Phenolic Compounds Extracted from Cherry Tree (Prunus avium) Branches: Impact of the Process on Cosmetic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Protocols
2.2.1. Conventional Extraction Process
2.2.2. Accelerated Solvent Extraction Process
2.3. Identification and Quantification of PCs by UHPLC-MS
2.3.1. Sample and Standard Preparation
2.3.2. LC-MS Conditions
2.3.3. Phenolic Compound Identification
2.4. Biological Assays
2.4.1. Antioxidant Activity
2.4.2. Anti-Tyrosinase Activities
2.4.3. Antimicrobial Activities
2.5. Statistical Analysis
3. Results
3.1. Phenolic Compound Identification and Quantification
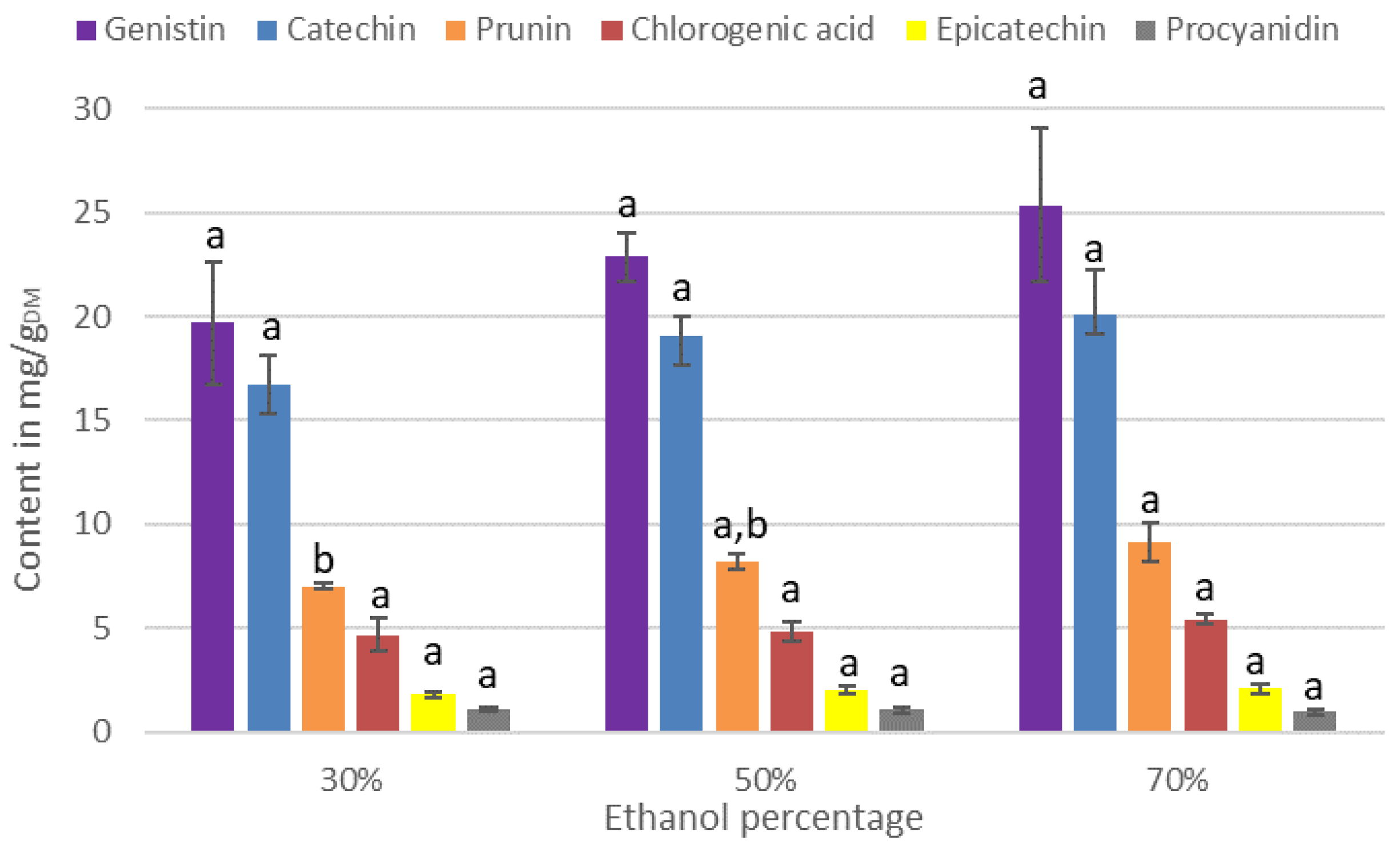
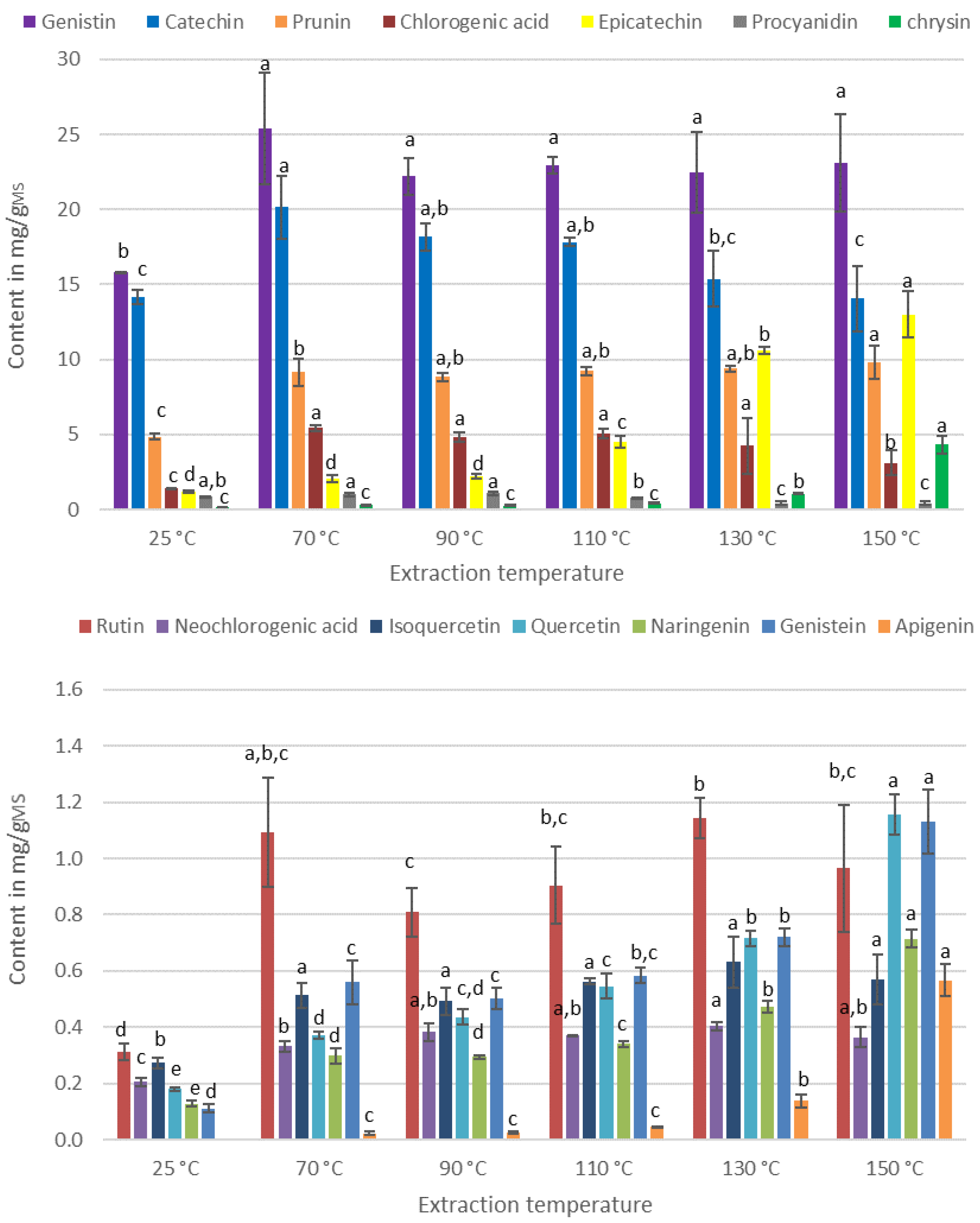
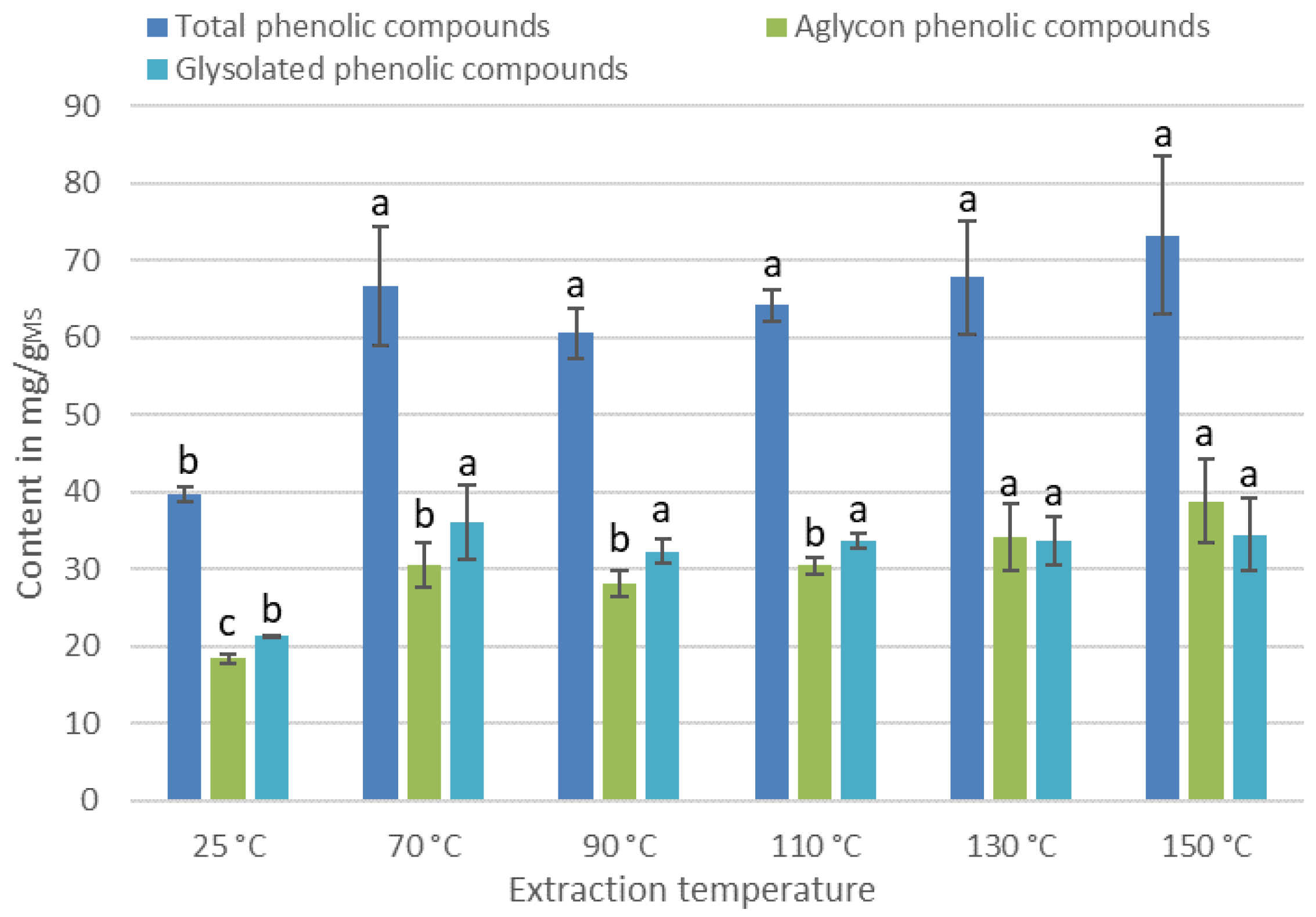
3.2. Effect of the Extraction Process on the IPC Content of the Extracts
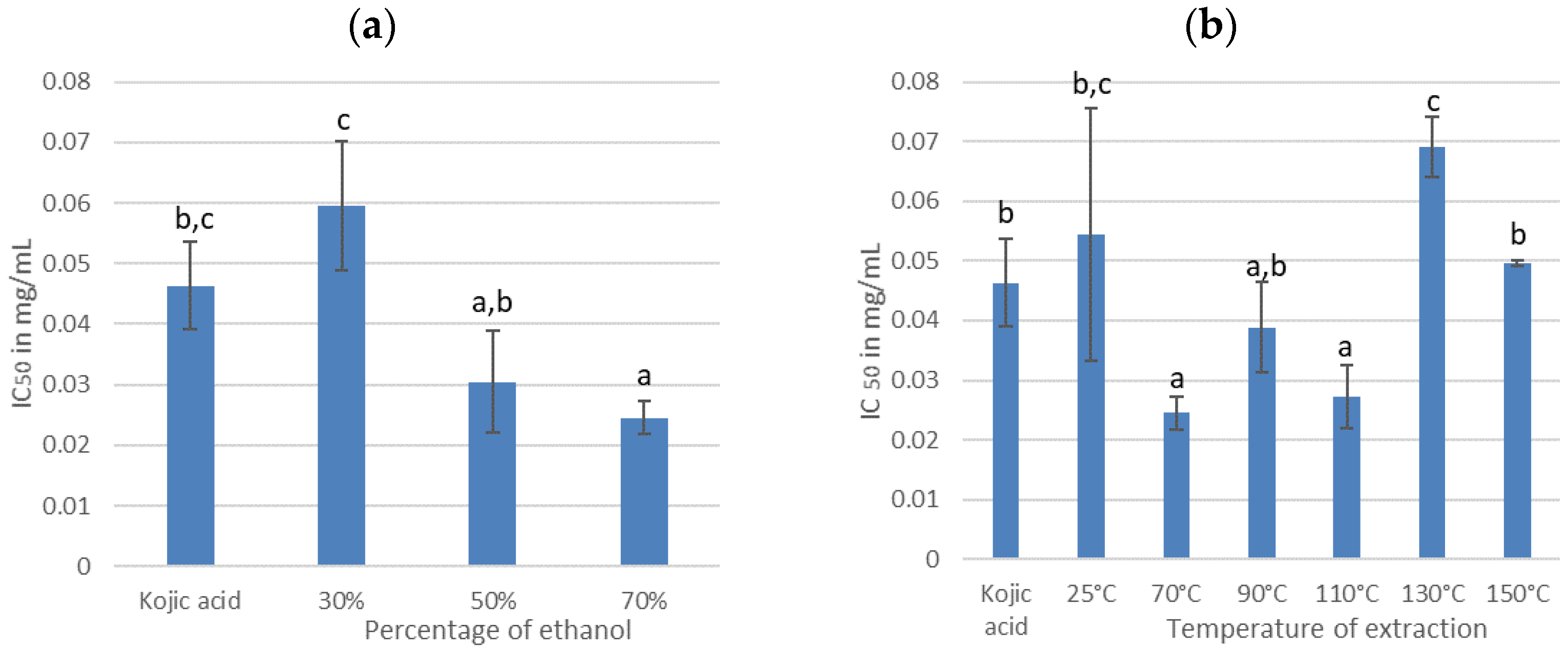
3.2.1. Effect of the Percentage of Ethanol
3.2.2. Effect of the Temperature of Extraction
3.3. Effect of the Process on Biological Activities of the Extracts
3.3.1. Antioxidant Activity Measured by DPPH
3.3.2. Anti-Tyrosinase Activity
3.3.3. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demir, T.; Akpınar, Ö.; Kara, H.; Güngör, H. Cherry stem phenolic compounds: Optimization of extraction conditions and in vitro evaluations of antioxidant, antimicrobial, antidiabetic, anti-inflammatory, and cytotoxic activities. J. Food Process. Preserv. 2020, 44, e14804. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, D. Sweet cherry phytochemicals: Identification and characterization by HPLC-DAD/ESI-MS in six sweet-cherry cultivars grown in Valle del Jerte (Spain). J. Food Compos. Anal. 2010, 23, 533–539. [Google Scholar] [CrossRef]
- Nascimento, L.B.D.; Gori, A.; Raffaelli, A.; Ferrini, F.; Brunetti, C. Phenolic Compounds from Leaves and Flowers of Hibiscus roseus: Potential Skin Cosmetic Applications of an Under-Investigated Species. Plants 2021, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.J.; Yang, L.; Bai, X.; Li, J.Y.; Yuan, M.Y.; Wang, Y.Q.; Xie, Y.; Hu, J.M.; Zhou, J. Phenolic Constituents with Antioxidative, Tyrosinase Inhibitory and Anti-aging Activities from Dendrobium loddigesii Rolfe. Nat. Prod. Bioprospect. 2019, 9, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Kolodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crops Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Asensio, E.; Vitales, D.; Perez, I.; Peralba, L.; Viruel, J.; Montaner, C.; Valles, J.; Garnatje, T.; Sales, E. Phenolic Compounds Content and Genetic Diversity at Population Level across the Natural Distribution Range of Bearberry (Arctostaphylos uva-ursi, Ericaceae) in the Iberian Peninsula. Plants 2020, 9, 1250. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhong, F.; Tian, R.; Zhang, K.; Zhang, X.; Li, T. Comparative Study of Phenolic Compounds and Antioxidant Activity in Different Species of Cherries. J. Food Sci. 2011, 76, C633–C638. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef]
- Yilmaz, F.M.; Gorguc, A.; Karaaslan, M.; Vardin, H.; Bilek, S.E.; Uygun, O.; Bircan, C. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3549–3563. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güçlü, S.F. Identification of polyphenols in homogenetic and heterogenetic combination of cherry graftings. Pak. J. Bot. 2019, 51, 2067–2072. [Google Scholar] [CrossRef]
- Nastić, N.; Lozano-Sánchez, J.; Borrás-Linares, I.; Švarc-Gajić, J.; Segura-Carretero, A. New technological approaches for recovering bioactive food constituents from sweet cherry (Prunus avium L.) stems. Phytochem. Anal. 2019, 31, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Svarc-Gajic, J.; Cerda, V.; Clavijo, S.; Suarez, R.; Maskovic, P.; Cvetanovic, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive compounds of sweet and sour cherry stems obtained by subcritical water extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Sun, R.; Yang, J.; Wang, L.; Zhao, P.; Chen, S. Characterization and Quantification of Phenolic Constituents in Peach Blossom by UPLC-LTQ-Orbitrap-MS and UPLC-DAD. Nat. Prod. Comm. 2020, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Masamoto, Y.; Ando, H.; Murata, Y.; Shimoishi, Y.; Tada, M.; Takahata, K. Mushroom Tyrosinase Inhibitory Activity of Esculetin Isolated from Seeds of Euphorbia lathyris L. Biosci. Biotechnol. Biochem. 2003, 67, 631–634. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Exploring the phenolic profile, antioxidant, antidiabetic and anti-hemolytic potential of Prunus avium vegetal parts. Food Res. Int. 2019, 116, 600–610. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Alves, G.; García-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical compositon of Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gerardin, C.; Paris, C.; Charbonnel, C.; Ghoul, M. Effect of heat processin on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Murakami, M.; Yamaguchi, T.; Takamura, H.; Matoba, T. Effects of thermal treatment on radical-scavenging activity of single and mixed polyphenolic compounds. Food Chem. Toxicol. 2004, 69, FCT7–FCT10. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-o-rhamnosylglucoside) in aqueous model. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Buchner, N.; Krumbein, A.; Rhon, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Hafsa, I.; Ghoul, M. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J. Food Eng. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroco, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant Activity Relationships of Flavonoids: A Re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, A.; Zhang, H.L.; Mizukami, H.; Okuyama, H.; Sakakibara, J.; Tokuda, H.; Nishino, H. Tyrosinase inhibitory and anti-tumor promoting activities of compounds isolated from safflower (Carthamus tinctorius L.) and cotton (Gossypium hirsutum L.) oil cakes. Nat. Prod. Res. 2000, 14, 153–158. [Google Scholar] [CrossRef]
- Dreno, B.; Zuberbier, T.; Gelmetti, C.; Gontijo, G.; Marinovich, M. Safety review of phenoxyethanol when used as a preservative in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Zgola-Grzeskowiak, A.; Werner, J.; Jeszka-Skowron, M.; Czarczynska-Golinska, B. Determination of parabens in cosmetic products using high performance liquid chromatography with fluorescence detection. Anal. Methods 2016, 19, 3903–3909. [Google Scholar] [CrossRef]
- Ali, R.M.; Houghton, P.J.; Ramarr, A.; Hoult, J.R.S. Antimicrobial and antiinflammatory activities of extracts and constituents of Oroxylum indicum (L.) Vent. Phytomedecine 1998, 5, 375–381. [Google Scholar]
- Fathima, A.; Rao, J.R. Selective toxicity of Catechin-a natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]

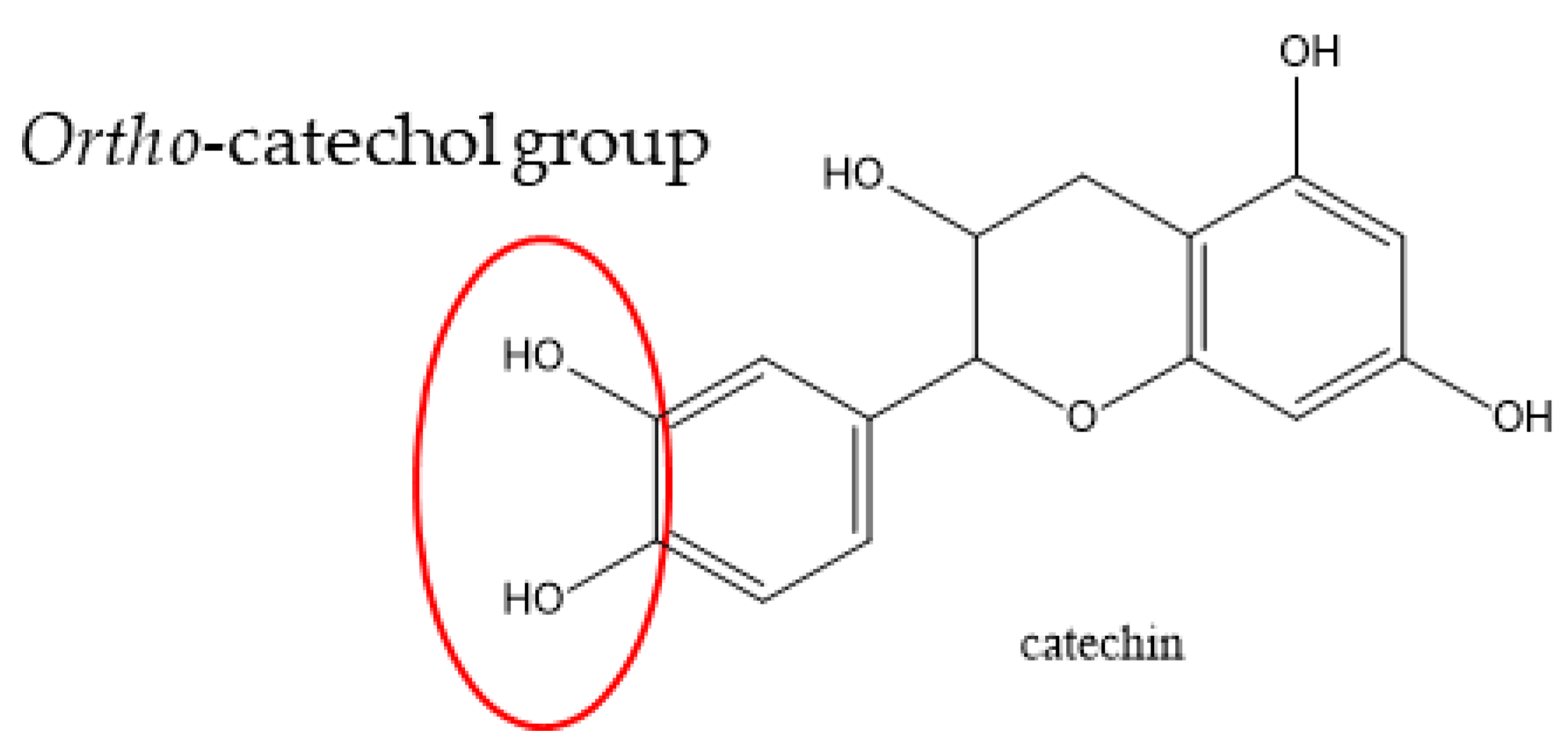
| PC | Retention Time (min) | UV Detection (nm) | Observed m/z (Da) | Content in mg/gDM |
|---|---|---|---|---|
| Neochlorogenic acid | 1.5 | 320 | 355.1021/163.0387 | 0.42 ± 0.02 |
| Catechin | 3.0 | 285 | 291.0867/139.0387 | 22.04 ± 0.53 |
| Chlorogenic acid | 3.3 | 320 | 355.1029/163.0390 | 3.81 ± 0.32 |
| Procyanidine B2 | 5.4 | 285 | 579.149 | 1.30 ± 0.05 |
| Epicatechin | 6.1 | 285 | 291.0867/139.0389 | 2.56 ± 0.05 |
| Rutin | 10.1 | 254 | 611.1616/303.0495/465.1026 | 1.19 ± 0.06 |
| Genistin | 10.2 | 254 | 433.1140/271.0599 | 27.22 ± 1.01 |
| Isoquercetin | 10.2 | 254 | 465.1035/303.0497 | 0.57 ± 0.02 |
| Prunin | 11.1 | 285 | 435.1283/273.0754/153.0182 | 10.87 ± 0.38 |
| Quercetin | 14.2 | 254 | 303.0509 | 0.41 ± 0.04 |
| Naringenin | 15.6 | 285 | 273.0756 | 0.33 ± 0.05 |
| Genistein | 15.8 | 254 | 271.0598 | 0.47 ± 0.03 |
| Apigenin | 16.3 | 320 | 271.0605 | 0.02 ± 0.01 |
| Chrysin | 20.4 | 254 | 255.0654 | 0.31 ± 0.01 |
| Factor Tested | Temperature (°C) | Ethanol Percentage | MIC | ||
|---|---|---|---|---|---|
| E. coli | C. albicans | B. subtilis | |||
| References | Ampicilline | 10 µg (+22 mm) | No inhibition | 10 µg (+22 mm) | |
| Nystatine | No inhibition | 100 UI (+26 mm) | No inhibition | ||
| Phenoxyethanol | 1.25% | No inhibition | 1.25% | ||
| Ethyl paraben | 1.25% | 1.25% | 1.25% | ||
| Variation of ethanol percentage | 70 °C | 30% | No inhibition | No inhibition | No inhibition |
| 70 °C | 50% | No inhibition | No inhibition | No inhibition | |
| 70 °C | 70% | No inhibition | No inhibition | No inhibition | |
| Variation of extraction temperature | 25 °C | 70% | No inhibition | No inhibition | 1.25% |
| 70 °C | 70% | No inhibition | No inhibition | No inhibition | |
| 90 °C | 70% | No inhibition | No inhibition | 1.25% | |
| 110 °C | 70% | 2.5% | No inhibition | No inhibition | |
| 130 °C | 70% | 1.25% | No inhibition | No inhibition | |
| 150 °C | 70% | 1.25% | 2.5% | No inhibition | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willig, G.; Brunissen, F.; Brunois, F.; Godon, B.; Magro, C.; Monteux, C.; Peyrot, C.; Ioannou, I. Phenolic Compounds Extracted from Cherry Tree (Prunus avium) Branches: Impact of the Process on Cosmetic Properties. Antioxidants 2022, 11, 813. https://doi.org/10.3390/antiox11050813
Willig G, Brunissen F, Brunois F, Godon B, Magro C, Monteux C, Peyrot C, Ioannou I. Phenolic Compounds Extracted from Cherry Tree (Prunus avium) Branches: Impact of the Process on Cosmetic Properties. Antioxidants. 2022; 11(5):813. https://doi.org/10.3390/antiox11050813
Chicago/Turabian StyleWillig, Gaëlle, Fanny Brunissen, Fanny Brunois, Blandine Godon, Christian Magro, Charles Monteux, Cédric Peyrot, and Irina Ioannou. 2022. "Phenolic Compounds Extracted from Cherry Tree (Prunus avium) Branches: Impact of the Process on Cosmetic Properties" Antioxidants 11, no. 5: 813. https://doi.org/10.3390/antiox11050813






