Honey and Alzheimer’s Disease—Current Understanding and Future Prospects
Abstract
1. Introduction
2. Pathophysiology and Clinical Picture of Alzheimer’s Disease
3. Honey and Its Powerful Ingredients—The Phenolic Compounds
4. Therapeutic Potential of Flavonoids and Phenolic Acids
5. Effects of Honey on Memory, Cognition, and Behavior
6. Honey on Dopaminergic Neurons—Important Players in Memory Deficits in AD
7. Honey as a Nootropic Agent—Prevention, Treatment, or Both?
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Silva Filho, S.R.B.; Barbosa, J.H.O.; Rondinoni, C.; dos Santos, A.C.; Salmon, C.E.G.; da Costa Lima, N.K.; Ferriolli, E.; Moriguti, J.C. Neuro-Degeneration Profile of Alzheimer’s Patients: A Brain Morphometry Study. NeuroImage Clin. 2017, 15, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Klein-Koerkamp, Y.; Rolf, A.H.; Kylee, T.R.; Moreaud, O.; Keignart, S.; Krainik, A.; Hammers, A.; Baciu, M.; Hot, P.; Alzheimer’s Disease Neuroimaging Initiative. Amygdalar Atrophy in Early Alzheimer’s Disease. Curr. Alzheimer Res. 2014, 11, 239–252. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- WHO. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 16 November 2022).
- Mega, M.S.; Cummings, J.L.; Fiorello, T.; Gornbein, J. The Spectrum of Behavioral Changes in Alzheimer’s Disease. Neurology 1996, 46, 130–135. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in Moderate-to-Severe Alzheimer’s Disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef]
- Eyjolfsdottir, H.; Eriksdotter, M.; Linderoth, B.; Lind, G.; Juliusson, B.; Kusk, P.; Almkvist, O.; Andreasen, N.; Blennow, K.; Ferreira, D.; et al. Targeted Delivery of Nerve Growth Factor to the Cholinergic Basal Forebrain of Alzheimer’s Disease Patients: Application of a Second-Generation Encapsulated Cell Biodelivery Device. Alzheimers Res. Ther. 2016, 8, 30. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for Dementia Due to Alzheimer’s Disease. Cochrane Database Syst. Rev. 2018, 2018, CD001190. [Google Scholar] [CrossRef]
- Kennedy, M.E.; Stamford, A.W.; Chen, X.; Cox, K.; Cumming, J.N.; Dockendorf, M.F.; Egan, M.; Ereshefsky, L.; Hodgson, R.A.; Hyde, L.A.; et al. The BACE1 Inhibitor Verubecestat (MK-8931) Reduces CNS β-Amyloid in Animal Models and in Alzheimer’s Disease Patients. Sci. Transl. Med. 2016, 8, 363ra150. [Google Scholar] [CrossRef]
- Novak, P.; Schmidt, R.; Kontsekova, E.; Zilka, N.; Kovacech, B.; Skrabana, R.; Vince-Kazmerova, Z.; Katina, S.; Fialova, L.; Prcina, M.; et al. Safety and Immunogenicity of the Tau Vaccine AADvac1 in Patients with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Neurol. 2017, 16, 123–134. [Google Scholar] [CrossRef]
- Yamada, K.; Tanaka, T.; Han, D.; Senzaki, K.; Kameyama, T.; Nabeshima, T. Protective Effects of Idebenone and α-Tocopherol on β-Amyloid-(1–42)-Induced Learning and Memory Deficits in Rats: Implication of Oxidative Stress in β-Amyloid-Induced Neurotoxicity in Vivo. Eur. J. Neurosci. 1999, 11, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, T.; Isse, K.; Akazawa, K.; Hamamoto, M.; Yaoi, Y.; Hagino, N. Evaluation of Estrogen Treatment in Female Patients with Dementia of the Alzheimer Type. Endocr. J. 1994, 41, 361–371. [Google Scholar] [CrossRef]
- Ohkura, T.; Isse, K.; Akazawa, K.; Hamamoto, M.; Yaoi, Y.; Hagino, N. Long-Term Estrogen Replacement Therapy in Female Patients with Dementia of the Alzheimer Type: 7 Case Reports. Dement. Geriatr. Cogn. Disord. 1995, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. Honey as the Potential Natural Source of Cholinesterase Inhibitors in Alzheimer’s Disease. Plant Foods Hum. Nutr. 2020, 75, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. Honey as an Antioxidant Therapy to Reduce Cognitive Ageing. Iran. J. Basic Med. Sci. 2019, 22, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.; Saim, A.B.; Idrus, R.B.H. Honey Ameliorate Negative Effects in Neurodegenerative Diseases: An Evidence-Based Review. Sains Malays. 2021, 50, 791–801. [Google Scholar] [CrossRef]
- Kuns, B.; Rosani, A.; Varghese, D. Memantine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rossom, R.; Adityanjee; Dysken, M. Efficacy and Tolerability of Memantine in the Treatment of Dementia. Am. J. Geriatr. Pharmacother. 2004, 2, 303–312. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Selkoe, D. Verubecestat for Prodromal Alzheimer’s Disease. N. Engl. J. Med. 2019, 381, 388–389. [Google Scholar] [CrossRef]
- Sperling, R.; Salloway, S.; Brooks, D.J.; Tampieri, D.; Barakos, J.; Fox, N.C.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; et al. Amyloid-Related Imaging Abnormalities in Patients with Alzheimer’s Disease Treated with Bapineuzumab: A Retrospective Analysis. Lancet Neurol. 2012, 11, 241–249. [Google Scholar] [CrossRef]
- Farina, N.; Llewellyn, D.; Isaac, M.G.E.K.N.; Tabet, N. Vitamin E for Alzheimer’s Dementia and Mild Cognitive Impairment. Cochrane Database Syst. Rev. 2017, 4, CD002854. [Google Scholar] [CrossRef]
- Farkas, S.; Szabó, A.; Hegyi, A.E.; Török, B.; Fazekas, C.L.; Ernszt, D.; Kovács, T.; Zelena, D. Estradiol and Estrogen-like Alternative Therapies in Use: The Importance of the Selective and Non-Classical Actions. Biomedicines 2022, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; Tarín, J.J.; Cano, A. The Adverse Effects of Estrogen and Selective Estrogen Receptor Modulators on Hemostasis and Thrombosis. Semin. Thromb. Hemost. 2012, 38, 797–807. [Google Scholar] [CrossRef]
- Bauer, L.; Kohlich, A.; Hirschwehr, R.; Siemann, U.; Ebner, H.; Scheiner, O.; Kraft, D.; Ebner, C. Food Allergy to Honey: Pollen or Bee Products?: Characterization of Allergenic Proteins in Honey by Means of Immunoblotting. J. Allergy Clin. Immunol. 1996, 97, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, M.; De Paulis, N.; Peveri, S.; Montagni, M.; Canani, R.B.; Biasucci, G. Anaphylaxis Caused by Artisanal Honey in a Child: A Case Report. J. Med. Case Reports 2021, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.; Duarte, F.C.; Mendes, A.; Bartolomé, B.; Barbosa, M.P. Anaphylaxis Caused by Honey: A Case Report. Asia Pac. Allergy 2017, 7, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Protective Effects of Tualang Honey against Oxidative Stress and Anxiety-Like Behaviour in Stressed Ovariectomized Rats. Int. Sch. Res. Not. 2014, 2014, e521065. [Google Scholar] [CrossRef]
- Sairazi, N.S.M.; Sirajudeen, K.N.S.; Asari, M.A.; Mummedy, S.; Muzaimi, M.; Sulaiman, S.A. Effect of Tualang Honey against KA-Induced Oxidative Stress and Neurodegeneration in the Cortex of Rats. BMC Complement. Altern. Med. 2017, 17, 31. [Google Scholar] [CrossRef]
- Shafin, N.; Othman, Z.; Zakaria, R.; Hussain, N.H.N. Tualang Honey Supplementation Reduces Blood Oxidative Stress Levels/Activities in Postmenopausal Women. Int. Sch. Res. Not. 2014, 2014, e364836. [Google Scholar] [CrossRef]
- Candiracci, M.; Piatti, E.; Dominguez-Barragán, M.; García-Antrás, D.; Morgado, B.; Ruano, D.; Gutiérrez, J.F.; Parrado, J.; Castaño, A. Anti-Inflammatory Activity of a Honey Flavonoid Extract on Lipopolysaccharide-Activated N13 Microglial Cells. J. Agric. Food Chem. 2012, 60, 12304–12311. [Google Scholar] [CrossRef]
- Sairazi, N.S.M.; Sirajudeen, K.N.S.; Muzaimi, M.; Mummedy, S.; Asari, M.A.; Sulaiman, S.A. Tualang Honey Reduced Neuroinflammation and Caspase-3 Activity in Rat Brain after Kainic Acid-Induced Status Epilepticus. Evid. Based Complement. Alternat. Med. 2018, 2018, e7287820. [Google Scholar] [CrossRef]
- Arshad, N.A.; Lin, T.S.; Yahaya, M.F. Stingless Bee Honey Reduces Anxiety and Improves Memory of the Metabolic Disease-Induced Rats. CNS Neurol. Disord.-Drug Targets-CNS Neurol. Disord. 2020, 19, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Phyu, H.P.; Al-Ani, I.M.; Talib, N.A. Potential Protective Effect of Honey against Chronic Cerebral Hypoperfusion-Induced Neurodegeneration in Rats. J. Anat. Soc. India 2014, 63, 151–155. [Google Scholar] [CrossRef]
- Zaidi, H.; Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Debbache, N.; Pacheco, R.; Serralheiro, M.L.; Araujo, M.E. Biological Properties of Phenolic Compound Extracts in Selected Algerian Honeys—The Inhibition of Acetylcholinesterase and α-Glucosidase Activities. Eur. J. Integr. Med. 2019, 25, 77–84. [Google Scholar] [CrossRef]
- Al-Himyari, F.A. P1-241: The Use of Honey as a Natural Preventive Therapy of Cognitive Decline and Dementia in the Middle East. Alzheimers Dement. 2009, 5, P247. [Google Scholar] [CrossRef]
- Shafin, N.; Zakaria, R.; Othman, Z.; Nik, N.H. Improved Blood Oxidative Status Is Not Associated with Better Memory Performance in Postmenopausal Women Receiving Tualang Honey Supplementation. J. Biochem. Pharmacol. Res. 2014, 2, 110–116. [Google Scholar]
- Behl, C. Alzheimer’s Disease and Oxidative Stress: Implications for Novel Therapeutic Approaches. Prog. Neurobiol. 1999, 57, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Schönheit, B.; Zarski, R.; Ohm, T.G. Spatial and Temporal Relationships between Plaques and Tangles in Alzheimer-Pathology. Neurobiol. Aging 2004, 25, 697–711. [Google Scholar] [CrossRef]
- Vossel, K.A.; Zhang, K.; Brodbeck, J.; Daub, A.C.; Sharma, P.; Finkbeiner, S.; Cui, B.; Mucke, L. Tau Reduction Prevents Aβ-Induced Defects in Axonal Transport. Science 2010, 330, 198. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Evolutional Aspects of Alzheimer’s Disease Pathogenesis. J. Alzheimers Dis. 2013, 33, S155–S161. [Google Scholar] [CrossRef]
- Ellmerich, S.; Taylor, G.W.; Richardson, C.D.; Minett, T.; Schmidt, A.F.; Brayne, C.; Matthews, F.E.; Ince, P.G.; Wharton, S.B.; Pepys, M.B.; et al. Dementia in the Older Population Is Associated with Neocortex Content of Serum Amyloid P Component. Brain Commun. 2021, 3, fcab225. [Google Scholar] [CrossRef]
- Crawford, J.R.; Bjorklund, N.L.; Taglialatela, G.; Gomer, R.H. Brain Serum Amyloid P Levels Are Reduced in Individuals That Lack Dementia While Having Alzheimer’s Disease Neuropathology. Neurochem. Res. 2012, 37, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, K.V.; Wegmann, S.; Kopeikina, K.J.; Hawkes, J.; Rudinskiy, N.; Andermann, M.L.; Spires-Jones, T.L.; Bacskai, B.J.; Hyman, B.T. Neurofibrillary Tangle-Bearing Neurons Are Functionally Integrated in Cortical Circuits in Vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Haroutunian, V.; Schnaider-Beeri, M.; Schmeidler, J.; Wysocki, M.; Purohit, D.P.; Perl, D.P.; Libow, L.S.; Lesser, G.T.; Maroukian, M.; Grossman, H.T. Role of the Neuropathology of Alzheimer Disease in Dementia in the Oldest-Old. Arch. Neurol. 2008, 65, 1211–1217. [Google Scholar] [CrossRef]
- Li, T.; Braunstein, K.E.; Zhang, J.; Lau, A.; Sibener, L.; Deeble, C.; Wong, P.C. The Neuritic Plaque Facilitates Pathological Conversion of Tau in an Alzheimer’s Disease Mouse Model. Nat. Commun. 2016, 7, 12082. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.A.; Liu, L.; Provenzano, F.A.; Berman, D.E.; Profaci, C.P.; Sloan, R.; Mayeux, R.; Duff, K.E.; Small, S.A. Molecular Drivers and Cortical Spread of Lateral Entorhinal Cortex Dysfunction in Preclinical Alzheimer’s Disease. Nat. Neurosci. 2014, 17, 304–311. [Google Scholar] [CrossRef]
- Nelson, P.T.; Abner, E.L.; Schmitt, F.A.; Kryscio, R.J.; Jicha, G.A.; Santacruz, K.; Smith, C.D.; Patel, E.; Markesbery, W.R. Brains With Medial Temporal Lobe Neurofibrillary Tangles But No Neuritic Amyloid Plaques Are a Diagnostic Dilemma But May Have Pathogenetic Aspects Distinct From Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2009, 68, 774–784. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.; Wang, J.-Z.; Liu, R.; Wang, X. Tau Ubiquitination in Alzheimer’s Disease. Front. Neurol. 2022, 12, 786353. [Google Scholar] [CrossRef]
- Rodríguez-Martín, T.; Cuchillo-Ibáñez, I.; Noble, W.; Nyenya, F.; Anderton, B.H.; Hanger, D.P. Tau Phosphorylation Affects Its Axonal Transport and Degradation. Neurobiol. Aging 2013, 34, 2146–2157. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.-M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.-Y. Synapse Loss and Microglial Activation Precede Tangles in a P301S Tauopathy Mouse Model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Arnaud, L.; Robakis, N.K.; Figueiredo-Pereira, M.E. It May Take Inflammation, Phosphorylation and Ubiquitination to ‘Tangle’ in Alzheimer’s Disease. Neurodegener. Dis. 2006, 3, 313–319. [Google Scholar] [CrossRef]
- Lennol, M.P.; Sánchez-Domínguez, I.; Cuchillo-Ibañez, I.; Camporesi, E.; Brinkmalm, G.; Alcolea, D.; Fortea, J.; Lleó, A.; Soria, G.; Aguado, F.; et al. Apolipoprotein E Imbalance in the Cerebrospinal Fluid of Alzheimer’s Disease Patients. Alzheimers Res. Ther. 2022, 14, 161. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.; George-Hyslop, P.H.S.; Pericak-Vance, M.A.; Joo, S.H.; Rosi, B.L.; Gusella, J.F.; Crapper-MacLachlan, D.R.; Alberts, M.J.; et al. Association of Apolipoprotein E Allele Ε4 with Late-onset Familial and Sporadic Alzheimer’s Disease. Neurology 1993, 43, 1467–1472. [Google Scholar] [CrossRef]
- Miyata, M.; Smith, J.D. Apolipoprotein E Allele–Specific Antioxidant Activity and Effects on Cytotoxicity by Oxidative Insults and β–Amyloid Peptides. Nat. Genet. 1996, 14, 55–61. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Mattson, M.P. Apolipoprotein E and Oxidative Stress in Brain with Relevance to Alzheimer’s Disease. Neurobiol. Dis. 2020, 138, 104795. [Google Scholar] [CrossRef]
- Gralle, M.; Ferreira, S.T. Structure and Functions of the Human Amyloid Precursor Protein: The Whole Is More than the Sum of Its Parts. Prog. Neurobiol. 2007, 82, 11–32. [Google Scholar] [CrossRef]
- Rovelet-Lecrux, A.; Hannequin, D.; Raux, G.; Meur, N.L.; Laquerrière, A.; Vital, A.; Dumanchin, C.; Feuillette, S.; Brice, A.; Vercelletto, M.; et al. APP Locus Duplication Causes Autosomal Dominant Early-Onset Alzheimer Disease with Cerebral Amyloid Angiopathy. Nat. Genet. 2006, 38, 24–26. [Google Scholar] [CrossRef]
- Sleegers, K.; Brouwers, N.; Gijselinck, I.; Theuns, J.; Goossens, D.; Wauters, J.; Del-Favero, J.; Cruts, M.; van Duijn, C.M.; Broeckhoven, C.V. APP Duplication Is Sufficient to Cause Early Onset Alzheimer’s Dementia with Cerebral Amyloid Angiopathy. Brain 2006, 129, 2977–2983. [Google Scholar] [CrossRef]
- Wiseman, F.K.; Al-Janabi, T.; Hardy, J.; Karmiloff-Smith, A.; Nizetic, D.; Tybulewicz, V.L.J.; Fisher, E.M.C.; Strydom, A. A Genetic Cause of Alzheimer Disease: Mechanistic Insights from Down Syndrome. Nat. Rev. Neurosci. 2015, 16, 564–574. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yasvoina, M.; Shao, P.; Hitt, B.; O’Connor, T.; Logan, S.; Maus, E.; Citron, M.; Berry, R.; et al. β-Site Amyloid Precursor Protein Cleaving Enzyme 1 Levels Become Elevated in Neurons around Amyloid Plaques: Implications for Alzheimer’s Disease Pathogenesis. J. Neurosci. 2007, 27, 3639–3649. [Google Scholar] [CrossRef]
- Cai, Y.; An, S.S.A.; Kim, S. Mutations in Presenilin 2 and Its Implications in Alzheimer’s Disease and Other Dementia-Associated Disorders. Clin. Interv. Aging 2015, 10, 1163–1172. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Wang, M.; Jing, T.; Wang, X.; Yao, D. Beta-Secretase/BACE1 Promotes APP Endocytosis and Processing in the Endosomes and on Cell Membrane. Neurosci. Lett. 2018, 685, 63–67. [Google Scholar] [CrossRef]
- Siegel, G.; Gerber, H.; Koch, P.; Bruestle, O.; Fraering, P.C.; Rajendran, L. The Alzheimer’s Disease γ-Secretase Generates Higher 42:40 Ratios for β-Amyloid Than for P3 Peptides. Cell Rep. 2017, 19, 1967–1976. [Google Scholar] [CrossRef]
- Tyler, S.J.; Dawbarn, D.; Wilcock, G.K.; Allen, S.J. α- and β-Secretase: Profound Changes in Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2002, 299, 373–376. [Google Scholar] [CrossRef]
- Scahill, R.I.; Schott, J.M.; Stevens, J.M.; Rossor, M.N.; Fox, N.C. Mapping the Evolution of Regional Atrophy in Alzheimer’s Disease: Unbiased Analysis of Fluid-Registered Serial MRI. Proc. Natl. Acad. Sci. USA 2002, 99, 4703–4707. [Google Scholar] [CrossRef]
- Chetelat, G.A.; Baron, J.-C. Early Diagnosis of Alzheimer’s Disease: Contribution of Structural Neuroimaging. NeuroImage 2003, 18, 525–541. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal Synaptic Loss in Early Alzheimer’s Disease and Mild Cognitive Impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar] [CrossRef]
- Bell, K.F.S.; Ducatenzeiler, A.; Ribeiro-da-Silva, A.; Duff, K.; Bennett, D.A.; Cuello, A.C. The Amyloid Pathology Progresses in a Neurotransmitter-Specific Manner. Neurobiol. Aging 2006, 27, 1644–1657. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Lowe, V.J.; Weigand, S.D.; Wiste, H.J.; Senjem, M.L.; Knopman, D.S.; Shiung, M.M.; Gunter, J.L.; Boeve, B.F.; Kemp, B.J.; et al. Serial PIB and MRI in Normal, Mild Cognitive Impairment and Alzheimer’s Disease: Implications for Sequence of Pathological Events in Alzheimer’s Disease. Brain 2009, 132, 1355–1365. [Google Scholar] [CrossRef]
- Fitzjohn, S.M.; Morton, R.A.; Kuenzi, F.; Rosahl, T.W.; Shearman, M.; Lewis, H.; Smith, D.; Reynolds, D.S.; Davies, C.H.; Collingridge, G.L.; et al. Age-Related Impairment of Synaptic Transmission But Normal Long-Term Potentiation in Transgenic Mice That Overexpress the Human APP695SWE Mutant Form of Amyloid Precursor Protein. J. Neurosci. 2001, 21, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.S.; Wu, C.-C.; Redwine, J.M.; Comery, T.A.; Arias, R.; Bowlby, M.; Martone, R.; Morrison, J.H.; Pangalos, M.N.; Reinhart, P.H.; et al. Early-Onset Behavioral and Synaptic Deficits in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5161–5166. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Akaike, T.; Sokabe, M.; Nitta, A.; Iida, R.; Olariu, A.; Yamada, K.; Nabeshima, T. Impairments of Long-Term Potentiation in Hippocampal Slices of β-Amyloid-Infused Rats. Eur. J. Pharmacol. 1999, 382, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; An, K.; Kwon, O.B.; Kim, H.; Kim, J.-H. Pathway-Specific Alteration of Synaptic Plasticity in Tg2576 Mice. Mol. Cells 2011, 32, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Witton, J.; Brown, J.T.; Jones, M.W.; Randall, A.D. Altered Synaptic Plasticity in the Mossy Fibre Pathway of Transgenic Mice Expressing Mutant Amyloid Precursor Protein. Mol. Brain 2010, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Scheff, S.W. Oxidative Stress in the Progression of Alzheimer Disease in the Frontal Cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.T.; Perluigi, M.; De Marco, C.; Coccia, R.; Keller, J.N.; Markesbery, W.R.; Sultana, R. Elevated Levels of 3-Nitrotyrosine in Brain from Subjects with Amnestic Mild Cognitive Impairment: Implications for the Role of Nitration in the Progression of Alzheimer’s Disease. Brain Res. 2007, 1148, 243–248. [Google Scholar] [CrossRef]
- Bradley-Whitman, M.A.; Lovell, M.A. Biomarkers of Lipid Peroxidation in Alzheimer Disease (AD): An Update. Arch. Toxicol. 2015, 89, 1035–1044. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Lyra e Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; de Paula França Resende, E.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-Inflammatory Interleukin-6 Signaling Links Cognitive Impairments and Peripheral Metabolic Alterations in Alzheimer’s Disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Granic, I.; Dolga, A.M.; Nijholt, I.M.; van Dijk, G.; Eisel, U.L.M. Inflammation and NF-ΚB in Alzheimer’s Disease and Diabetes. J. Alzheimers Dis. 2009, 16, 809–821. [Google Scholar] [CrossRef]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Su, F.; Bai, F.; Zhang, Z. Inflammatory Cytokines and Alzheimer’s Disease: A Review from the Perspective of Genetic Polymorphisms. Neurosci. Bull. 2016, 32, 469–480. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine To Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef]
- Wang, P.; Wu, P.; Siegel, M.I.; Egan, R.W.; Billah, M.M. Interleukin (IL)-10 Inhibits Nuclear Factor KB (NFĸB) Activation in Human Monocytes: IL-10 AND IL-4 SUPPRESS CYTOKINE SYNTHESIS BY DIFFERENT MECHANISMS (∗). J. Biol. Chem. 1995, 270, 9558–9563. [Google Scholar] [CrossRef]
- Friedberg, J.S.; Aytan, N.; Cherry, J.D.; Xia, W.; Standring, O.J.; Alvarez, V.E.; Nicks, R.; Svirsky, S.; Meng, G.; Jun, G.; et al. Associations between Brain Inflammatory Profiles and Human Neuropathology Are Altered Based on Apolipoprotein E Ε4 Genotype. Sci. Rep. 2020, 10, 2924. [Google Scholar] [CrossRef]
- Tai, L.M.; Ghura, S.; Koster, K.P.; Liakaite, V.; Maienschein-Cline, M.; Kanabar, P.; Collins, N.; Ben-Aissa, M.; Lei, A.Z.; Bahroos, N.; et al. APOE-Modulated Aβ-Induced Neuroinflammation in Alzheimer’s Disease: Current Landscape, Novel Data, and Future Perspective. J. Neurochem. 2015, 133, 465–488. [Google Scholar] [CrossRef]
- Jessen, F.; Feyen, L.; Freymann, K.; Tepest, R.; Maier, W.; Heun, R.; Schild, H.-H.; Scheef, L. Volume Reduction of the Entorhinal Cortex in Subjective Memory Impairment. Neurobiol. Aging 2006, 27, 1751–1756. [Google Scholar] [CrossRef]
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical Values of Natural Honey and Its Contribution to Human Health and Wealth. Nutr. Metab. 2012, 9, 61. [Google Scholar] [CrossRef]
- Kreider, R.B.; Rasmussen, C.J.; Lancaster, S.L.; Kerksick, C.; Greenwood, M. Honey: An Alternative Sports Gel. Strength Cond. J. 2002, 24, 50–51. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical Composition of Honey. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 43–82. ISBN 978-3-319-59689-1. [Google Scholar]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic Acids and Flavonoids Profiles of Commercial Honey from Different Floral Sources and Geographic Sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Sousa, J.M.; de Souza, E.L.; Marques, G.; Meireles, B.; de Magalhães Cordeiro, Â.T.; Gullón, B.; Pintado, M.M.; Magnani, M. Polyphenolic Profile and Antioxidant and Antibacterial Activities of Monofloral Honeys Produced by Meliponini in the Brazilian Semiarid Region. Food Res. Int. 2016, 84, 61–68. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A.M.; Hamid, H.A.; Khazaai, H. Malaysian Stingless Bee and Tualang Honeys: A Comparative Characterization of Total Antioxidant Capacity and Phenolic Profile Using Liquid Chromatography-Mass Spectrometry. LWT 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis Spp. and Trigona Spp. Bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Malaysian Honeys Produced by Apis Cerana, Apis Dorsata and Apis Mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- Kishore, R.K.; Halim, A.S.; Syazana, M.S.N.; Sirajudeen, K.N.S. Tualang Honey Has Higher Phenolic Content and Greater Radical Scavenging Activity Compared with Other Honey Sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and Its Neuroprotective Effects on Brain Ischemia and Neurodegenerative Diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef]
- Mohd Kamal, D.A.; Ibrahim, S.F.; Kamal, H.; Kashim, M.I.A.M.; Mokhtar, M.H. Physicochemical and Medicinal Properties of Tualang, Gelam and Kelulut Honeys: A Comprehensive Review. Nutrients 2021, 13, 197. [Google Scholar] [CrossRef]
- Floyd, R.A. Neuroinflammatory Processes Are Important in Neurodegenerative Diseases: An Hypothesis to Explain the Increased Formation of Reactive Oxygen and Nitrogen Species as Major Factors Involved in Neurodegenerative Disease Development. Free Radic. Biol. Med. 1999, 26, 1346–1355. [Google Scholar] [CrossRef]
- Calabrese, V.; Bates, T.E.; Stella, A.M.G. NO Synthase and NO-Dependent Signal Pathways in Brain Aging and Neurodegenerative Disorders: The Role of Oxidant/Antioxidant Balance. Neurochem. Res. 2000, 25, 1315–1341. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Myricetin as a Promising Molecule for the Treatment of Post-Ischemic Brain Neurodegeneration. Nutrients 2021, 13, 342. [Google Scholar] [CrossRef]
- Ramezani, M.; Darbandi, N.; Khodagholi, F.; Hashemi, A. Myricetin Protects Hippocampal CA3 Pyramidal Neurons and Improves Learning and Memory Impairments in Rats with Alzheimer’s Disease. Neural Regen. Res. 2016, 11, 1976–1980. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin Ameliorates Scopolamine-Induced Memory Impairment in Mice via Inhibiting Acetylcholinesterase and down-Regulating Brain Iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Multifunction of Myricetin on Aβ: Neuroprotection via a Conformational Change of Aβ and Reduction of Aβ via the Interference of Secretases. J. Neurosci. Res. 2008, 86, 368–377. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Cheng, H.; Che, Z. Ameliorating Effect of Luteolin on Memory Impairment in an Alzheimer’s Disease Model. Mol. Med. Rep. 2016, 13, 4215–4220. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, J.; Guo, L.; Xu, Y.; Sun, L.; Wang, S.; Feng, Y.; Gou, L.; Zhang, L.; Liu, Y. Protective Role of Luteolin against Cognitive Dysfunction Induced by Chronic Cerebral Hypoperfusion in Rats. Pharmacol. Biochem. Behav. 2014, 126, 122–130. [Google Scholar] [CrossRef]
- Jang, S.; Dilger, R.N.; Johnson, R.W. Luteolin Inhibits Microglia and Alters Hippocampal-Dependent Spatial Working Memory in Aged Mice. J. Nutr. 2010, 140, 1892–1898. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, L.; Wang, Q.; Gao, Y. Naringenin Alleviates Cognition Deficits in High-Fat Diet-Fed SAMP8 Mice. J. Food Biochem. 2020, 44, e13375. [Google Scholar] [CrossRef]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Siddiqui, R.A.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin Protects AlCl3/D-Galactose Induced Neurotoxicity in Rat Model of AD via Attenuation of Acetylcholinesterase Levels and Inhibition of Oxidative Stress. PLOS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin Improves Learning and Memory in an Alzheimer’s Disease Rat Model: Insights into the Underlying Mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Khan, M.M.; Khan, A.; Ahmed, E.; Ishrat, T.; Tabassum, R.; Vaibhav, K.; Ahmad, A.; Islam, F. Naringenin Ameliorates Alzheimer’s Disease (AD)-Type Neurodegeneration with Cognitive Impairment (AD-TNDCI) Caused by the Intracerebroventricular-Streptozotocin in Rat Model. Neurochem. Int. 2012, 61, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Fu, M.; Wang, S.; Chen, W.; Wang, J.; Zhang, N. Naringin Ameliorates Memory Deficits and Exerts Neuroprotective Effects in a Mouse Model of Alzheimer’s Disease by Regulating Multiple Metabolic Pathways. Mol. Med. Rep. 2021, 23, 332. [Google Scholar] [CrossRef]
- Tongjaroenbuangam, W.; Ruksee, N.; Chantiratikul, P.; Pakdeenarong, N.; Kongbuntad, W.; Govitrapong, P. Neuroprotective Effects of Quercetin, Rutin and Okra (Abelmoschus Esculentus Linn.) in Dexamethasone-Treated Mice. Neurochem. Int. 2011, 59, 677–685. [Google Scholar] [CrossRef]
- Ashrafpour, M.; Parsaei, S.; Sepehri, H. Quercetin Improved Spatial Memory Dysfunctions in Rat Model of Intracerebroventricular Streptozotocin-Induced Sporadic Alzheimer’sdisease. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 411–415. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The Flavonoid Quercetin Ameliorates Alzheimer’s Disease Pathology and Protects Cognitive and Emotional Function in Aged Triple Transgenic Alzheimer’s Disease Model Mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Tota, S.; Awasthi, H.; Kamat, P.K.; Nath, C.; Hanif, K. Protective Effect of Quercetin against Intracerebral Streptozotocin Induced Reduction in Cerebral Blood Flow and Impairment of Memory in Mice. Behav. Brain Res. 2010, 209, 73–79. [Google Scholar] [CrossRef]
- Choi, G.N.; Kim, J.H.; Kwak, J.H.; Jeong, C.-H.; Jeong, H.R.; Lee, U.; Heo, H.J. Effect of Quercetin on Learning and Memory Performance in ICR Mice under Neurotoxic Trimethyltin Exposure. Food Chem. 2012, 132, 1019–1024. [Google Scholar] [CrossRef]
- Wang, D.-M.; Li, S.-Q.; Wu, W.-L.; Zhu, X.-Y.; Wang, Y.; Yuan, H.-Y. Effects of Long-Term Treatment with Quercetin on Cognition and Mitochondrial Function in a Mouse Model of Alzheimer’s Disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Beg, T.; Jyoti, S.; Naz, F.; Rahul; Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord.-Drug Targets-CNS Neurol. Disord. 2018, 17, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol Attenuates Cognitive Deficit via Regulating Oxidative Stress and Neuroinflammation in an Ovariectomized Rat Model of Sporadic Dementia. Neural Regen. Res. 2018, 13, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P.; Eyvani, K.; Kouhestani, S. Sex-Independent Cognition Improvement in Response to Kaempferol in the Model of Sporadic Alzheimer’s Disease. Neurochem. Res. 2021, 46, 1480–1486. [Google Scholar] [CrossRef]
- Deshmukh, R.; Kaundal, M.; Bansal, V. Samardeep Caffeic Acid Attenuates Oxidative Stress, Learning and Memory Deficit in Intra-Cerebroventricular Streptozotocin Induced Experimental Dementia in Rats. Biomed. Pharmacother. 2016, 81, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Kumar, N.; Nayak, P.G.; Nampoothiri, M.; Shenoy, R.R.; Krishnadas, N.; Rao, C.M.; Mudgal, J. Impact of Caffeic Acid on Aluminium Chloride-Induced Dementia in Rats. J. Pharm. Pharmacol. 2013, 65, 1745–1752. [Google Scholar] [CrossRef]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective Effect of Caffeic Acid against Alzheimer’s Disease Pathogenesis via Modulating Cerebral Insulin Signaling, β-Amyloid Accumulation, and Synaptic Plasticity in Hyperinsulinemic Rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Kadar, N.N.M.A.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Comparable Benefits of Stingless Bee Honey and Caffeic Acid in Mitigating the Negative Effects of Metabolic Syndrome on the Brain. Antioxidants 2022, 11, 2154. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, J.; Hua, L.; Han, B.; Zhang, Y.; Yang, X.; Zeng, Z.; Bai, H.; Yin, H.; et al. Effects of Caffeic Acid on Learning Deficits in a Model of Alzheimer’s Disease. Int. J. Mol. Med. 2016, 38, 869–875. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some Pro-Oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Saenno, R.; Dornlakorn, O.; Anosri, T.; Kaewngam, S.; Sirichoat, A.; Aranarochana, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats Induced by D-Galactose. Nutrients 2022, 14, 2169. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Aβ25-35-Induced Autophagy and Cognitive Impairment via the MTOR/TFEB Signaling Pathway. Drug Des. Devel. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic Acid Inhibits LPS-Induced Microglial Activation and Improves Survival of Dopaminergic Neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y.; et al. Neuroprotective Effects of Chlorogenic Acid on Scopolamine-Induced Amnesia via Anti-Acetylcholinesterase and Anti-Oxidative Activities in Mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kiasalari, Z.; Heydarifard, R.; Khalili, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Zahedi, E.; Sanaierad, A.; Roghani, M. Ellagic Acid Ameliorates Learning and Memory Deficits in a Rat Model of Alzheimer’s Disease: An Exploration of Underlying Mechanisms. Psychopharmacology 2017, 234, 1841–1852. [Google Scholar] [CrossRef]
- Harakeh, S.; Qari, M.H.; Ramadan, W.S.; Al Jaouni, S.K.; Almuhayawi, M.S.; Al Amri, T.; Ashraf, G.M.; Bharali, D.J.; Mousa, S.A. A Novel Nanoformulation of Ellagic Acid Is Promising in Restoring Oxidative Homeostasis in Rat Brains with Alzheimer’s Disease. Curr. Drug Metab. 2021, 22, 299–307. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, H.; Zhang, W.; Liu, X.; Jiang, B.; Fei, H.; Sun, Z. Ellagic Acid Ameliorates Learning and Memory Impairment in APP/PS1 Transgenic Mice via Inhibition of Β-amyloid Production and Tau Hyperphosphorylation. Exp. Ther. Med. 2018, 16, 4951–4958. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic Acid Is a Dual α/β-Secretase Modulator That Reverses Cognitive Impairment and Remediates Pathology in Alzheimer Mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Ramadan, W.S.; Alkarim, S. Ellagic Acid Modulates the Amyloid Precursor Protein Gene via Superoxide Dismutase Regulation in the Entorhinal Cortex in an Experimental Alzheimer’s Model. Cells 2021, 10, 3511. [Google Scholar] [CrossRef]
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic Acid: Insights into Its Neuroprotective and Cognitive Enhancement Effects in Sporadic Alzheimer’s Disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46. [Google Scholar] [CrossRef]
- Wang, N.-Y.; Li, J.-N.; Liu, W.-L.; Huang, Q.; Li, W.-X.; Tan, Y.-H.; Liu, F.; Song, Z.-H.; Wang, M.-Y.; Xie, N.; et al. Ferulic Acid Ameliorates Alzheimer’s Disease-like Pathology and Repairs Cognitive Decline by Preventing Capillary Hypofunction in APP/PS1 Mice. Neurotherapeutics 2021, 18, 1064–1080. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combined Treatment with the Phenolics (−)-Epigallocatechin-3-Gallate and Ferulic Acid Improves Cognition and Reduces Alzheimer-like Pathology in Mice. J. Biol. Chem. 2019, 294, 2714–2731. [Google Scholar] [CrossRef]
- Yan, J.-J.; Jung, J.-S.; Kim, T.-K.; Hasan, M.A.; Hong, C.-W.; Nam, J.-S.; Song, D.-K. Protective Effects of Ferulic Acid in Amyloid Precursor Protein Plus Presenilin-1 Transgenic Mouse Model of Alzheimer Disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.-V.; Tan, J.; Town, T. Ferulic Acid Is a Nutraceutical β-Secretase Modulator That Improves Behavioral Impairment and Alzheimer-like Pathology in Transgenic Mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Kim, H.-S.; Kim, D.-H.; Yan, J.-J.; Lee, H.-K.; Suh, H.-W.; Song, D.-K. Inhibitory Effects of Long-Term Administration of Ferulic Acid on Astrocyte Activation Induced by Intracerebroventricular Injection of β-Amyloid Peptide (1–42) in Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 901–907. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cho, J.; Kim, D.-H.; Yan, J.-J.; Lee, H.-K.; Suh, H.-W.; Song, D.-K. Inhibitory Effects of Long-Term Administration of Ferulic Acid on Microglial Activation Induced by Intracerebroventricular Injection of β-Amyloid Peptide (1—42) in Mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Seong, A.-R.; Yoo, J.-Y.; Jin, C.-H.; Lee, Y.-H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.-G. Gallic Acid, a Histone Acetyltransferase Inhibitor, Suppresses β-Amyloid Neurotoxicity by Inhibiting Microglial-Mediated Neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808. [Google Scholar] [CrossRef]
- Ogunlade, B.; Adelakun, S.A.; Agie, J.A. Nutritional Supplementation of Gallic Acid Ameliorates Alzheimer-Type Hippocampal Neurodegeneration and Cognitive Impairment Induced by Aluminum Chloride Exposure in Adult Wistar Rats. Drug Chem. Toxicol. 2022, 45, 651–662. [Google Scholar] [CrossRef]
- Ogunsuyi, O.B.; Oboh, G.; Oluokun, O.O.; Ademiluyi, A.O.; Ogunruku, O.O. Gallic Acid Protects against Neurochemical Alterations in Transgenic Drosophila Model of Alzheimer’s Disease. Adv. Tradit. Med. 2020, 20, 89–98. [Google Scholar] [CrossRef]
- Hajipour, S.; Sarkaki, A.; Farbood, Y.; Eidi, A.; Mortazavi, P.; Valizadeh, Z. Effect of Gallic Acid on Dementia Type of Alzheimer Disease in Rats: Electrophysiological and Histological Studies. Basic Clin. Neurosci. 2016, 7, 97–106. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y.; Sarkaki, A.; Bavarsad, K. Gallic Acid Prevents Memory Deficits and Oxidative Stress Induced by Intracerebroventricular Injection of Streptozotocin in Rats. Pharmacol. Biochem. Behav. 2013, 111, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Rashno, M.; Gholipour, P.; Salehi, I.; Komaki, A.; Rashidi, K.; Khoshnam, S.E.; Ghaderi, S. P-Coumaric Acid Mitigates Passive Avoidance Memory and Hippocampal Synaptic Plasticity Impairments in Aluminum Chloride-Induced Alzheimer’s Disease Rat Model. J. Funct. Foods 2022, 94, 105117. [Google Scholar] [CrossRef]
- Ghaderi, S.; Gholipour, P.; Komaki, A.; Salehi, I.; Rashidi, K.; Khoshnam, S.E.; Rashno, M. P-Coumaric Acid Ameliorates Cognitive and Non-Cognitive Disturbances in a Rat Model of Alzheimer’s Disease: The Role of Oxidative Stress and Inflammation. Int. Immunopharmacol. 2022, 112, 109295. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Lee, S.; Hwang, E.-S.; Maeng, S.; Park, J.-H. P-Coumaric Acid Enhances Long-Term Potentiation and Recovers Scopolamine-Induced Learning and Memory Impairments. Biochem. Biophys. Res. Commun. 2017, 492, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Valentao, P.; Ferreres, F.; Andrade, P.B. The Use of Flavonoids in Central Nervous System Disorders. Curr. Med. Chem. 2013, 20, 4694–4719. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Bilan, A.R. Natural Polyphenols Effects on Protein Aggregates in Alzheimer’s and Parkinson’s Prion-like Diseases. Neural Regen. Res. 2018, 13, 955. [Google Scholar] [CrossRef]
- Fernandes, M.Y.D.; Dobrachinski, F.; Silva, H.B.; Lopes, J.P.; Gonçalves, F.Q.; Soares, F.A.A.; Porciúncula, L.O.; Andrade, G.M.; Cunha, R.A.; Tomé, A.R. Neuromodulation and Neuroprotective Effects of Chlorogenic Acids in Excitatory Synapses of Mouse Hippocampal Slices. Sci. Rep. 2021, 11, 10488. [Google Scholar] [CrossRef]
- Matsui, T.; Ingelsson, M.; Fukumoto, H.; Ramasamy, K.; Kowa, H.; Frosch, M.P.; Irizarry, M.C.; Hyman, B.T. Expression of APP Pathway MRNAs and Proteins in Alzheimer’s Disease. Brain Res. 2007, 1161, 116–123. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Shytle, R.D.; Bai, Y.; Tian, J.; Hou, H.; Mori, T.; Zeng, J.; Obregon, D.; Town, T.; Tan, J. Flavonoid-Mediated Presenilin-1 Phosphorylation Reduces Alzheimer’s Disease β-Amyloid Production. J. Cell. Mol. Med. 2009, 13, 574–588. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Hole, K.L.; Williams, R.J. Flavonoids as an Intervention for Alzheimer’s Disease: Progress and Hurdles Towards Defining a Mechanism of Action. Brain Plast. 2020, 6, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Fan, Y.; Yan, E.; Liu, Z.; Zong, Z.; Qi, Z. Effects of Sodium Ferulate on Amyloid-Beta-Induced MKK3/MKK6-P38 MAPK-Hsp27 Signal Pathway and Apoptosis in Rat Hippocampus. Acta Pharmacol. Sin. 2006, 27, 1309–1316. [Google Scholar] [CrossRef]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin Reduces IL-6 Production in Microglia by Inhibiting JNK Phosphorylation and Activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef]
- Zheng, Q.; Kebede, M.T.; Kemeh, M.M.; Islam, S.; Lee, B.; Bleck, S.D.; Wurfl, L.A.; Lazo, N.D. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules 2019, 24, 2316. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic Compounds Prevent Alzheimer’s Pathology through Different Effects on the Amyloid-β Aggregation Pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef]
- Ochiai, R.; Saitou, K.; Suzukamo, C.; Osaki, N.; Asada, T. Effect of Chlorogenic Acids on Cognitive Function in Mild Cognitive Impairment: A Randomized Controlled Crossover Trial. J. Alzheimers Dis. 2019, 72, 1209–1216. [Google Scholar] [CrossRef]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of Chlorogenic Acid Intake on Cognitive Function in the Elderly: A Pilot Study. Evid. Based Complement. Alternat. Med. 2018, 2018, e8608497. [Google Scholar] [CrossRef]
- Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1337. [Google Scholar] [CrossRef]
- Root, M.; Ravine, E.; Harper, A. Flavonol Intake and Cognitive Decline in Middle-Aged Adults. J. Med. Food 2015, 18, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R.; Aziz, C.B.A.; Othman, Z. Tualang Honey Attenuates Noise Stress-Induced Memory Deficits in Aged Rats. Oxid. Med. Cell. Longev. 2016, 2016, e1549158. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R.; Othman, Z.; Aziz, C.B.A. Neuroprotective Effects of Tualang Honey against Oxidative Stress and Memory Decline in Young and Aged Rats Exposed to Noise Stress. J. Taibah Univ. Sci. 2018, 12, 273–284. [Google Scholar] [CrossRef]
- al-rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A. The Effects of Tualang Honey Supplement on Medial Prefrontal Cortex Morphology and Cholinergic System in Stressed Ovariectomised Rats. Int. J. Appl. Res. Nat. Prod. 2014, 7, 28–36. [Google Scholar]
- Kamarulzaidi, M.A.; Yusoff, M.Y.Z.M.; Mohamed, A.M.; Adli, D.S.H. Tualang Honey Consumption Enhanced Hippocampal Pyramidal Count and Spatial Memory Performance of Adult Male Rats. Sains Malays. 2016, 45, 215–220. [Google Scholar]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ismail, Z.I.M.; Muthuraju, S. Tualang Honey Supplement Improves Memory Performance and Hippocampal Morphology in Stressed Ovariectomized Rats. Acta Histochem. 2014, 116, 79–88. [Google Scholar] [CrossRef]
- Adeniyi, I.A.; Babalola, K.T.; Adekoya, V.A.; Oyebanjo, O.; Ajayi, A.M.; Onasanwo, S.A. Neuropharmacological Effects of Honey in Lipopolysaccharide-Induced Neuroinflammation, Cognitive Impairment, Anxiety and Motor Impairment. Nutr. Neurosci. 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Cai, M.; Shin, B.Y.; Kim, D.H.; Kim, J.M.; Park, S.J.; Park, C.S.; Won, D.H.; Hong, N.D.; Kang, D.H.; Yutaka, Y.; et al. Neuroprotective Effects of a Traditional Herbal Prescription on Transient Cerebral Global Ischemia in Gerbils. J. Ethnopharmacol. 2011, 138, 723–730. [Google Scholar] [CrossRef]
- Oyefuga, O.H.; Ajani, E.O.; Salau, B.A.; Agboola, F.; Adebawo, O.O. Honey Consumption and Its Anti-Ageing Potency in White Wister Albino Rats. Sch. J. Biol. Sci. 2012, 1, 15–19. [Google Scholar]
- Djordjevic, J.; Jones-Gotman, M.; De Sousa, K.; Chertkow, H. Olfaction in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Neurobiol. Aging 2008, 29, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Royet, J.P.; Croisile, B.; Williamson-Vasta, R.; Hibert, O.; Serclerat, D.; Guerin, J. Rating of Different Olfactory Judgements in Alzheimer’s Disease. Chem. Senses 2001, 26, 409–417. [Google Scholar] [CrossRef]
- Li, W.; Howard, J.D.; Gottfried, J.A. Disruption of Odour Quality Coding in Piriform Cortex Mediates Olfactory Deficits in Alzheimer’s Disease. Brain 2010, 133, 2714–2726. [Google Scholar] [CrossRef]
- Devanand, D.P.; Liu, X.; Tabert, M.H.; Pradhaban, G.; Cuasay, K.; Bell, K.; de Leon, M.J.; Doty, R.L.; Stern, Y.; Pelton, G.H. Combining Early Markers Strongly Predicts Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. Biol. Psychiatry 2008, 64, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.; Chen, L.; Nguyen, V.T.; Al-Harthi, L.; Hu, X.-T. Medial Prefrontal Cortex Pyramidal Neurons Exhibit Functional Defects during Early Stage of Alzheimer’s Disease in 3xTg-AD Mice. Alzheimers Dement. 2021, 17, e057589. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Li, A.; Yao, M.; Liu, G.; Chen, S.; Luo, Y.; Wang, Z.; Gong, H.; Li, X.; et al. Acetylcholine Deficiency Disrupts Extratelencephalic Projection Neurons in the Prefrontal Cortex in a Mouse Model of Alzheimer’s Disease. Nat. Commun. 2022, 13, 998. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Li, L.; Zhang, Y.; Yang, M.; Liang, S.; Li, L.; Dai, Y.; Chen, L.; Jia, W.; et al. Enhanced Medial Prefrontal Cortex and Hippocampal Activity Improves Memory Generalization in APP/PS1 Mice: A Multimodal Animal MRI Study. Front. Cell. Neurosci. 2022, 16, 848967. [Google Scholar] [CrossRef]
- Roostaei, T.; Nazeri, A.; Felsky, D.; De Jager, P.L.; Schneider, J.A.; Pollock, B.G.; Bennett, D.A.; Voineskos, A.N. Genome-Wide Interaction Study of Brain Beta-Amyloid Burden and Cognitive Impairment in Alzheimer’s Disease. Mol. Psychiatry 2017, 22, 287–295. [Google Scholar] [CrossRef]
- Basu, J.; Siegelbaum, S.A. The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb. Perspect. Biol. 2015, 7, a021733. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.J. The Role of the Perirhinal Cortex and Hippocampus in Learning, Memory, and Perception. Q. J. Exp. Psychol. Sect. B 2005, 58, 246–268. [Google Scholar] [CrossRef] [PubMed]
- Janeczek, M.; Gefen, T.; Samimi, M.; Kim, G.; Weintraub, S.; Bigio, E.; Rogalski, E.; Mesulam, M.-M.; Geula, C. Variations in Acetylcholinesterase Activity within Human Cortical Pyramidal Neurons Across Age and Cognitive Trajectories. Cereb. Cortex 2018, 28, 1329–1337. [Google Scholar] [CrossRef]
- Rogers, J.L.; Kesner, R.P. Cholinergic Modulation of the Hippocampus during Encoding and Retrieval. Neurobiol. Learn. Mem. 2003, 80, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Cho, C.; Jassar, B.; Harris, K.; MacTavish, D.; Easaw, J. Cellular Mechanisms for Amyloid β-Protein Activation of Rat Cholinergic Basal Forebrain Neurons. J. Neurophysiol. 2001, 86, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Potapov, K.; Kim, S.; Peppercorn, K.; Tate, W.P.; Ábrahám, I.M. Treatment of Beta Amyloid 1–42 (Aβ1–42)-Induced Basal Forebrain Cholinergic Damage by a Non-Classical Estrogen Signaling Activator in Vivo. Sci. Rep. 2016, 6, 21101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-H.; Bastianetto, S.; Mennicken, F.; Ma, W.; Kar, S. Amyloid β Peptide Induces Tau Phosphorylation and Loss of Cholinergic Neurons in Rat Primary Septal Cultures. Neuroscience 2002, 115, 201–211. [Google Scholar] [CrossRef]
- Zambrzycka, A.; Alberghina, M.; Strosznajder, J.B. Effects of Aging and Amyloid-β Peptides on Choline Acetyltransferase Activity in Rat Brain. Neurochem. Res. 2002, 27, 277–281. [Google Scholar] [CrossRef]
- Vaucher, E.; Aumont, N.; Pearson, D.; Rowe, W.; Poirier, J.; Kar, S. Amyloid β Peptide Levels and Its Effects on Hippocampal Acetylcholine Release in Aged, Cognitively-Impaired and -Unimpaired Rats. J. Chem. Neuroanat. 2001, 21, 323–329. [Google Scholar] [CrossRef]
- Campanari, M.-L.; Navarrete, F.; Ginsberg, S.D.; Manzanares, J.; Sáez-Valero, J.; García-Ayllón, M.-S. Increased Expression of Readthrough Acetylcholinesterase Variants in the Brains of Alzheimer’s Disease Patients. J. Alzheimers Dis. 2016, 53, 831–841. [Google Scholar] [CrossRef]
- Berson, A.; Knobloch, M.; Hanan, M.; Diamant, S.; Sharoni, M.; Schuppli, D.; Geyer, B.C.; Ravid, R.; Mor, T.S.; Nitsch, R.M.; et al. Changes in Readthrough Acetylcholinesterase Expression Modulate Amyloid-Beta Pathology. Brain 2008, 131, 109–119. [Google Scholar] [CrossRef]
- Macdonald, I.R.; Maxwell, S.P.; Reid, G.A.; Cash, M.K.; DeBay, D.R.; Darvesh, S. Quantification of Butyrylcholinesterase Activity as a Sensitive and Specific Biomarker of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 491–505. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent Progress in the Identification of Selective Butyrylcholinesterase Inhibitors for Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Mushtaq, G.; Nigel, H.G.; Jalaluddin, A.K.; Mohammad, A.K. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord.-Drug Targets-CNS Neurol. Disord. 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Gajowniczek-Ałasa, D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients 2022, 14, 2969. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective Butyrylcholinesterase Inhibition Elevates Brain Acetylcholine, Augments Learning and Lowers Alzheimer β-Amyloid Peptide in Rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Chepulis, L.M.; Starkey, N.J.; Waas, J.R.; Molan, P.C. The Effects of Long-Term Honey, Sucrose or Sugar-Free Diets on Memory and Anxiety in Rats. Physiol. Behav. 2009, 97, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R.; AbdAziz, C.; Othman, Z.; Al-Rahbi, B. Tualang Honey Improves Memory Performance and Decreases Depressive-like Behavior in Rats Exposed to Loud Noise Stress. Noise Health 2015, 17, 83–89. [Google Scholar] [CrossRef]
- Akouchekian, S.; Omranifard, V.; Maracy, M.R.; Pedram, A.; Zefreh, A.A. Efficacy of Herbal Combination of Sedge, Saffron, and Astragalus Honey on Major Neurocognitive Disorder. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2018, 23, 58. [Google Scholar] [CrossRef]
- Mustafa, M.Z.; Zulkifli, F.N.; Fernandez, I.; Mariatulqabtiah, A.R.; Sangu, M.; Nor Azfa, J.; Mohamed, M.; Roslan, N. Stingless Bee Honey Improves Spatial Memory in Mice, Probably Associated with Brain-Derived Neurotrophic Factor (BDNF) and Inositol 1,4,5-Triphosphate Receptor Type 1 (Itpr1) Genes. Evid. Based Complement. Alternat. Med. 2019, 2019, e8258307. [Google Scholar] [CrossRef]
- Oyekunle, O.A.; Ogundeji, T.P.; Okojie, A.K. Behavioral Modifications Related to Consumption of a “Soft” Adaptogen, Bee Honey, by Rats. Neurophysiology 2011, 43, 38–41. [Google Scholar] [CrossRef]
- Warad, V.B.; Shastri, R.; Habbu, P.; Katti, P.; Jagannath, A.B.; Kulkarni, V.H.; Chakraborty, M. Preparation and Screening of Swarnaprashana for Nootropic Activity. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 170. [Google Scholar] [CrossRef]
- Akuchekian, S.; Layegh, E.; Najafi, M.; Barekatein, M.; Maracy, M.; Zomorodi, M.H. Effect of Herbal Medicine on Memory Impairment in Electroconvulsive Therapy (ECT)—ProQuest. J. Res. Med. Sci. 2012, 17, S59–S64. [Google Scholar]
- Badrasawi, M.M.; Shahar, S.; Manaf, Z.A.; Haron, H. Effect of Talbinah Food Consumption on Depressive Symptoms among Elderly Individuals in Long Term Care Facilities, Randomized Clinical Trial. Clin. Interv. Aging 2013, 8, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Tsetsenis, T.; Badyna, J.K.; Wilson, J.A.; Zhang, X.; Krizman, E.N.; Subramaniyan, M.; Yang, K.; Thomas, S.A.; Dani, J.A. Midbrain Dopaminergic Innervation of the Hippocampus Is Sufficient to Modulate Formation of Aversive Memories. Proc. Natl. Acad. Sci. USA 2021, 118, e2111069118. [Google Scholar] [CrossRef] [PubMed]
- Rosen, Z.B.; Cheung, S.; Siegelbaum, S.A. Midbrain Dopamine Neurons Bidirectionally Regulate CA3-CA1 Synaptic Drive. Nat. Neurosci. 2015, 18, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Kempadoo, K.A.; Mosharov, E.V.; Choi, S.J.; Sulzer, D.; Kandel, E.R. Dopamine Release from the Locus Coeruleus to the Dorsal Hippocampus Promotes Spatial Learning and Memory. Proc. Natl. Acad. Sci. USA 2016, 113, 14835–14840. [Google Scholar] [CrossRef]
- Smith, C.C.; Greene, R.W. CNS Dopamine Transmission Mediated by Noradrenergic Innervation. J. Neurosci. 2012, 32, 6072–6080. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Okuyama, T.; Sun, C.; Smith, L.M.; Abe, K.; Tonegawa, S. Locus Coeruleus Input to Hippocampal CA3 Drives Single-Trial Learning of a Novel Context. Proc. Natl. Acad. Sci. USA 2018, 115, E310–E316. [Google Scholar] [CrossRef]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernández, G.; Deisseroth, K.; Greene, R.W.; et al. Locus Coeruleus and Dopaminergic Consolidation of Everyday Memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef]
- Duszkiewicz, A.J.; McNamara, C.G.; Takeuchi, T.; Genzel, L. Novelty and Dopaminergic Modulation of Memory Persistence: A Tale of Two Systems. Trends Neurosci. 2019, 42, 102–114. [Google Scholar] [CrossRef]
- Shohamy, D.; Wagner, A.D. Integrating Memories in the Human Brain: Hippocampal-Midbrain Encoding of Overlapping Events. Neuron 2008, 60, 378–389. [Google Scholar] [CrossRef]
- McNamara, C.G.; Tejero-Cantero, Á.; Trouche, S.; Campo-Urriza, N.; Dupret, D. Dopaminergic Neurons Promote Hippocampal Reactivation and Spatial Memory Persistence. Nat. Neurosci. 2014, 17, 1658–1660. [Google Scholar] [CrossRef]
- Lisman, J.E.; Grace, A.A. The Hippocampal-VTA Loop: Controlling the Entry of Information into Long-Term Memory. Neuron 2005, 46, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Guitart-Masip, M.; Bunzeck, N.; Dolan, R.J.; Düzel, E. Dopamine Modulates Episodic Memory Persistence in Old Age. J. Neurosci. 2012, 32, 14193–14204. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, J.; Björkholm, C.; Feltmann, K.; Malmlöf, T.; Mishra, D.; Gonzales, C.B.; Schilström, B.; Konradsson-Geuken, Å. The Importance of Ventral Hippocampal Dopamine and Norepinephrine in Recognition Memory. Front. Behav. Neurosci. 2021, 15, 667244. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.S.; Lima, K.R.; Ramborger, B.P.; Roehrs, R.; Izquierdo, I.; Mello-Carpes, P.B. Catecholaminergic Hippocampal Activation Is Necessary for Object Recognition Memory Persistence Induced by One-Single Physical Exercise Session. Behav. Brain Res. 2020, 379, 112356. [Google Scholar] [CrossRef]
- Peterson, A.C.; Zhang, S.; Hu, S.; Chao, H.H.; Li, C.R. The Effects of Age, from Young to Middle Adulthood, and Gender on Resting State Functional Connectivity of the Dopaminergic Midbrain. Front. Hum. Neurosci. 2017, 11, 52. [Google Scholar] [CrossRef]
- Noda, S.; Sato, S.; Fukuda, T.; Tada, N.; Hattori, N. Aging-Related Motor Function and Dopaminergic Neuronal Loss in C57BL/6 Mice. Mol. Brain 2020, 13, 46. [Google Scholar] [CrossRef]
- Simon, J.R.; Howard, J.H., Jr.; Howard, D.V. Adult Age Differences in Learning from Positive and Negative Probabilistic Feedback. Neuropsychology 2010, 24, 534–541. [Google Scholar] [CrossRef]
- Krashia, P.; Nobili, A.; D’Amelio, M. Unifying Hypothesis of Dopamine Neuron Loss in Neurodegenerative Diseases: Focusing on Alzheimer’s Disease. Front. Mol. Neurosci. 2019, 12, 123. [Google Scholar] [CrossRef]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine Neuronal Loss Contributes to Memory and Reward Dysfunction in a Model of Alzheimer’s Disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef]
- Cordella, A.; Krashia, P.; Nobili, A.; Pignataro, A.; La Barbera, L.; Viscomi, M.T.; Valzania, A.; Keller, F.; Ammassari-Teule, M.; Mercuri, N.B.; et al. Dopamine Loss Alters the Hippocampus-Nucleus Accumbens Synaptic Transmission in the Tg2576 Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2018, 116, 142–154. [Google Scholar] [CrossRef]
- Moreno-Castilla, P.; Rodriguez-Duran, L.F.; Guzman-Ramos, K.; Barcenas-Femat, A.; Escobar, M.L.; Bermudez-Rattoni, F. Dopaminergic Neurotransmission Dysfunction Induced by Amyloid-β Transforms Cortical Long-Term Potentiation into Long-Term Depression and Produces Memory Impairment. Neurobiol. Aging 2016, 41, 187–199. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Venneri, A. Volume and Connectivity of the Ventral Tegmental Area Are Linked to Neurocognitive Signatures of Alzheimer’s Disease in Humans. J. Alzheimers Dis. 2018, 63, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Ma, D.; Chen, G.; Wang, W.; Fu, S. Myricetin Prevents Dopaminergic Neurons from Undergoing Neuroinflammation-Mediated Degeneration in a Lipopolysaccharide-Induced Parkinson’s Disease Model. J. Funct. Foods 2018, 45, 452–461. [Google Scholar] [CrossRef]
- Bureau, G.; Longpré, F.; Martinoli, M.-G. Resveratrol and Quercetin, Two Natural Polyphenols, Reduce Apoptotic Neuronal Cell Death Induced by Neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, D.; Yang, Z.; Hu, X.; Qian, S.; Liu, J.; Wilson, B.; Block, M.; Hong, J.-S. Curcumin Protects Dopaminergic Neuron Against LPS Induced Neurotoxicity in Primary Rat Neuron/Glia Culture. Neurochem. Res. 2008, 33, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Plouffe, M.; Renaud, J.; Provencher, C.; Martinoli, M.-G. Quercetin and Sesamin Protect Dopaminergic Cells from MPP+-Induced Neuroinflammation in a Microglial (N9)-Neuronal (PC12) Coculture System. Oxid. Med. Cell. Longev. 2012, 2012, e921941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Q.; Zhang, L.; Wang, Q.; Yang, Z.; Liu, J.; Feng, L. Caffeic Acid Reduces A53T α-Synuclein by Activating JNK/Bcl-2-Mediated Autophagy in Vitro and Improves Behaviour and Protects Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease. Pharmacol. Res. 2019, 150, 104538. [Google Scholar] [CrossRef]
- Kim, J.E.; Shrestha, A.C.; Kim, H.S.; Ham, H.N.; Kim, J.H.; Kim, Y.J.; Noh, Y.J.; Kim, S.J.; Kim, D.K.; Jo, H.K.; et al. WS-5 Extract of Curcuma Longa, Chaenomeles Sinensis, and Zingiber Officinale Contains Anti-AChE Compounds and Improves β-Amyloid-Induced Memory Impairment in Mice. Evid.-Based Complement. Altern. Med. ECAM 2019, 2019, 5160293. [Google Scholar] [CrossRef]
- He, X.; Yang, X.; Ou, R.; Ouyang, Y.; Wang, S.; Chen, Z.; Wen, S.; Pi, R. Synthesis and Evaluation of Multifunctional Ferulic and Caffeic Acid Dimers for Alzheimer’s Disease. Nat. Prod. Res. 2017, 31, 734–737. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Y.; Dong, X. Design of Gallic Acid–Glutamine Conjugate and Chemical Implications for Its Potency Against Alzheimer’s Amyloid-β Fibrillogenesis. Bioconjug. Chem. 2022, 33, 677–690. [Google Scholar] [CrossRef]
- Jeon, S.G.; Song, E.J.; Lee, D.; Park, J.; Nam, Y.; Kim, J.; Moon, M. Traditional Oriental Medicines and Alzheimer’s Disease. Aging Dis. 2019, 10, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Z.-Y.; Zheng, X.; Li, D.-L.; Zhou, R.-P.; Zhang, K. Use of Curcumin in Diagnosis, Prevention, and Treatment of Alzheimer’s Disease. Neural Regen. Res. 2018, 13, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Palanivelu, K. The Effect of Curcumin (Turmeric) on Alzheimer’s Disease: An Overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. (Berl.) 2017, 133, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Murata, M.; Kawanishi, S.; Oikawa, S. Polyphenols with Anti-Amyloid β Aggregation Show Potential Risk of Toxicity Via Pro-Oxidant Properties. Int. J. Mol. Sci. 2020, 21, 3561. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Dhana, K.; Leurgans, S.E.; Shea, K.; Booth, S.L.; Rajan, K.; Schneider, J.A.; Barnes, L.L. Association of Dietary Intake of Flavonols With Changes in Global Cognition and Several Cognitive Abilities. Neurology 2022. [Google Scholar] [CrossRef]
- Shishtar, E.; Rogers, G.T.; Blumberg, J.B.; Au, R.; Jacques, P.F. Long-Term Dietary Flavonoid Intake and Risk of Alzheimer Disease and Related Dementias in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2020, 112, 343–353. [Google Scholar] [CrossRef]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.; Morris, M.C. Association of Strawberries and Anthocyanidin Intake with Alzheimer’s Dementia Risk. Nutrients 2019, 11, 3060. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary Intakes of Berries and Flavonoids in Relation to Cognitive Decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Yahya, H.M.; Day, A.; Lawton, C.; Myrissa, K.; Croden, F.; Dye, L.; Williamson, G. Dietary Intake of 20 Polyphenol Subclasses in a Cohort of UK Women. Eur. J. Nutr. 2016, 55, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- García-Ayllón, M.-S.; Riba-Llena, I.; Serra-Basante, C.; Alom, J.; Boopathy, R.; Sáez-Valero, J. Altered Levels of Acetylcholinesterase in Alzheimer Plasma. PLOS ONE 2010, 5, e8701. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, A.; Darreh-Shori, T.; Peskind, E.; Soininen, H.; Mousavi, M.; Eagle, G.; Lane, R. Different Cholinesterase Inhibitor Effects on CSF Cholinesterases in Alzheimer Patients. Curr. Alzheimer Res. 2009, 6, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, P.; Blennow, K.; Andreasen, N.; Eriksson, B.; Minthon, L.; Hesse, C. Differential Increase in Cerebrospinal Fluid-Acetylcholinesterase after Treatment with Acetylcholinesterase Inhibitors in Patients with Alzheimer’s Disease. Neurosci. Lett. 2001, 300, 157–160. [Google Scholar] [CrossRef]
- Guzmán-Ramos, K.; Moreno-Castilla, P.; Castro-Cruz, M.; McGaugh, J.L.; Martínez-Coria, H.; LaFerla, F.M.; Bermúdez-Rattoni, F. Restoration of Dopamine Release Deficits during Object Recognition Memory Acquisition Attenuates Cognitive Impairment in a Triple Transgenic Mice Model of Alzheimer’s Disease. Learn. Mem. 2012, 19, 453–460. [Google Scholar] [CrossRef]
- Tsunekawa, H.; Noda, Y.; Mouri, A.; Yoneda, F.; Nabeshima, T. Synergistic Effects of Selegiline and Donepezil on Cognitive Impairment Induced by Amyloid Beta (25–35). Behav. Brain Res. 2008, 190, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Di Lorenzo, F.; Bonnì, S.; Giacobbe, V.; Bozzali, M.; Caltagirone, C.; Martorana, A. Dopaminergic Modulation of Cortical Plasticity in Alzheimer’s Disease Patients. Neuropsychopharmacology 2014, 39, 2654–2661. [Google Scholar] [CrossRef]
- Nam, G.S.; Nam, K.-S.; Park, H.-J. Caffeic Acid Diminishes the Production and Release of Thrombogenic Molecules in Human Platelets. Biotechnol. Bioprocess Eng. 2018, 23, 641–648. [Google Scholar] [CrossRef]
- Abdel-Moneim, W.M.; Ghafeer, H.H. The Potential Protective Effect of Natural Honey Against Cadmium-Induced Hepatotoxicity and Nephrotoxicity. Mansoura J. Forensic Med. Clin. Toxicol. 2007, 15, 75–98. [Google Scholar] [CrossRef]
- Khalil, M.L.; Sulaiman, S.A. The Potential Role of Honey and Its Polyphenols in Preventing Heart Disease: A Review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
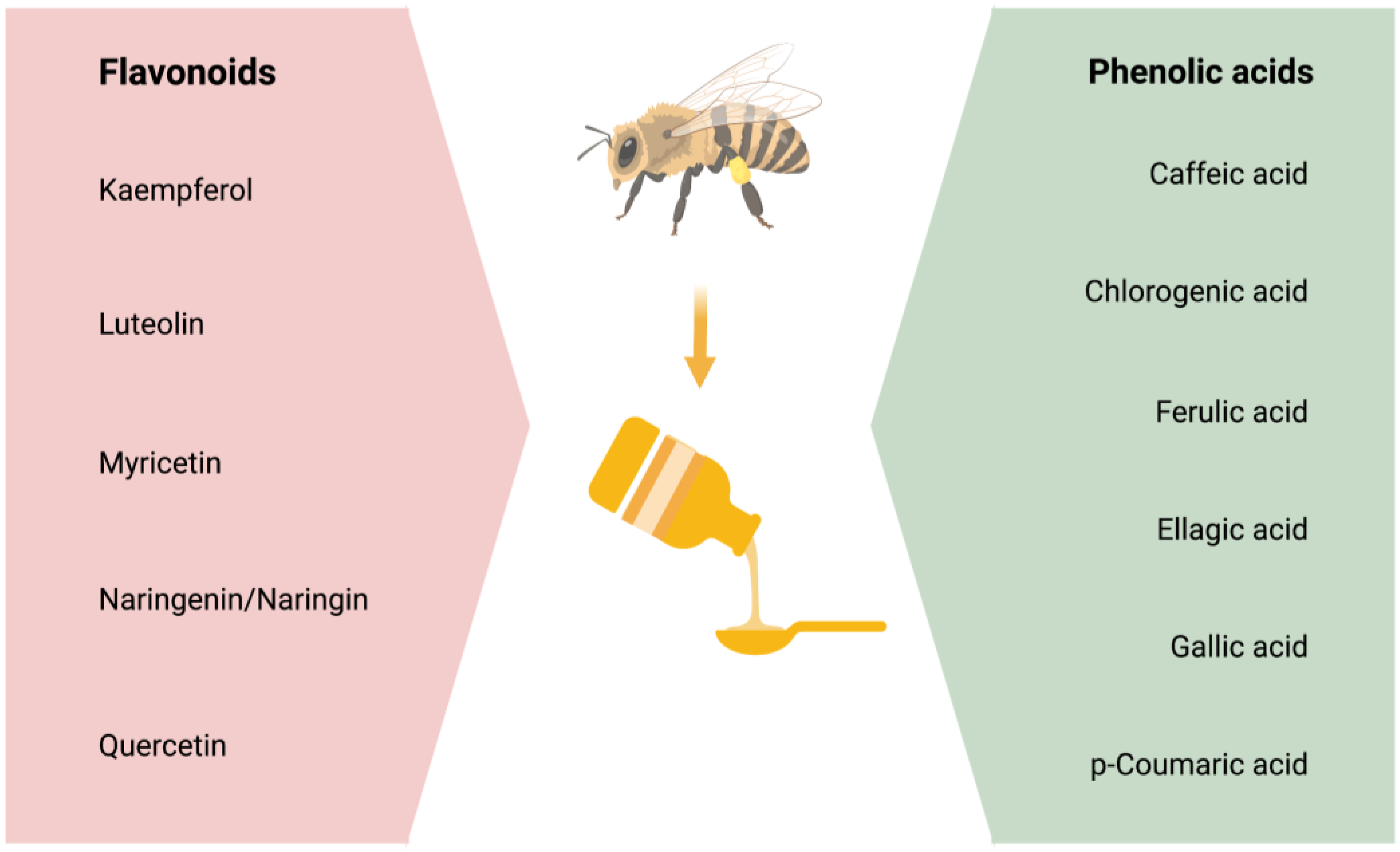
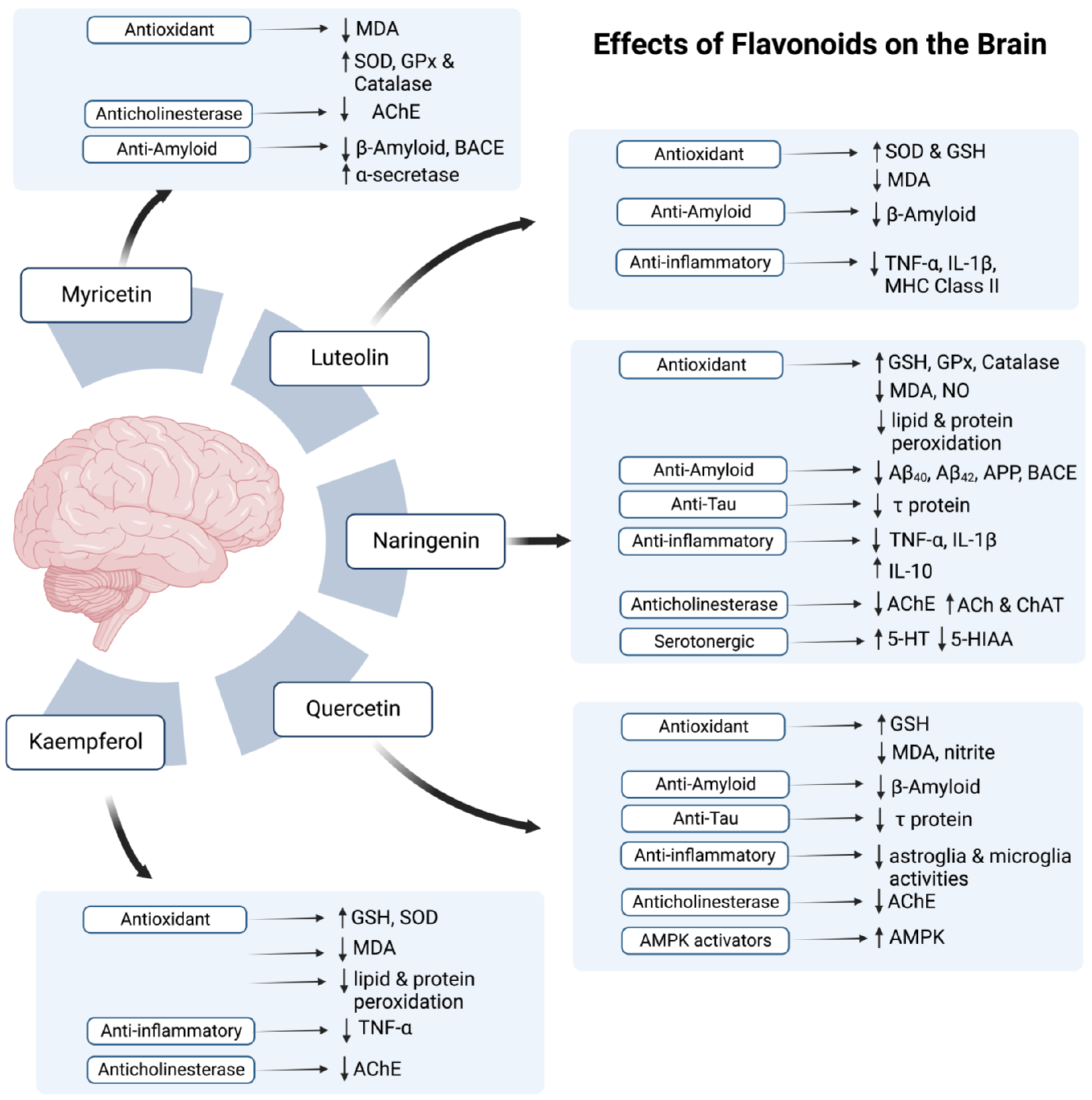
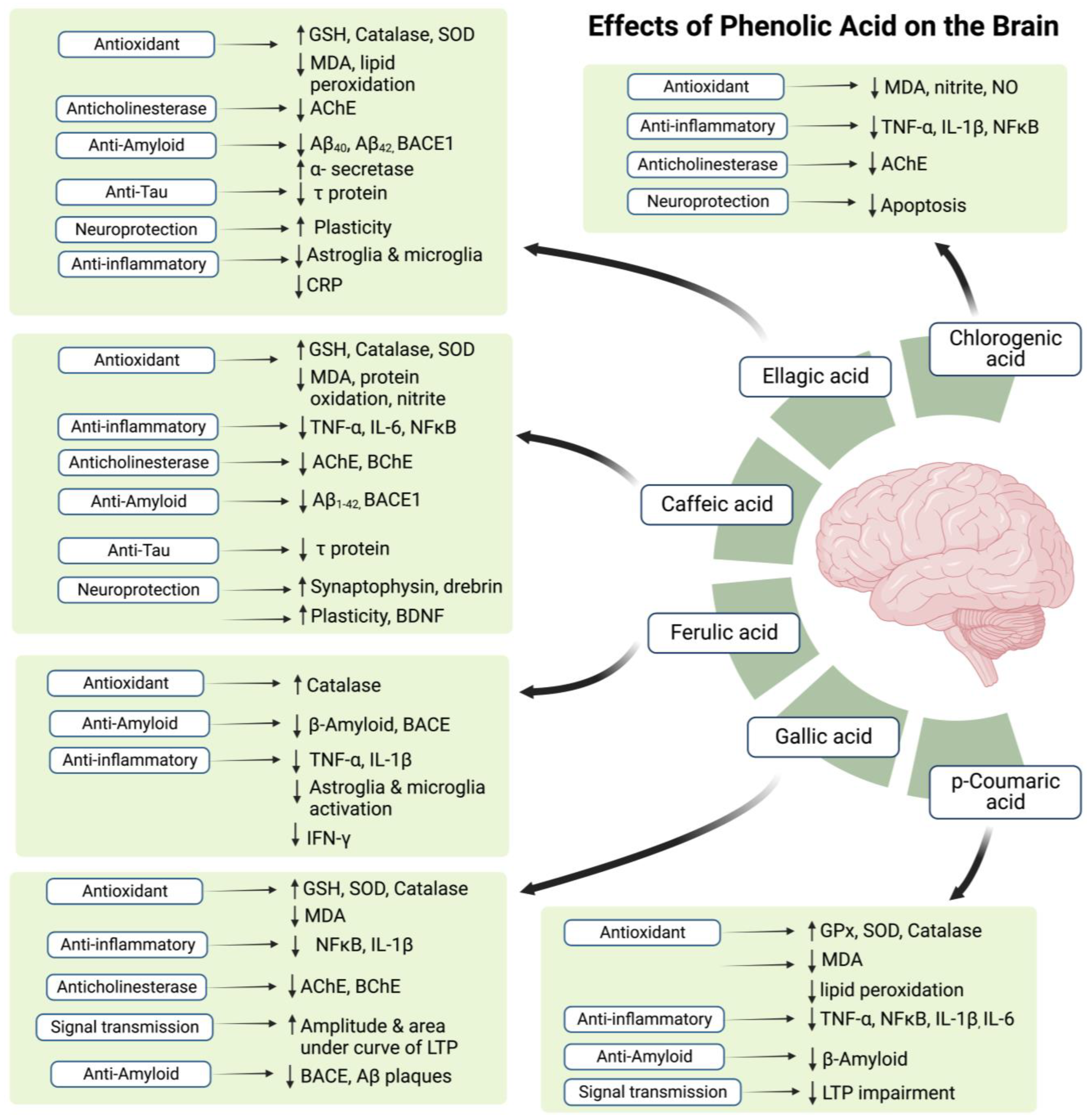
| Flavonoid Component | Studied Model | Testing Method | Time of Starting Administration | Potential to Act As | Studied Region in Brain | Important Findings of the Study | References |
|---|---|---|---|---|---|---|---|
| Myricetin | STZ induced AD (Wistar) rat model | Passive avoidance test IHC | 1 day before stereotactic surgery (STZ exposure) | Neuroprotective agent | Hippocampus (area CA3) | Myricetin (at 10 mg/kg i.p.,) resulted in a better performance in avoidance test with decreased STL and increased TDC, along with increasing number of intact neurons in CA3 layer. | [109] |
| Kunming Mice | MWM test, and brain tissue analysis | Together with i.p. injection of scopolamine | Antioxidant and anti-AChE agent | Hippocampus | Myricetin decreased escape latency and increased time spent in target quadrant, and number of platform crossings. Decreased the amount of MDA while improving antioxidant enzyme activities; it also sustained the concentration of ACh in the hippocampus. | [110] | |
| Neurons from fetal rat cerebral cortex (E18) | IHC, Immunoblotting, spectroscopy, and activity assays | 1 day before Aβ1–42 exposure | Anti-amyloid and neuroprotective agent | Not applicable | Myrecetin protects neurons from Aβ1–42 induced injury and cell death. It decreases production and aggregation of Aβ1–42 and Aβ1–40 (only at higher dose) that is also proved by increased activity of α-secretase and decreased activity of BACE1 (in a concentration-dependent manner). | [111] | |
| Luteolin | ICV-STZ induced AD (Wistar) rat model | MWM task and probe tests; IHC | 3 days before injection of STZ | Neuroprotective agent | Hippocampus (area CA1) | Luteolin pre-treatment resulted in:
| [112] |
| Sprague–Dawley rats (chronic hypoperfusion injury model) | MWM task; Brain tissue analysis | On 5th post-operative day of (bilateral common carotid arter) ligation surgery | Anti-inflammatory, antioxidant, and anti- amyloid agent | Cortex and hippocampus | Luteolin-treated rats showed:
| [113] | |
| Adult male Balb/c mice; Murine Neuro.2a, and LPS stimulated BV-2 (murine microglia cell line) | MWM task; Brain tissue analysis | 4 weeks before experiment | Anti-inflammatory (aged mice), and neuroprotective (before LPS induction) agent | Hippocampus | Pretreatment with luteolin reduces pro-inflammatory mediators in microglia, therefore, prevents Neuro.2a cell death. Reduction in mRNA levels of IL-1β and MHC class II, and TNFα (only with higher intake). Better performance of aged mice fed with luteolin in MWM task. | [114] | |
| Naringenin/Naringin | High-fat-diet fed SAMP8 mice (a model of AD) | MWM task and Barnes Maze test; Brain tissue analysis | Along with the high-fat diet | Anti-inflammatory, anti-amyloid, anti-tau, and neuroprotective agent | Cortex, hippocampus, and white matter | Naringenin treatment resulted in:
| [115] |
| AlCl3+D-gal induced AD (Wistar) rat model | Behavioral tests; Brain tissue analysis | Two weeks before AlCl3+D-gal induction | Antioxidant, anti-AChE, Serotonin- enhancer, and neuroprotective agent | Cortex and hippocampus | Naringenin pre-treatment resulted in:
| [116] | |
| Intra- hippocampal Aβ1–40 induced (Wistar) rat model | Y-maze, Radial arm maze task, passive avoidance test; Brain tissue analysis | 1 h before injecting Aβ1–40 bilaterally in the dorsal hippocampus | Antioxidant and neuroprotective agent | Hippocampus | Pre-treatment of rats with naringenin caused:
| [117] | |
| ICV-STZ induced AD rat model | Passive avoidance test, MWM task; Brain tissue analysis | 14 days before ICV-STZ injection | Antioxidant and neuroprotective agent | Hippocampus | Pre-treatment with naringenin resulted in:
| [118] | |
| Hydrocortisone injected AD mice model | MWM task NOR test and step-down test;$$$$$Brain tissue analysis | 21 days before hydrocortisone injection | Anti-amyloid, anti-tau, anti-AChE, antioxidant, and neuroprotective agent | Hippocampus and hypothalamus | The results of the pre-treatment of mice with Naringin were:
| [119] | |
| Quercetin | ICR mice subjected to dexamethasone | MWM task | 3 h before dexamethasone i.p. injection | Neuroprotective agent | Hippocampus (area CA3 and DG) | More number of cells in DG in quercetin-treated group. | [120] |
| ICV-STZ induced AD rat model | MWM task | After 1 week of ICV-STZ induction | Neuroprotective agent | Not mentioned | Decreased escape latency, and increased time spent in target quadrant. | [121] | |
| Homozygous 3xTg-AD mice | MWM task, elevated plus maze; Brain tissue analysis | Quercetin injected i.p., every 48 h for 3 months in AD mice before experimentation | Anti-amyloid, anti-tau, anti-inflammatory and neuroprotective agent | Subiculum, area CA1, entorrhinal cortex and amygdala | Quercertin-treated group showed:
| [122] | |
| I.C.-STZ induced (Swiss) albino mice | MWM task Passive avoidance test; Brain tissue analysis | Just after I.C.- STZ injection | Antioxidant and anti-AChE agent | Whole brain (homogenate) | Reduced mean latency in MWM task and increased TLT. Reduction in MDA and nitrite levels, and inhibition of AChE activity (with higher dose of quercetin). Increased GSH levels in quercetin-treated mice. | [123] | |
| ICR mice subjected to TMT-induced neuronal deficits | Y-maze and passive avoidance test; Brain tissue analysis | 21 days before the TMT induction | Antioxidant and anti-AChE agent | Whole brain (homogenate) | Quercetin pre-treatment resulted in:
| [124] | |
| APPswe/PS1dE9 (C57/BL) transgenic mice | NOR test, MWM test; Brain tissue analysis | 16 weeks before sacrifice | Antioxidant, anti-amyloid, and neuroprotective agent | Hippocampus and cortex | Mice treated with quercetin showed an increased recognition index in NOR test, decreased escape latency in MWM task. Quercetin increases AMPK, prevents the formation of amyloid plaques, and alleviates hippocampal-mitochondria dysfunction. | [125] | |
| Kaempferol | Transgenic Aβ flies (DS model) | Climbing assay; Brain tissue analysis | 30 days before behavioral tests | Antioxidant, anti-AChE, and neuroprotective agent | Whole brain (homogenate) | Dose-dependent increase in GSH content, and decrease in LPO, PC, GST, and AChE activity after kaempferol treatment compared with unexposed Aβ-flies. Decreased apoptosis (evident by lower level of caspase enzymes) compared with the unexposed Aβ-flies. | [126] |
| Ovariectomized ICV-STZ induced AD (Wistar) rat model | MWM test; Brain tissue analysis | On the same day as 2nd dose of STZ, and continued for 21 days | Antioxidant and anti-inflammatory agent | Hippocampus | Kaempferol consumption caused:
| [127] | |
| ICV-STZ induced AD (Wistar) rat model | [128] |
| Phenolic Acid Component | Studied Model | Testing Method | Time of Starting Administration | Potential to Act As | Studied Region in Brain | Important Findings of Study | References |
|---|---|---|---|---|---|---|---|
| Caffeic acid | ICV-STZ induced AD (Wistar) rat model | MWM, NOR test and spontaneous locomotor activity; Brain tissue analysis | 1 h after first dose of ICV-STZ | Antioxidant and anti-AChE agent | Cerebral cortex and hippocampus | Caffeic acid-treated rats showed:
| [129] |
| AlCl3-induced AD (Wistar) rat model | MWM; Brain tissue analysis | 20 days after AlCl3 (daily) injection | Antioxidant and anti-AChE agent | Whole brain (homogenate) | Reversal of AlCl3 –induced memory deficits. Inhibition of AChE activity, and nitrite levels in brain. Increased levels of catalase, GSH, and GST in caffeic acid-treated group. | [130] | |
| High-fat-diet-induced AD (Sprague-Dawley) rat model | MWM; Brain tissue analysis | Along with the high-fat diet | Antioxidant, anti-amyloid and$$$$$anti-tau agent | Cerebral cortex and hippocampus | Reversal of memory deficits in caffeic acid group. Increased SOD, and decreased level of APP expression, β-Amyloid(1–42) content and BACE1 levels. Decreased in p-Tau (Thr181) expression. Increased synaptophysin expression in cortex, and drebrin expression after caffeic acid treatment. | [131] | |
| High carbohydrate high fructose (HCHF) diet induced metabolic syndrome (Wistar) rat model | Brain tissue analysis | After consumption of HCFC diet for 8 weeks | Anti-inflammatory agent | Hippocampus (area CA1 and DG) | Reduced TNF-α levels, and higher BDNF concentration compared with HCHF-only fed group. | [132] | |
| Intrahippocampally- Aβ1–40-induced AD (Sprague-Dawley) rat model | MWM; Brain tissue analysis | After injecting Aβ1–40 | Antioxidant, anti-inflammatory, anti-AChE, and neuroprotective agent | Hippocampus | Caffeic acid-treated group showed:
| [133] | |
| Wistar rats (whole brain in-vitro) | Not applicable | Added to the supernatant of the homogenate | Antioxidant, anti-AChE, and anti-BChE agent | Whole brain homogenate | Addition of caffeic acid caused:
| [134] | |
| i.p. D-gal induced aging (Sprague-Dawley) rat model | Novel Object Location, NOR; Brain tissue analysis | Along with D-gal | Neuroprotective agent | Hippocampus | Co-treatment with caffeic acid displayed:
| [135] | |
| Chlorogenic acid | APP/PS1 double transgenic mice | MWM;Brain tissue analysis | At 3-month of age | Neuroprotective agent | Brain (including histological evaluation of hippocampal CA1 area) | Chlorogenic acid-treated mice showed:
| [136] |
| C57BL/6 mice + Primary neuro-glia cultures | Brain tissue analysis + Neuro-glia analysis and assays | 7 days before LPS injection + 2 h before incubation with LPS | Anti-inflammatory and neuroprotective agent | Substantia nigra | Pre-treatment with chlorogenic acid:
| [137] | |
| Scopolamine-induced AD (ICR) mice model | Y-maze test, passive avoidance test, MWM; Brain tissue analysis | 30-min before scopolamine injection | Antioxidant, and anti-AChE agent | Whole brain (homogenate), and frontal cortex and hippocampus (homogenate) | Cholorogenic acid pre-treatment resulted in:
| [138] | |
| Ellagic acid | Intrahippocampal microinjection Aβ25–35 induced AD (Wistar) rat model | NOR, Y-maze, passive avoidance and radial arm maze tasks; Brain tissue analysis | One week before Aβ-induction surgery | Anti-inflammatory, antioxidant, anti-AChE, and neuroprotective agent | Hippocampus (including histological evaluation of CA1 area) | Ellagic acid pre-treatment caused:
| [139] |
| AlCl3-induced AD (Wistar albino) rat model | NOR test; Brain tissue analysis | After stopping AlCl3 | Antioxidant, anti-amyloid, anti-tau, and neuroprotective agent | Whole brain | The results of ellagic acid treatment were:
| [140] | |
| APP/PS1 double-transgenic mice | MWM; Brain tissue analysis | 1 week after acclimatization | Anti-amyloid, anti-tau, and neuroprotective agent | Hippocampus | Improved learning and memory in the ellagic acid-treated group. More number of neurons with reduced expression level of caspase-3 in hippocampus. Decreased Aβ plaque deposition along with reduced levels of both Aβ40 and Aβ42 which is also confirmed by reduction in pThr668-APP and BACE1 expression. Down-regulation of p-tau (pSer199-tau and pSer396-tau) by mediating AKT/GSK3β signaling pathway (increasing pSer473-AKT and lowering pTyr216-GSK3β). | [141] | |
| NOR, Y-maze, radial arm water- maze tasks; Brain tissue analysis | At 12 months of age | Antioxidant, anti-inflammatory, and anti-amyloid agent | Whole brain (including study on EC, RSC, and hippocampus) | Treatment with ellagic acid resulted in:
| [142] | ||
| Oral AlCl3- induced AD (Wistar) rat model | NOR test; Brain tissue analysis | 4 weeks after the beginning of oral AlCl3 dosage | Antioxidant, anti-amyloid, anti-tau, and neuroprotective agent | EC | Ellagic acid-treated group showed:
| [143] | |
| ICV-STZ induced AD (Wistar) rat model | Radial arm maze and Y-maze tasks; Brain tissue analysis | 1 day after STZ administration | Antioxidant, anti-inflammatory, anti-amyloid, and neuroprotective agent | Cerebral cortex (homogenate), EC and hippocampus proper (area CA1, CA2, CA3, and DG) | Ellagic acid treatment caused:
| [144] | |
| Ferulic acid | APP/PS1 (transgenic) mice | MWM task; Brain tissue analysis | In AD mice of 6 months age | Anti-amyloid, neurovascular protective agent | Whole brain (including study on cerebral cortex and hippocampus) | Ferulic acid treatment effects on APP/PS1 mice were:
| [145] |
| NOR, Y-maze, radial arm-water maze tasks; Brain tissue analysis | In 1-year-old AD- mice model | Anti-inflammatory, antioxidant, and anti-amyloid agent | Whole brain (including study on RSC, EC, hippocampus) | Treatment with Ferulic acid resulted in:
| [146] | ||
| NOR and Y-maze tasks; Brain tissue analysis | In 6-month old AD mice | Anti-amyloid, anti-inflammatory agent | Frontal cortex and hippocampus |
Ferulic acid treatment:
| [147] | ||
| PSAPP mice (AD-model) | NOR, Y-maze and MWM tasks; Brain tissue analysis | In 6-month-old AD mice | Antioxidant, anti-inflammatory and anti-amyloid agent | Cingulate cortex, EC and hippocampus + Whole brain (homogenate) |
Ferulic acid-treated PSAPP mice displayed:
| [148] | |
| ICR mice (ICV-induced Aβ1–42 AD-model) | Brain tissue analysis | 4 weeks before ICV injection of Aβ1–42 | Antioxidant and anti-inflammatory agent | Hippocampus | Ferulic acid pre-treatment mitigated oxidative stress and neuroinflammation by blocking astroglial activation evident by double staining of 3-nitrotyrosine and endothelial nitric oxide synthase immunoreactive cells with GFAP. | [149] | |
| Brain tissue analysis | 4 weeks before ICV injection of Aβ1–42 | Antioxidant and anti-inflammatory agent | Hippocampus | Ferulic acid pre-treatment inhibited microglial activation evident by blocking of OX-42 (marker of activated microglia) and IFN-γ immunoreactivity. | [150] | ||
| Gallic acid | ICV Aβ1–42 induced AD (ICR) mice model | Y-maze and passive avoidance test; Brain tissue analysis | 3-weeks before ICV injection of Aβ | Anti-inflammatory and neuroprotective agent | Whole brain (homogenate), Cerebral cortex and hippocampus | Gallic acid pre-treatment:
| [151] |
| Oral AlCl3-induced AD (Wistar) rat model | Y-maze and MWM tests; Brain tissue analysis | Together with AlCl3 | Antioxidant and neuroprotective agent | Hippocampus | Gallic acid co-ingestion group showed:
| [152] | |
| APP: BACE [high] transgenic Drosophila AD-model | Brain homogenate analysis | 5 days before sacrifice | Antioxidant, anti-inflammatory, anti-AChE, and anti-BChE, and anti-amyloid agent | Whole brain (homogenate) | Gallic acid caused:
| [153] | |
| Intrahippocampal Aβ1–42 induced AD (Wistar) rat model | Electrophysiological analysis; Brain tissue analysis | The 2nd day after intrahippocampal injection | Anti-amyloid | Hippocampus (area CA1 and DG) | Improved amplitude and area under curve of LTP as recorded from DG in gallic acid-treated flies. Reduced burden of Aβ plaques in area CA1 in treated group. | [154] | |
| ICV-STZ induced AD (Wistar) rat model | Passive avoidance and MWM tests; Brain tissue analysis | 5 days before ICV-STZ injection | Antioxidant agent | Cerebral cortex and hippocampus | Pre-treatment with Gallic acid resulted in:
| [155] | |
| p-Coumaric acid (p-CA) | AlCl3-induced AD (Wistar) rat model | Passive avoidance test; Electrophysiological analysis; Histological analysis | 1-h prior to AlCl3-induction | Anti-amyloid and neuroprotective agent | Hippocampus | p-CA pretreatment resulted in:Improved memory retrieval in behavioral test. Mitigation of LTP impairment that is evident by increased amplitude and area under curve for the population spike, and the field excitatory postsynaptic potentials slope in electrophysiological recordings. Reduced burden of Aβ-plaques in DG. | [156] |
| OFT, elevated plus maze, MWM, and forced swimming tests; Brain tissue analysis | Antioxidant, anti-inflammatory, and neuroprotective agent | Cerebral cortex and hippocampus (histological studies on area CA1, CA3 and DG) | p-CA pre-treatment improved memory, decreased anxiety and depression-like behavior, and increased exploratory activity. p-CA increased SOD, GPx, and Catalase activities and decreased MDA NFκB, TNF-α, IL-1β, and IL-6 levels. Increased number of intact neurons. | [157] | |||
| Scopolamine-induced AD (Sprague-Dawley) rat model | Passive avoidance and MWM test Electrophysiological analysis | 1-h before scopolamine administration | Neuroprotective agent | Hippocampus (area CA1) | p-CA causes the following changes:
| [158] |
| Studied Model | Dosage | Duration of Exposure | Findings | Reference |
|---|---|---|---|---|
| Wistar rats induced with metabolic syndrome effects (MetS) by feeding high carbohydrate high fructose (HCHF) diet | 15 mL of Kelulut honey dissolved in 15 mL of distilled water given at 0.1 mL/kg of body weight daily | 35 days (after 16 weeks of HCFC diet) | Reduced anxiety compared with the MetS group Enhanced memory efficiency than both control and MetS groups Increased number of pyramidal cells in the hippocampus compared with the MetS group | [34] |
| Female Swiss albino mice (age = 2.5 months) | A total concentration of 750 mg/kg and 2000 mg/kg among groups—with 0.3 mL of Stingless bee honey dilution forced-fed daily | 7 days (acute) and 35 days (semichronic) | Improvement in learning and spatial reference memory | [211] |
| Stressed ovariectomized Sprague–Dawley rats (approximately 8 weeks old) | 0.2 g/kg body weight daily of Tualang honey diluted with 1ml of distilled water | 18 days (started 3 days before stress induction) | Decreased anxiety-like behavior in the stressed ovariectomized rats (StOE) that is comparable to β-estradiol (E2) treatment | [29] |
| Improved short-term and long-term memory in the StOE rats Increased pyramidal cells in CA2, CA3, and DG of hippocampus in the StOE rats Results were comparable to that after E2 treatment | [180] | |||
| Sprague–Dawley rats (approximately 2 months old) | Experimental diet of containing 100 g/kg honeydew honey that was available ad libitum | 3, 6, 9, and 12 months | Improved spatial memory and decreased anxiety-like behavior | [208] |
| Sprague–Dawley rats (2 months old) | 0.2 g/kg body weight Tualang honey dissolved in distilled water/daily | 35 days | Improved short-term and long-term memory Decreased depressive-like symptoms | [209] |
| Sprague–Dawley rats (16 months old) | 200 mg/kg body weight of Tualang honey/daily | 28 days (started 14 days prior to stress procedures) | Improvement in both short-term and long-term memory More Nissl-positive cells in the mPFC and hippocampus Greater number of pyramidal cells in the mPFC and hippocampus exhibiting normal shape and structure | [176] |
| Male Sprague–Dawley rats Young (2 months old), and aged (16 months old) | 200 mg/kg body weight of Tualang honey/daily | 28 days (started 14 days prior to stress procedures) | [177] | |
| Wistar rats | 0.5, 1.0, and 2.0 g/kg body weight | Singly dose (1 h before behavioral tests) | Honey, in a dose-dependent manner, ameliorates the anxiety-like behavior and possibly also acts as an anti-depressant | [212] |
| Swiss albino mice Young (3–4 months), aged (12–15 months) | The formulation containing honey (400 mL), ghee (800 mL) and gold (288 mg) was given at the dose of 30 mg/kg/daily | 15 days | The formulation intake improved learning and memory in young and aged mice Decreased activity of AChE in brain | [213] |
| Subjects | Type of Study | Dosage | Duration of Exposure | Findings | Reference |
|---|---|---|---|---|---|
| Mild cognitively impaired, and Cognitively intact controls (all over 65 years old) | Randomized, placebo control, double-blind | 1 tablespoon daily | 5 years—tested every 6 months | About 28% of the placebo-given subjects, while less than 7% of the honey-ingested subjects developed dementia. | [37] |
| Postmenopausal women (aged between 45 and 60 years) | Cohort | 20 g Tualang honey (sachet) daily | 16 weeks | Improved learning and memory scores in the auditory verbal learning test | [38] |
| Patients diagnosed with mood disorders and candidates for electroconvulsive therapy (ECT) (aged > 18 years) | Randomized, double-blind | 9 g of herbal combination of Crocus sativus, Cyperus rotundus, and honey/twice daily | 40 days (after initiation of ECT) | Improvement of ECT-induced memory improvement, especially after one to two months of the last ECT session. | [214] |
| Depressed elderly individuals (aged 60 or more) | Crossover randomized | 25 g Talbinah honey in 100 mL of water daily | 6 weeks (3 weeks + 1 week break + 3 weeks) | Improvement in depression, stress, and mood disturbances scores. | [215] |
| Patients diagnosed with mild to moderate major neurocognitive disorder | Randomized, double-blind | 10 g Asparagalus honey with 1000 mg of sedge and 60 mg of saffron extracts daily | 3 months | Improved attention, memory and cognition compared with the placebo-given group. | [210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, A.; Ahmad, F.; Teoh, S.L.; Kumar, J.; Yahaya, M.F. Honey and Alzheimer’s Disease—Current Understanding and Future Prospects. Antioxidants 2023, 12, 427. https://doi.org/10.3390/antiox12020427
Shaikh A, Ahmad F, Teoh SL, Kumar J, Yahaya MF. Honey and Alzheimer’s Disease—Current Understanding and Future Prospects. Antioxidants. 2023; 12(2):427. https://doi.org/10.3390/antiox12020427
Chicago/Turabian StyleShaikh, Ammara, Fairus Ahmad, Seong Lin Teoh, Jaya Kumar, and Mohamad Fairuz Yahaya. 2023. "Honey and Alzheimer’s Disease—Current Understanding and Future Prospects" Antioxidants 12, no. 2: 427. https://doi.org/10.3390/antiox12020427
APA StyleShaikh, A., Ahmad, F., Teoh, S. L., Kumar, J., & Yahaya, M. F. (2023). Honey and Alzheimer’s Disease—Current Understanding and Future Prospects. Antioxidants, 12(2), 427. https://doi.org/10.3390/antiox12020427










