particularly, 308 ppm of c: ca: ra could considerably reduce its concentration, even if this reduction was significantly (
p < 0.05) lower than that of 200 ppm BHA: BHT, which exerted a superior effect compared to all the different phenolic concentrations.
Hexanal is recognized as one of the main lipid oxidation indicators in many samples as it is usually present in high concentrations throughout storage [
28,
33]. Hexanal is formed via the oxidation of C18:2 by the 13-LOOH [
33]. According to Beliz et al. [
35] the autoxidation of 2,4-decadienal also leads to the formation of hexanal and additional volatile compounds. Pentanal, which also arises from the oxidation of C18:2 in this case from the decomposition of 14-LOOH was also present in substantial quantities in all the treatments [
35]. Oxidatively derived volatile compounds have been ascribed a range of odour descriptors and their potency is dependent on their odour threshold. For example, hexanal has been ascribed as an off-flavor of “grassy” [
33] and its odour thresholds vary significantly in different matrices [
35]. The odour threshold for hexanal in H
2O is equal to 4.5 μg/kg [
33], whereas a higher value of 12 μg/kg has been reported by Beliz et al. [
35]. On the other hand, the odour threshold of pentanal in H
2O has been reported to be 18 μg/kg [
35]. However, the odour threshold of various volatile compounds is greater in oil and fat in comparison to H
2O and air, probably as due to the matrix complexity and binding [
36]. The threshold of hexanal in oil is equal to 320 or 75 μg/kg depending on whether it is determined orthonasally or retronasally, and an odour described as “tallowy” or “green leafy” [
35]. The same authors have reported an odour threshold for pentanal in oil that is equal to 240 μg/kg (nasal) or 150 μg/kg (retronasal), described as “pungent” or “bitter almonds” [
35]. At the same time, odour thresholds of pentanal and hexanal in infant formulas have exhibited odour thresholds as low as 12 and 4.5 μg/kg, respectively [
37], which were substantially lower than the concentrations determined in this study (hexanal: 730 to 92,143 μg/kg, pentanal: 63 to 101,202 μg/kg).
Hexanal started to increase sharply after 69 days of accelerated storage and increased further after 90 days (
Figure 3h,
Table A9 and
Table A10). After 69 days, the maximum levels of the compound were observed for the control samples (24,737 μg/kg) and were significantly (
p < 0.05) higher than all the supplemented with natural and synthetic antioxidants FFMPs, in which lower rates of hexanal formation were observed. The potential of the added antioxidants in FFMPs to control the rate of lipid oxidation could be ranked in the following decreasing order: 77 ppm of c: ca: ra (9675 μg/kg) > 200 ppm of c: ca: ra (7666 μg/kg) > 308 ppm of c: ca: ra (5541 μg/kg) > 200 ppm of BHA: BHT (3306 μg/kg), whereas 308 ppm of c: ca: ra did not differ significantly (
p > 0.05) from the samples with 200 ppm of BHA: BHT. The highest hexanal concentration was observed for control samples (92,143 μg/kg) after 90 days of storage, followed by 77 ppm of c: ca: ra (84,077 μg/kg), 200 ppm of c: ca: ra (66,064 μg/kg), 308 ppm of c: ca: ra (39,213 μg/kg) and finally the lowest in samples with 200 ppm of BHA: BHT (21,672 μg/kg). However, again the high variability of the control samples after 90 days of accelerated storage led to a lack of significance (
p > 0.05) after comparison with the supplemented with antioxidants samples. Even though, the same apparent trend was observed concerning the effectiveness and potency of all the different antioxidant combinations to control hexanal formation. In addition, in that case, 308 ppm of c: ca: ra substantially reduced its formation, but to a lesser extent than 200 ppm of BHA: BHT (
p < 0.05). Olmedo et al. [
38] also observed that hexanal was present at very high levels of 10,000 to 80,000 µg/kg in sunflower oil samples that were supplemented with laurel, oregano, and rosemary EOs as natural antioxidants [
38]. Therefore, the high levels of hexanal in this study fall within the range reported by the former authors. As it could be further speculated, the high variability of pentanal and hexanal in the highly oxidized control samples could be potentially attributed to the presence of extremely high levels of these volatile compounds, which could have led to insufficient time for equilibration in the headspace, as well as saturation of the SPME fibre. In addition, in a number of cases (i.e., for hexanal after 5 days of accelerated storage), a higher standard deviation compared to the mean was observed (
Table A5 of
Appendix A). Other studies, as of Stewart et al. [
39] have found high variability in the levels of volatile compounds in milk and milk powder samples. This can again be attributable to the inherent variability of food systems that in parallel to the high sensitivity of GC-MS analysis, can lead to these effects.
The remaining eight volatile indicators also increased in all FFMPs during accelerated storage, following the same trends as discussed for hexanal and pentanal (
Figure 3a–j and
Table A4,
Table A5,
Table A6,
Table A7,
Table A8,
Table A9 and
Table A10). Even if these aldehydes, ketones, ketenes and alcohol compounds were present in lower concentrations compared to pentanal and hexanal, their presence at substantial levels is potentially significant and many of these, particularly those with one or more double bonds have much lower odour thresholds than their saturated equivalents. For instance, as a result of the presence of carbon-to-carbon double bonds, even relatively low concentrations 2,4-decadienal in a product can result in its unacceptability [
40], as the compounds has a low threshold of 2 μg/kg in skim milk and is related to a “fried fatty” and “painty odour” [
41]. 2-heptanone has been established as a good indicator of “grassy flavor”, while 3-octen-2-one and octanal have been established as accurate indicators of “painty flavor” in whole milk powders [
42]. The odour of heptanal is described as “oily” or “fishy” [
35], as well as “woody” [
37], whereas its threshold is 3200 μg/kg (nasal) or 50 μg/kg (retronasal) [
35]. In respect to 1-pentanol, a low threshold (0.47 mg/kg) has also been found in oil [
43]. Volatile compounds such as 3-octen-2-one and octanal that originate from C18:2 and C18:1 FAs, respectively, are not only formed due to the spontaneous decomposition of LOOH, but also due to the autoxidation throughout the production and storage of milk powders [
34].
At this point, it is important to highlight that the HS-SPME-GC-MS method in this study has been designed to accurately quantify the determined volatiles in a range from 0 to 3000 μg/kg, which is normally sufficient for most matrices with present oxidized lipids. However, samples stored for 41, 69 and 90 days exceeded this range. This was especially evident for hexanal and pentanal after 69 and 90 days of accelerated storage. Hence, a higher concentration of internal standard was utilized to quantify these samples. Even so, there is a possibility of overloading of the SPME fibre, and, therefore, an underestimation of the levels of aldehydes after 69 and 90 days of accelerated storage. However, the limited capacity of SPME fibres is a known weakness of this technique [
44].