Abstract
Ethanol consumption triggers oxidative stress by generating reactive oxygen species (ROS) through its metabolites. This process leads to steatosis and liver inflammation, which are critical for the development of alcoholic liver disease (ALD). Autophagy is a regulated dynamic process that sequesters damaged and excess cytoplasmic organelles for lysosomal degradation and may counteract the harmful effects of ROS-induced oxidative stress. These effects include hepatotoxicity, mitochondrial damage, steatosis, endoplasmic reticulum stress, inflammation, and iron overload. In liver diseases, particularly ALD, macroautophagy has been implicated as a protective mechanism in hepatocytes, although it does not appear to play the same role in stellate cells. Beyond the liver, autophagy may also mitigate the harmful effects of alcohol on other organs, thereby providing an additional layer of protection against ALD. This protective potential is further supported by studies showing that drugs that interact with autophagy, such as rapamycin, can prevent ALD development in animal models. This systematic review presents a comprehensive analysis of the literature, focusing on the role of autophagy in oxidative stress regulation, its involvement in organ–organ crosstalk relevant to ALD, and the potential of autophagy-targeting therapeutic strategies.
Keywords:
autophagy; oxidative stress; alcoholic liver disease; alcohol; ethanol; macroautophagy; mitophagy; redox; antioxidant; rapamycin 1. Introduction
Alcohol consumption causes multi-organ damage and is linked to a wide variety of diseases. In 2016, ethanol intake caused an estimated 3 million alcohol-related deaths and resulted in 132.6 million alcohol-related disability-adjusted life years worldwide [1]. Alcohol intake causes a wide spectrum of damage in the liver, ranging from steatosis to alcoholic hepatitis, cirrhosis, and hepatocellular carcinoma [2,3,4]. One-quarter of cirrhosis deaths and one-fifth of liver cancer deaths worldwide in 2019 were associated with alcohol toxicity [4]. Some of the mechanisms underlying this toxicity are: (1) ethanol metabolism due to acetaldehyde accumulation, (2) an increase in the nicotinamide adenine dinucleotide (NAD)H/NAD+ ratio, and/or (3) ROS generation [5]. Oxidative stress may be involved in functional and structural changes in mitochondria, leading to impaired oxidative phosphorylation, increased mitochondrial DNA (mtDNA) damage, and changes in mitochondrial protein profiles [6,7,8]. An increase in ROS levels leads to lipid peroxidation, as lipids accumulate in the liver during ethanol metabolism, which can escalate organ damage [9,10].
Simultaneously, oxidative stress can induce autophagy via different pathways. Autophagy is a dynamic, evolutionarily conserved process that aims to maintain cellular homeostasis, in which defective organelles, toxic proteins, and various other aggregates on the cytoplasmic surface are degraded and recycled in the effort to promote cell survival [11]. Ethanol-induced autophagy in the liver does not appear to be related to long-lived proteins; however, it does seem to be selective for damaged mitochondria and accumulated lipid droplets [12,13,14]. The stimulation and suppression of autophagy have been found to alleviate and exacerbate acute and chronic alcohol-related liver damage, respectively [15,16,17], paving the way for potential treatment development. In addition to affecting autophagic processes and oxidative stress in the liver, alcohol can dysregulate autophagy in several other organs, including the gut [18,19], adipose tissue [20], pancreas [21], brain [22], skeletal muscle [23], and heart [24]. Thus, organ–organ crosstalk—defined as complex, mutual, signaling factor-mediated communication between distant organs [25] such as the liver and the gut [26], adipose tissue [27,28], and brain [29]—has received increasing research interest. In this review, we summarize the current evidence available regarding the involvement of autophagic pathways in alcoholic liver disease (ALD) and their relationship with oxidative stress, as well as organ–organ crosstalk and treatment options ALD.
2. Materials and Methods
2.1. Search Strategy
We conducted a systematic review of publications on the mechanisms of oxidative stress and autophagy, as well as organ–organ crosstalk and related therapeutic strategies, in the context of ALD. Two investigators (CC and DS) independently performed the bibliographic search, and any divergence of opinion was resolved by consensus with the senior author (MM). We searched the PubMed, Web of Science, Scopus, and Embase electronic databases for publications up until 15 November 2022 using the following search terms alone and in combination as medical subject headings: “autophagy” or “p62” or “SQSTM1” or “autophagy-lysosome system” and “oxidative stress” or “redox” or “reactive oxygen species” or “cellular stress” or “antioxidants” and “alcoholic liver disease” or “alcoholic diseases” or “alcohol” or “ethanol”. No language restriction was applied to the initial search, although only the abstracts of articles published in languages other than English were considered. We manually scanned the reference lists of the retrieved publications to identify additional relevant articles. In addition, the search was supplemented by using the Medline “related articles” option and consulting review articles on the topic. Abstracts in meeting proceedings were not evaluated. Rayyan software (https://www.rayyan.ai/, accessed on 13 January 2023) was used to manage citations and identify and remove duplicates [30]. The protocol for the systematic review was registered with PROSPERO (ID: CRD42023435089).
2.2. Article Selection and Data Extraction
Published articles offering information on the mechanisms of oxidative stress and autophagy pathways in the presence of alcohol were selected. Studies with human samples, cell lines, and animal models were considered. Studies that did not directly evaluate the effects of alcohol, including autophagy, were excluded. Data recorded from each article included methodological details (cell type, murine model, or pharmacological drug used) and the molecular effects on autophagy and oxidative stress; these data were extracted independently by two authors for each dataset (DS, DPM, CC, and VJVR). The literature was summarized in tables, where appropriate.
3. Results and Literature Review
3.1. Studies Selected
Our initial search yielded 2127 articles, of which 996 were excluded and 200 were assessed for eligibility (Figure 1). In total, 78 articles were included in this systematic review. The literature regarding the roles of autophagy in hepatocytes and liver lysates, as well as in macrophages and hepatic stellate cells (HSCs) under both chronic and acute ethanol intake conditions, is comprehensively summarized in Table 1, Table 2, Table 3, Table 4 and Table 5. Acute ethanol models were defined as the exposure of cells for 24 h or less or intake of ethanol on one occasion or for less than 24 h. Studies focusing on the effects of specific therapeutic agents rather than describing the novel mechanisms involved in ethanol-induced autophagy are summarized in Table 6.
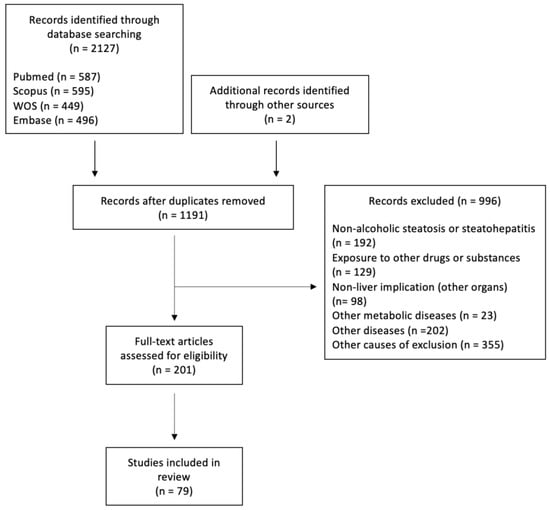
Figure 1.
Flow of study selection.

Table 1.
Studies included in our systematic review that focused on the effects of acute ethanol exposure on cell models.

Table 2.
Studies included in our systematic review that focused on the effects of chronic ethanol exposure on cell models.

Table 3.
Studies included in our systematic review focusing on the effects of acute ethanol intake and exposure on animal models.

Table 4.
Studies included in our systematic review that focused on the effects of chronic ethanol intake and exposure on animal models.

Table 5.
Studies included in our systematic review that focused on effects of ethanol intake or exposure in non-hepatocyte liver cells.

Table 6.
Potential therapeutic approaches for alcoholic liver disease by targeting autophagy pathways.
3.2. Ethanol, ALD, and Oxidative Stress
3.2.1. Ethanol Metabolism
Hepatocytes remove ethanol from the bloodstream via non-oxidative and oxidative metabolism. Ethanol is metabolized oxidatively via two major pathways: alcohol dehydrogenase (ADH) and cytochrome P450 enzymes (in this case, CYP2E1) [108]. These pathways produce ethanol metabolites, such as acetaldehyde and ROS, and deplete the stores of some antioxidants such as glutathione (GSH) [109,110]. The metabolites produced induce oxidative stress [including endoplasmic reticulum (ER) stress and mitochondrial damage], autophagy, and inflammation, which add to the hepatic inflammation caused by the action of bacterial lipopolysaccharide (LPS) [111,112,113,114].
The main alcohol metabolism pathway is initiated by ADH [113], an NAD+-requiring enzyme that is strongly expressed in hepatocytes and oxidizes ethanol to acetaldehyde in the cytosol [108], decreasing the NAD+/NADH ratio [115]. In normal liver, acetaldehyde is quickly metabolized to acetate by aldehyde dehydrogenase (ALDH). In chronic alcohol users, the ADH/ALDH pathway becomes saturated and generates reactive aldehydes and lipid hydroperoxides that can bind to DNA and proteins, producing adducts that further induce hepatocyte damage and inflammation [116,117]. The decrease in the NAD+/NADH ratio also promotes fat accumulation in the liver by reducing fatty acid oxidation (through peroxisome proliferator–activated receptor-α target genes) and enhancing fatty acid synthesis (by increasing the expression levels of lipogenic genes such as SREBP1c) [118,119,120], reducing sirtuin-1 (SIRT1)-related autophagy by decreasing transcription factor EB (TFEB) deacetylation [121], and increasing oxidative stress, as the re-oxidation of NADH to NAD+ in mitochondria requires ROS production [122].
The second major pathway centers around CYP2E1, a nicotinamide adenine dinucleotide phosphate (NADPH)-requiring enzyme, and gains relevance over the ADH pathway with chronic ethanol intake [113]. CYP2E1 metabolizes ethanol to acetaldehyde by converting NADPH and oxygen to NADP+ and water, resulting in the generation of ROS such as H2O2, hydroxyl (HO•−), and carbon-centered HO•− [123,124]. Through this process, CYP2E1 facilitates DNA and protein adduct formation, activates stress proteins, induces ER stress, and affects lysosomal function and autophagy, leading to mitochondrial damage, hepatocellular death, and hepatic carcinogenesis via oxidative DNA damage [113,125].
Nicotinamide N-methyltransferase (NNMT) is another important enzyme involved in liver metabolism and alcoholic liver disease [126,127]. NNMT is highly expressed in the human liver and plays a crucial role in maintaining NAD+ homeostasis [128]. It catalyzes the methylation of nicotinamide and similar compounds using the methyl donor S-adenosyl methionine (SAM-e) to produce S-adenosyl-L-homocysteine (SAH) and 1-methylnicotinamide. NNMT can affect the autophagic pathway [129,130] and may counteract oxidative stress in liver vessels due to its putative protective role in the endothelium [131]. Increased NNMT in the liver is associated with a better metabolic profile, including reduced serum triglyceride and free fatty acid levels [132]. In the liver, 1-methylnicotinamide produced by the NNMT degradation of nicotinamide increases sirtuin 1 (SIRT1) by inhibiting its degradation [132].
Thus, ROS generation through alcohol metabolism, with a reduction in the NAD+/NADH ratio and the activation of CYP2E1, is a major hallmark of alcohol-related oxidative stress. One of the best indicators of ROS overproduction is an increase in hepatic CYP2E1 levels. ROS-induced oxidative stress is closely related to protein modification, lipid peroxidation, mitochondrial damage, inflammation, iron overload, and antioxidant responses.
3.2.2. Protein Modifications
ROS induce reversible protein modifications, mainly at the level of the sulfur-containing residues cysteine and methionine, indicating a redox-based signal. Oxidative stress can cause changes in protein structure, localization, physical interactions, and post-translational modifications through the oxidative modification of reactive cysteines [133]. These changes can affect the immune response against neoantigens [134]. In liver sinusoidal endothelial cells, oxidative stress can also alter the proper functioning of fenestrae through spectrin oxidation [135]. For instance, oxidative stress reduces disulfide bond formation and causes the accumulation of unfolded proteins, triggering additional ER stress [136]. Cells counteract these protein modifications via three main signaling pathways: inositol-requiring transmembrane kinase/endoribonuclease 1 (IRE1), activating transcription factor 6, and protein kinase RNA-like ER kinase. Collectively, they form the unfolded protein response (UPR), which occurs mainly via the ubiquitin–proteasome pathway [15].
Alcohol consumption also causes the synthesis of reactive nitrogen species (RNS) in the liver [137]. The RNS NO causes S-nitrosylation in proteins [e.g., c-Jun N-terminal kinase 1 (JNK1) and inhibitor κB kinase β] and lysosomal enzymes (i.e., hexosaminidase B and cathepsin B), which impair hepatic autophagy, especially mitophagy (mitochondrial macroautophagy) [138,139].
3.2.3. Lipid Peroxidation and DNA Alteration
ROS can react with lipid species to promote lipid peroxidation, inducing apoptosis and ferroptosis [9,140], among other changes. Lipid peroxidation products can bind to DNA and enhance carcinogenesis by producing etheno-DNA adducts and mutations in oncogenes and onco-suppressor genes [10,141,142]. These adducts have been identified in the livers of patients with ALD [143]. In addition, ROS can directly modify DNA, thereby affecting cell viability. HO•− can directly attack the DNA backbone, mainly guanine, due to its low redox potential. The main products of its oxidation are 8-hydroxyguanine and 8-hydroxydeoxyguanosine, which are mutagenic and carcinogenic [144].
3.2.4. Mitochondrial Damage
ALD is characterized by structural and functional abnormalities in hepatic mitochondria, including enlargement [145], mtDNA damage [6], reduced adenosine triphosphate (ATP) levels [146], mitochondrial protein synthesis [147], increased ROS production [7,8], and alterations in mitochondrial membrane permeability and mitochondrial permeability transition, resulting in intrinsic and extrinsic apoptosis [124]. In intrinsic apoptosis, cellular death occurs via the release of cytochrome C and other pro-apoptotic factors that interact with apoptotic protease activating factor 1 and caspase-9 [148]. In the extrinsic pathway, cell death is triggered by the ROS-induced release of apoptosis signal-regulating kinase 1, a member of the mitogen-activated protein kinase (MAPK) family, resulting in the cleavage of pro-caspase-3 to active caspase-3 [149,150]. Mitochondrial permeability transition has been found to activate caspase-3 in hepatocytes in a p38 MAPK–dependent manner [151].
ROS also harm mtDNA integrity by affecting mtDNA-coded proteins and RNA transcription, which in turn regulates the mitochondrial respiratory chain. A vicious cycle is established in which mitochondria with oxidized mtDNA and limited repair mechanisms [152] become dysfunctional and produce abundant ROS, leading to further mitochondrial impairment. This loop can ultimately result in severe nuclear DNA damage and cell death [153]. Mitophagy degrades mitochondria with damaged DNA [154] and enhances longevity in rodent models [155]. However, the autophagy rate declines with age and chronic alcohol intake [53], promoting the accumulation of mtDNA mutations and a decline in mitochondrial function [156].
3.2.5. Inflammation
ROS also play a key role in the development of ethanol-induced inflammation. The depletion of mitochondrial GSH by CYP2E1 activation [157,158] is one factor that links inflammation with oxidative stress as it impairs hepatocyte tolerance to tumor necrosis factor-α (TNF-α) [115]. TNF-α exacerbates oxidative damage and inflammation and stimulates MAPK activation [159], resulting in ROS accumulation through superoxide (O2•−) generation. This process causes oxidative damage and eventual TNF-α production, perpetuating the cycle [150,160]. The overexpression of CYP2E1 due to ethanol-induced oxidative stress also induces inflammation via the Notch1 pathway [161], and ROS mediate interleukin (IL)-1β and IL-18 signaling via inflammasome NLRP3 activation [162,163].
Nicotinamide adenine dinucleotide phosphate oxidases (NOXs) are also important sources of inflammation-related ROS generation, as they generate O2•− from oxygen using NAD(P)H. In mice, chronic alcohol consumption increased NOX4 expression in the mitochondrial fraction and NOX4 inhibition ameliorated alcohol-induced liver damage [164]. Upon ethanol administration, NOX-derived ROS are key mediators of nuclear factor-κB activation and subsequent TNF-α production [165], sensitizing Kupffer cells to LPS, thereby contributing to ALD [166].
3.2.6. Iron Overload
Metals such as zinc and iron are involved in ROS-induced oxidative stress caused by ethanol intake. Hepatic iron overload has been observed in approximately 50% of patients with ALD [167]. It causes cellular damage and ferroptosis, which may contribute to ROS-associated alcohol toxicity through Fenton reactions [168] and cause lipid peroxidation and subsequent cell membrane damage and rupture, thereby promoting the autophagy-dependent release of damage-associated molecular patterns [169].
3.2.7. Protective Mechanisms and the Antioxidant Response
Several mechanisms, including the involvement of antioxidants, counteract the harmful effects of ethanol-induced oxidative stress. The antioxidant enzymes superoxide dismutase (SOD), catalases, and GSH peroxidases, in concert with other proteins, are responsible for ROS removal and restoring the reduced protein and lipid pool [170]. Ethanol inhibits the expression of antioxidant enzymes (e.g., SOD1) and depletes the levels of non-enzyme antioxidants (e.g., GSH), thereby reducing the cellular ability to modulate oxidative stress [171,172]. ROS weaken this antioxidant response by S-glutathionylation, which reduces the expression of downstream antioxidants, such as SOD2, catalases, and sestrin 3 via the phosphoinositide 3 kinase (PI3K)/AKT pathway [173]. This process also increases mitochondrial generation of H2O2, facilitating further oxidative damage [174].
3.3. Autophagy and ALD
The three main types of autophagy which have different means of cargo delivery to the lysosome are macroautophagy, microautophagy, and chaperone-mediated autophagy [15,175]. In macroautophagy, cytosolic materials are sequestered by autophagosomes and then fused with lysosomes for degradation. In microautophagy, cytoplasmic cargo is engulfed directly into the lysosomes [176]. Chaperone-mediated autophagy involves the direct shuttling of specific proteins across the lysosomal membrane for lumen degradation [177].
Macroautophagy can be classified according to the type of organelle or structure that is selectively degraded as mitophagy (mitochondria), lipophagy (lipid droplets) [178,179,180], ribophagy (ribosomes), ER-phagy (ER), and pexophagy (peroxisomes) [181], among others. This selective form of autophagy is made possible by adaptor proteins such as p62 and specific receptors [182].
3.3.1. The Macroautophagy Pathway
Several multi-molecular complexes contribute to autophagosome formation in macroautophagy: (1) the uncoordinated-51-like kinase (ULK) and Beclin-1 complexes, (2) the antithymocyte globulin (Atg)9–Atg2–Atg18 complex, and (3) the Atg5–Atg12–Atg16 and Atg7–Atg3–Atg8/microtubule-associated protein light chain 3 (LC3) conjugation systems [15,16,17]. The initiation of the macroautophagic pathway involves assembly-site phagophore formation, which is regulated by the ULK complex (composed of ULK), the focal adhesion kinase family-interacting protein of 200 kDa, and Atg13 [183]. This complex is activated by adenosine monophosphate-activated protein kinase (AMPK) and inhibited by the mammalian target of rapamycin complex 1 (mTORC1). In turn, mTORC1 is inhibited by tuberous sclerosis complex 1/2 activation or p27 phosphorylation [184,185,186]. After ULK complex formation, Beclin-1 (Atg6) is recruited by the ER-resident soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein syntaxin 17 (STX17) [187] and phosphorylated by ULK1 to initiate the formation of its complex, which is composed of Beclin-1, Atg14L, p150, and Vps34 (class III PI3K subunits) [188,189]. This complex produces phosphatidylinositol-3-phosphate [190], which recruits Atg18 (WD repeat domain phosphoinositide-interacting protein 1/2 in mammals) [191]. The Atg9–Atg2–Atg18 complex acts as a cycling system and contributes to phagophore expansion and elongation via membrane addition [192]. Then, phagophore spread continues via the ubiquitin-like Atg5–Atg12–Atg16 and Atg-7–Atg3–Atg8/LC3 complexes, which are required for the generation of membrane-associated LC3-II and the stabilization of the nascent autophagosome membrane [193]. Nascent LC3 is first processed by the protease Atg4 and cytosol-resident LC3-I, which is activated by Atg7 and transferred to Atg3. Finally, the Atg12–Atg5–Atg16 complex conjugates LC3-I with phosphatidylethanolamine to form LC3-II [194,195], which, together with p62 and other cargo receptors, is indispensable for the selectivity of autophagy [196]. Eventually, the expanding membrane closes around its cargo to form a complete autophagosome, which may require charged multivesicular body protein 2A (an endosomal sorting complex required for transport III subunit), vacuolar protein sorting-associated protein (VPS) 4 (an AAA ATPase), and VPS37A [197,198].
Autophagosomes migrate to the lysosomes and fuse with them to form autolysosomes for cargo degradation. The cytoskeleton and proteins such as STX17, Rab7, lysosomal-associated membrane protein (LAMP)1/2, [20], synaptosomal-associated protein 29 (SNAP29), and vesicle-associated membrane protein 8 (VAMP8) [199,200] seem to be involved in this migration. The deacetylation of STX17 by the inactivation of the cyclic adenosine monophosphate response element-binding protein promotes the formation of the STX17–SNAP29–VAMP8 SNARE complex, leading to autolysosome formation [199,200]. LAMP2 appears to be key to proper STX17 function [201]. Another key process for autophagy regulation is lysosomal biogenesis, which appears to be regulated by TFEB [202].
3.3.2. Mitophagy
Mitophagy is a selective form of macroautophagy that mediates mitochondrial degradation in the autolysosomes. In type-1 mitophagy, small (0.2–0.3-μm) pre-autophagosomal structures grow into cup-shaped phagophores that envelop and sequester mitochondria into mitophagosomes, often in coordination with mitochondrial fission. In type 2 mitophagy, cup-shaped phagophores are not formed; rather, LC3 aggregates sequester individual mitochondria into mitophagosomes. In both types of mitophagy, mitophagosomes form, acidify, fuse with lysosomes, and degrade their contents [154,156]. While type 1 is primarily related to physiological mechanisms, such as nutrient deprivation, type 2 is related to (and activated by) sensors of mitochondrial damage, such as those caused by oxidative stress [203]. The main oxidative-stress-dependent pathway is that of phosphatase and the tensin homolog-induced putative kinase 1 (PINK1)/Parkin/p62 [203,204,205].
PINK1 is a serine/threonine kinase that translocates to the outer mitochondrial membrane, where it is stabilized by a low mitochondrial transmembrane potential and senses mitochondrial depolarization [206]. It recruits and phosphorylates Parkin (a ubiquitin E3 ligase) [207], which ubiquitylates several proteins on the outer mitochondrial membrane, including voltage-dependent anion channels. p62 recognizes ubiquitylated proteins [204] which trigger their degradation through the lysosome pathway via autophagy via LC3-II interaction [208,209].
3.3.3. Acute Alcohol Intake and Autophagy in Hepatocytes
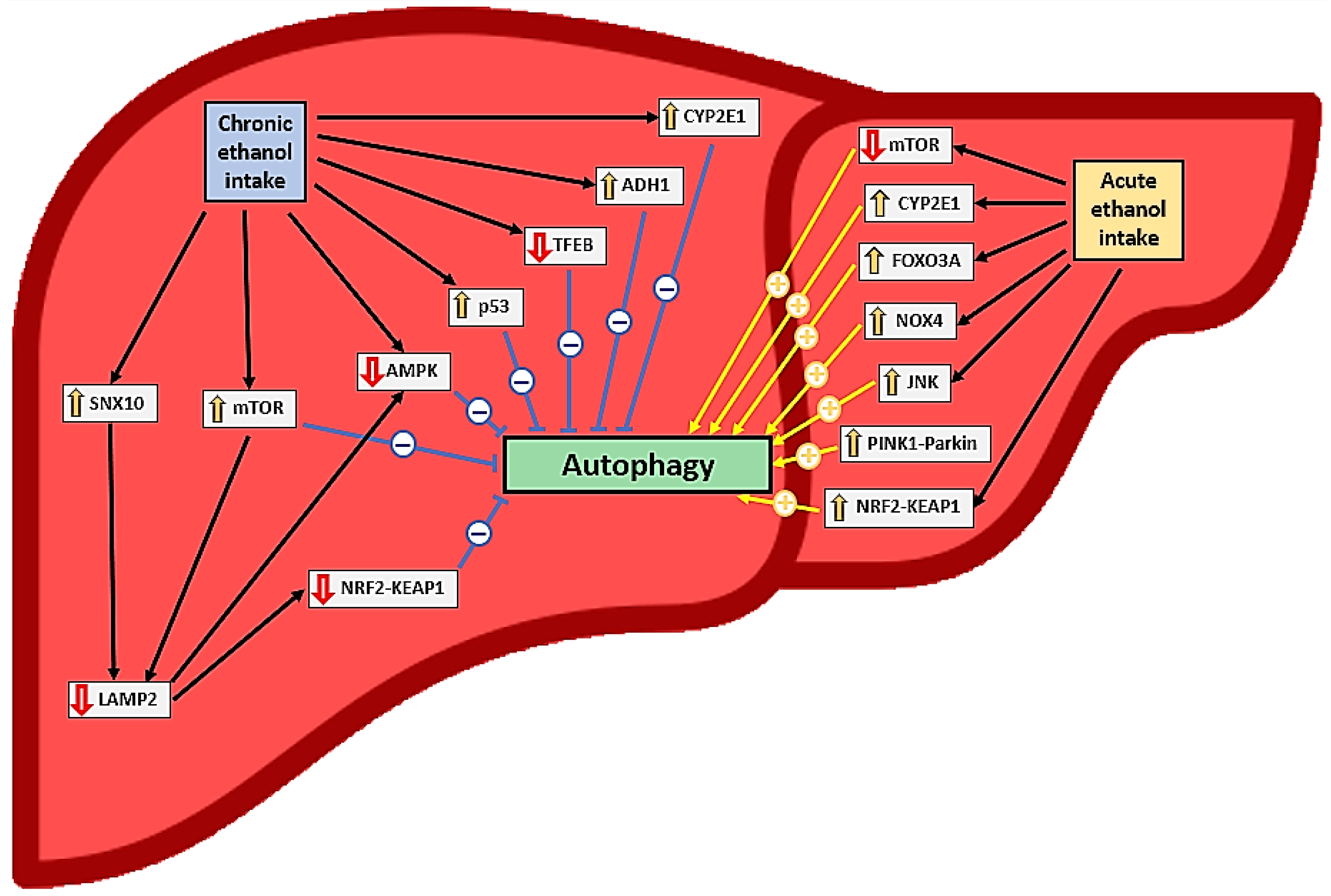
Current evidence clearly indicates that acute alcohol intake and exposure activate autophagy in hepatocytes in vivo and in vitro, respectively [12,32,33,37,38,42,44,46,47,49] (Table 1 and Table 3, Figure 2); a reduction in autophagy after ethanol intake has been documented in only one study [48]. This activation depends on ethanol oxidation, ROS generation [37], mTOR [38], and proteasome inhibition [49] but not on acetaldehyde [32]. In this context, the induction of autophagy is probably more closely related to CYP2E1 pathway activation than to NOX4 [32], JNK [36], and forkhead box O3a (FOXO3A) [45,46] activation (Figure 2). Autophagy in hepatocytes appears to play a protective role against oxidative stress, steatosis, and inflammation caused by acute alcohol consumption [34,36,38,47]. Antioxidants may help reduce oxidative stress, but they may also indirectly reduce autophagy induction [32], mitigating its protective role.
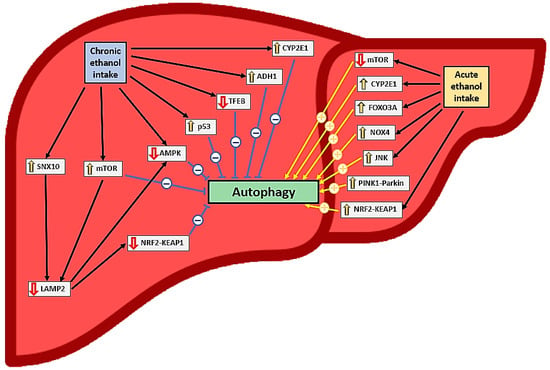
Figure 2.
Effects of ethanol consumption on liver autophagy and relationships to oxidative stress. The symbol “↑” represents an increase in expression or activation while the symbol “↓“ represents a decrease in expression or inactivation.
The nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant response to acute alcohol exposure in hepatocytes is controversial; it may induce p62-dependent autophagy, which may have a protective role by increasing chaperone-mediated autophagy [35]. However, some researchers have found that this activation may worsen the negative effects of alcohol ingestion by activating ferroptosis [33] (Figure 2). Alcohol exposure also activated type 2 mitophagy in hepatocytes through the PINK1-Parkin-LC3 pathway and mitochondrial depolarization [12,42] (Table 1 and Table 3, Figure 2).
3.3.4. Acute Alcohol Intake and Autophagy in Other Cell Types
No study included in our systematic review evaluated autophagy in Kupffer cells (KCs) after acute ethanol intake or exposure. A model in which HSCs were acutely exposed to ethanol revealed increased autophagy, oxidative stress, and cellular activation, with the former regulating the latter two processes via the Nrf2–Keap1–antioxidant response element pathway [62]. The inhibition of autophagy reversed HSC activation and suppressed oxidative stress [62] (Table 5).
3.3.5. Chronic Ethanol Intake and Autophagy in Hepatocytes
However, the effect of chronic ethanol intake on autophagy in hepatocytes remains unclear. Some studies have shown that chronic ethanol intake or exposure activates autophagy in these cells [47,52,58] and has a protective role similar to acute intake [47]. In these studies, CYP2E1 expression or the reduction of proteasome activity appeared to induce autophagy (e.g., through an increase in LC3 level) [41,49]. Babuta et al. [52] observed decreased LAMP1/2 and lysosomal marker expression, despite increased autophagy [with reduced mTOR and Ras homolog enriched in brain (Rheb) and increased LC3-II expression] in mouse hepatocytes after chronic ethanol intake.
In contrast, chronic alcohol consumption reduced autophagy in hepatocytes in several studies [31,35,39,40,40,50,51,52,53,54,55,56,57,59] (Table 2 and Table 4, Figure 2). This effect was accompanied by decreased levels of LC3 [39,40,59], Beclin-1 [59], Atg5 [54], Atg7 [57], Atg3, Atg12 [59], TFEB [55], AMPK [59], and LAMP1/2 [50,51,52] and increased p62 levels [40,54,57,59], mTOR pathway activation [50,55], and iron overload [59]. This reduction in autophagy has negative effects (e.g., as hepatotoxicity, steatosis, and oxidative stress) and appears to be related mainly to CYP2E1 [40,57] and ADH1 [39] activation. Ethanol, acetaldehyde, and hepatic free fatty acids may reduce autophagy in hepatocytes after chronic ethanol treatment [39,50]. This treatment also appears to induce ER stress [50,51,59].
The inhibition of chronic intake exposure-induced autophagy in hepatocytes and the development of steatosis, inflammation, and oxidative stress can be attenuated by ALDH2 expression [39], CYP2E1 inhibition [40], and LAMP2A overexpression [50,51]. LAMP2A plays a protective role by activating the Nrf2 and AMPK pathways by increasing chaperone-mediated autophagy [35]. LAMP2 suppression, which is probably mTORC1-dependent, has been observed in patients with severe alcoholic hepatitis [50] (Table 2 and Table 4, Figure 2). Mitophagy in hepatocytes is suppressed after chronic ethanol exposure, probably due to the upregulation of the DNA-dependent protein kinase catalytic subunit and p53 activation [53]. In this context, AMPK has been shown to enhance mitophagy via the Nrf2–ubiquinol–cytochrome C reductase core protein 2 pathway [31].
Mice lacking proteins required for LC3, Atg5, and Atg7 lipidation show different vulnerabilities to acute and chronic alcohol exposure. For instance, Atg5-knockout (KO) mice are more susceptible to liver damage with acute alcohol treatment, whereas Atg7-KO mice are more sensitive to liver damage after chronic plus binge ethanol intake [43]. Atg5 inhibition appears to improve chronic ethanol consumption-induced liver damage [43]. Similarly, CYP2E1 activation is associated with increased autophagy after acute ethanol intake [43] but reduced autophagy after chronic ethanol intake (Figure 2). The mechanisms involved in these differences are not adequately understood [43].
Nevertheless, autophagy activation seems to protect against liver damage induced by chronic and acute ethanol exposure [43] as well as increased apoptosis [33,54,59] (Table 2 and Table 4, Figure 2). In addition, selective autophagic pathways, such as mitophagy, which allows hepatocytes to adapt to chronic ethanol exposure, may improve hepatocyte biology [31,44,53] (Table 2 and Table 4).
3.3.6. Chronic Ethanol Intake and Autophagy in Other Cell Types
In KCs exposed chronically to alcohol, autophagy seems to have a protective role associated with decreased Myeloid differentiation factor 2/Toll-like receptor 4 expression [56] and the mediation of the anti-inflammatory and anti-steatogenic effects of the cannabinoid 2 receptor [63]. The inhibition of autophagy in macrophages after chronic ethanol treatment appeared to increase ethanol-induced liver damage, inflammation, ROS generation, interferon regulatory factor 1 accumulation, and the induction of hepatic C-C motif chemokine ligand 5 and C-X-C motif chemokine ligand 10 expression [60,61] (Table 5).
HSCs from ethanol-fed mice had an increased UPR, which triggered autophagy and induced an Nrf2-mediated antioxidant response under ER stress conditions [146]. The IRE1α pathway blockade significantly reduced autophagy activation in a p38 MAPK-dependent manner, thereby reducing the fibrogenic response [64] (Table 5).
3.4. Alcohol-Induced Organ–Organ Crosstalk and Autophagy
Information on the autophagic pathways involved in organ–organ crosstalk in ALD is scarcely available, and the studies that have been performed have focused mainly on the crosstalk between adipose and liver tissue. Rodriguez et al. [210] showed that the ablation of the mTOR (Raptor) pathway in adipocytes (but not hepatocytes) contributed to acute alcohol treatment-induced liver damage with increased inflammation, suggesting the implication of adipose tissue in the development of alcoholic steatohepatitis. Consistently, Li et al. [153] reported exacerbated alcohol-induced hepatic steatosis in adipocyte-specific Raptor–KO mice. Adipocyte-specific Atg5–KO mice had increased circulating levels of fibroblast growth factor 21 (FGF21) and adiponectin and were resistant to chronic alcohol treatment-induced adipose tissue atrophy and liver damage [211]. Although this area of research, particularly in the context of ALD, is relatively new, current evidence suggests that autophagy modulates the crosstalk between adipose tissue and the liver by controlling the synthesis of FGF21 and adiponectin. Such autophagy-mediated crosstalk has been detected in other liver diseases, such as non-alcoholic fatty liver disease, in which autophagy inhibition in white adipose tissue after four months of a high-fat diet ameliorated liver pathology in mice [212].
3.5. Autophagy-Targeting Treatments for ALD
Effective therapies for ALD are lacking, and given its important role in this disease, autophagy is a potential therapeutic target. The roles of several pharmacological agents in the prevention or amelioration of ALD through autophagic modulation have been examined (Table 6). Most of these studies have been performed using mouse models, although other animal models (i.e., rat and zebrafish) have also been used. The mTOR pathway is one of the most relevant potential therapeutic targets in this context. The activation of mTOR and AMPK signaling is involved in ethanol-induced autophagy under oxidative stress [14,213]. Rapamycin (also known as sirolimus) is a lipophilic macrolide antibiotic that was first isolated from Streptomyces hygroscopicus and has been shown to inhibit mTORC1, thereby reversing alcohol-induced mTOR activation and attenuating related liver damage [15,40,51]. Torin 1 is a selective ATP-competitive small molecule that inhibits the mTOR pathway through direct inhibition of the mTORC1 and mTORC2 complexes [55,105], and it may also ameliorate ALD. Upstream of mTOR, the pathway can be modulated by the inhibition of AMPK. The activation of AMPK inhibits mTOR-dependent signaling through different molecules, such as calcitriol (the active form of vitamin D) [69] and palmatine (a protoberberine alkaloid found in several plants) [87]. Although no clinical trials have been performed to study the effects of these drugs on ALD, studies are being undertaken to examine their effects on Sjögren syndrome (ClinicalTrials.gov identifier: NCT05605665) and Alzheimer’s disease (ClinicalTrials.gov identifier: NCT04629495). The possibility of rapamycin delivery via nanoparticles, which may reduce side effects and have been shown to ameliorate metabolic fatty liver disease in a mouse model [214], adds to the interest in testing the effect of this drug on ALD.
Ethanol intake activates FOXO3a, which transcriptionally regulates several autophagy genes [46]. The SIRT1/FOXO pathway is involved in the alleviation of chronic alcoholic liver damage by preventing fat accumulation and reducing ROS production, inflammation, and cell death [215]. Modulators of autophagy through this pathway, such as resveratrol, quercetin, and salvianolic acid A [46,90,91,92,93,94,96,97,98], have shown promise for the treatment of ALD in animal models. We are not aware of a clinical trial examining the effects of these products, but potential interest due to the promising experimental results and favorable side effect profiles, particularly with resveratrol, is attenuated by the lack of efficacy against non-alcoholic fatty liver disease [216].
Alcohol-induced autophagy can also be suppressed by antioxidants such as N-acetylcysteine (NAC) [38,40]. NAC and the CYP2E1 inhibitor chlormethiazole [40,72,73] appear to attenuate the toxic effects of ethanol in the liver [40]. NAC has been tested in a clinical trial conducted in patients with alcoholic hepatitis; although infections were less frequent and 1-month mortality was reduced in the prednisolone-NAC group relative to the prednisolone-only group, other side effects and the 6-month survival rate were similar across the two groups [217]. Another clinical trial examining the efficacy of NAC against alcoholic hepatitis is ongoing (ClinicalTrials.gov identifier: NCT03069300), given that the body of evidence provides a rationale for the use of this drug in patients with ALD and/or alcohol use disorders [218].
Other products, such as zinc and carbamazepine, appear to be involved in autophagy activation after ethanol intake or exposure; thus, they could be tested as potential treatments for ALD. Interestingly, zinc exposure stimulates autophagy, with an additive effect of co-stimulation with ethanol for 24 h [107]. Carbamazepine, a mood-stabilizing drug, induces autophagy by reducing the level of intracellular inositol [47]. The administration of Nrf2 activators, such as sulforaphane [102] from vegetables of the genus Brassica and glycycoumarin from Glycyrrhiza uralensis [81], is also of potential interest in the treatment of this disease. Data from animal models suggest that these agents improve alcohol-induced liver steatosis and oxidative stress and promote autophagy (Table 6). The activation of the Nrf2 pathway appears to protect against alcohol-induced liver fibrosis and hepatotoxicity, whereas Nrf2 knockdown is associated with increased alcohol-induced hepatocyte damage [81,219,220].
4. Discussion
Acute ethanol consumption increases autophagy activation in the liver, whereas chronic alcohol intake or exposure decreases autophagy activation. The mechanisms underlying this difference have not yet been fully elucidated. Increased autophagy in hepatocytes and KCs seems to have a protective role against the pathogenesis of alcohol-related liver damage and ALD, and the inhibition of autophagy makes hepatocytes susceptible to hepatotoxicity, steatosis, and oxidative stress. Autophagy leads to HSC activation and oxidative stress in these cells.
Most studies in this field have examined the classical components of autophagic pathways (e.g., p62, LC3, and Beclin 1) in liver cells; expanding the scope of research to examine other components is desirable. For instance, autolysosome formation, a key step in autophagy, has not been analyzed in detail in the context of ALD. In addition, autophagy in different organs and tissues, especially in cells of the immune system, needs to be assessed because of the involvement of these cells and tissues in inflammation and oxidative stress, which play key roles in ALD development. Selective autophagy pathways such as mitophagy and lipophagy should also be analyzed further. Autophagy-related organ–organ crosstalk in ALD has received little research attention despite the growing interest in and potential of this field. Regarding clinical applications, autophagy-targeting therapies have not been tested successfully in clinical trials, although data from animal and in vitro models suggest potential roles for several drugs, including those involved in the mTOR pathway and antioxidants that modulate autophagy. Although a large number of drugs could potentially be useful in ALD treatment, based on basic research, the potential difficulties of carrying out large clinical trials, particularly those not funded by drug companies, could prove difficult to navigate, in spite of the fact that autophagy targeting may have protective effects against several liver diseases [47]. We hope to see expansion in this area in the near future.
It is important to acknowledge that the variations observed in the definition of acute or chronic ethanol intake or exposure, as well as the diverse experimental protocols employed across the included studies, have posed challenges in clearly elucidating the underlying mechanisms associated with acute and chronic ethanol exposure and have prevented us from employing a meta-analysis or other numerical approaches to demonstrate the extent of variability (e.g., classic coefficient of variation or robust coefficient of variation estimators [221].
5. Conclusions
In conclusion, autophagy activation in hepatocytes and KCs appears to have a protective role against alcohol-induced liver damage and oxidative stress. However, further research is needed to elucidate these mechanisms and develop potential clinical applications.
Author Contributions
M.M. conceived this project; M.M., D.S.-G. and C.C. designed the study; C.C., D.P.-M., V.-J.V.-R. and D.S.-G. searched the literature data; D.S.-G. and C.C. extracted and interpreted the data; D.S.-G., C.C. and M.M. drafted the manuscript; D.P.-M., V.-J.V.-R., M.G.-M. and A.B.H. critically revised the manuscript for important intellectual content; D.S.-G. and C.C. have equally contributed as the primary authors to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Instituto de Salud Carlos III (ISCIII) and co-funded by The European Union through the project PI20/00743 and INT21/00065 (awarded to M.M.) and by Junta de Castilla y León, Spain, through projects GRS 2388/A/21 and GRS 2648/A/22 to M.M. Other funding sources include Institute of Biomedical Research of Salamanca (IBSAL) grant IBI19/00013 (awarded to M.M.) and Sociedad Castellano-Leonesa-Cántabra de Medicina Interna (SOCALMI) 2022 grant (awarded to M.M.). D. Salete-Granado holds a PFIS contract funded by Instituto de Salud Carlos III (ISCIII) through the project FI21/00189 and co-funded by The European Union. Cristina Carbonell holds a Río Hortega contract funded by Instituto de Salud Carlos III (ISCIII) through the project CM22/00033 and co-funded by The European Union—Next GenerationEU.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- WHO. Global Status Report on Alcohol and Health 2018; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Zakhari, S.; Li, T.-K. Determinants of Alcohol Use and Abuse: Impact of Quantity and Frequency Patterns on Liver Disease. Hepatology 2007, 46, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Bataller, R. Trends in the Management and Burden of Alcoholic Liver Disease. J. Hepatol. 2015, 62, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global Epidemiology of Alcohol-Associated Cirrhosis and HCC: Trends, Projections and Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. [Google Scholar] [CrossRef]
- Williams, J.A.; Manley, S.; Ding, W.-X. New Advances in Molecular Mechanisms and Emerging Therapeutic Targets in Alcoholic Liver Diseases. World J. Gastroenterol. 2014, 20, 12908–12933. [Google Scholar] [CrossRef]
- Venkatraman, A.; Landar, A.; Davis, A.J.; Chamlee, L.; Sanderson, T.; Kim, H.; Page, G.; Pompilius, M.; Ballinger, S.; Darley-Usmar, V.; et al. Modification of the Mitochondrial Proteome in Response to the Stress of Ethanol-Dependent Hepatotoxicity. J. Biol. Chem. 2004, 279, 22092–22101. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Alcohol-Induced Oxidative Stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and Mitochondrial Effects of Alcohol Consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Mueller, S.; Peccerella, T.; Qin, H.; Glassen, K.; Waldherr, R.; Flechtenmacher, C.; Straub, B.K.; Millonig, G.; Stickel, F.; Bruckner, T.; et al. Carcinogenic Etheno DNA Adducts in Alcoholic Liver Disease: Correlation with Cytochrome P-4502E1 and Fibrosis. Alcohol. Clin. Exp. Res. 2018, 42, 252–259. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Cho, G.-W. Autophagy: An Evolutionarily Conserved Process in the Maintenance of Stem Cells and Aging. Cell Biochem. Funct. 2019, 37, 452–458. [Google Scholar] [CrossRef]
- Eid, N.; Ito, Y.; Horibe, A.; Otsuki, Y. Ethanol-Induced Mitophagy in Liver Is Associated with Activation of the PINK1-Parkin Pathway Triggered by Oxidative DNA Damage. Histol. Histopathol. 2016, 31, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Rasineni, K.; Donohue, T.M.; Thomes, P.G.; Yang, L.; Tuma, D.J.; McNiven, M.A.; Casey, C.A. Ethanol-induced Steatosis Involves Impairment of Lipophagy, Associated with Reduced Dynamin2 Activity. Hepatol. Commun. 2017, 1, 501–512. [Google Scholar] [CrossRef]
- Ding, W.-X.; Li, M.; Yin, X.-M. Selective Taste of Ethanol-Induced Autophagy for Mitochondria and Lipid Droplets. Autophagy 2011, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Khambu, B.; Wang, L.; Zhang, H.; Yin, X.-M. The Activation and Function of Autophagy in Alcoholic Liver Disease. Curr. Mol. Pharmacol. 2017, 10, 165–171. [Google Scholar] [CrossRef]
- Chao, X.; Ding, W.-X. Role and Mechanisms of Autophagy in Alcohol-Induced Liver Injury. Adv. Pharmacol. 2019, 85, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Ding, W.-X. Role of Autophagy in Alcohol and Drug-Induced Liver Injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 136, 111075. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, C.; Zhao, W.; He, C.; Ding, J.; Dai, R.; Xu, K.; Xiao, L.; Luo, L.; Liu, S.; et al. Impaired Autophagy in Intestinal Epithelial Cells Alters Gut Microbiota and Host Immune Responses. Appl. Environ. Microbiol. 2018, 84, e00880-18. [Google Scholar] [CrossRef]
- Foerster, E.G.; Mukherjee, T.; Cabral-Fernandes, L.; Rocha, J.D.B.; Girardin, S.E.; Philpott, D.J. How Autophagy Controls the Intestinal Epithelial Barrier. Autophagy 2022, 18, 86–103. [Google Scholar] [CrossRef]
- Li, Y.; Ding, W.-X. Adipose Tissue Autophagy and Homeostasis in Alcohol-Induced Liver Injury. Liver Res. 2017, 1, 54–62. [Google Scholar] [CrossRef]
- Rasineni, K.; Srinivasan, M.P.; Balamurugan, A.N.; Kaphalia, B.S.; Wang, S.; Ding, W.-X.; Pandol, S.J.; Lugea, A.; Simon, L.; Molina, P.E.; et al. Recent Advances in Understanding the Complexity of Alcohol-Induced Pancreatic Dysfunction and Pancreatitis Development. Biomolecules 2020, 10, 669. [Google Scholar] [CrossRef]
- Yang, F.; Luo, J. Endoplasmic Reticulum Stress and Ethanol Neurotoxicity. Biomolecules 2015, 5, 2538–2553. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Qin, X.; Chen, S.; Ceylan, A.F.; Dong, M.; Lin, Z.; Ren, J. Parkin Deficiency Accentuates Chronic Alcohol Intake-Induced Tissue Injury and Autophagy Defects in Brain, Liver and Skeletal Muscle. Acta Biochim. Biophys. Sin. 2020, 52, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Ni, H.-M.; Huang, H.; Ding, W.-X. Autophagy in Alcohol-Induced Multiorgan Injury: Mechanisms and Potential Therapeutic Targets. BioMed Res. Int. 2014, 2014, 498491. [Google Scholar] [CrossRef] [PubMed]
- Armutcu, F. Organ Crosstalk: The Potent Roles of Inflammation and Fibrotic Changes in the Course of Organ Interactions. Inflamm. Res. 2019, 68, 825–839. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Chiang, J.Y.L. Bile Acid Receptors and Signaling Crosstalk in the Liver, Gut and Brain. Liver Res. 2021, 5, 105–118. [Google Scholar] [CrossRef]
- Poole, L.G.; Dolin, C.E.; Arteel, G.E. Organ-Organ Crosstalk and Alcoholic Liver Disease. Biomolecules 2017, 7, 62. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Garcia-Macia, M.; Sahu, S.; Athonvarangkul, D.; Liebling, E.; Merlo, P.; Cecconi, F.; Schwartz, G.J.; Singh, R. Autophagy in the CNS and Periphery Coordinate Lipophagy and Lipolysis in the Brown Adipose Tissue and Liver. Cell Metab. 2016, 23, 113–127. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. Available online: https://www.rayyan.ai/ (accessed on 13 January 2023). [CrossRef]
- Lu, X.; Xuan, W.; Li, J.; Yao, H.; Huang, C.; Li, J. AMPK Protects against Alcohol-Induced Liver Injury through UQCRC2 to up-Regulate Mitophagy. Autophagy 2021, 17, 3622–3643. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Yu, L.; Mueller, J.; Fortunato, F.; Rausch, V.; Mueller, S. H2O2-Mediated Autophagy during Ethanol Metabolism. Redox Biol. 2021, 46, 102081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, J.; Mao, A.; Zhang, R.; Guan, S. Autophagy Inhibition Plays a Protective Role in Ferroptosis Induced by Alcohol via the P62–Keap1–Nrf2 Pathway. J. Agric. Food Chem. 2021, 69, 9671–9683. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, P.; Li, W.; Wu, Y.; An, W. Augmenter of Liver Regeneration Protects against Ethanol-Induced Acute Liver Injury by Promoting Autophagy. Am. J. Pathol. 2019, 189, 552–567. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Li, W.-Z.; Zhang, S.; Hu, B.; Li, Y.-X.; Li, H.-D.; Tang, H.-H.; Li, Q.-W.; Guan, Y.-Y.; Liu, L.-X.; et al. SNX10 Mediates Alcohol-Induced Liver Injury and Steatosis by Regulating the Activation of Chaperone-Mediated Autophagy. J. Hepatol. 2018, 69, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Mahli, A.; Thasler, W.E.; Patsenker, E.; Müller, S.; Stickel, F.; Müller, M.; Seitz, H.K.; Cederbaum, A.I.; Hellerbrand, C. Identification of Cytochrome CYP2E1 as Critical Mediator of Synergistic Effects of Alcohol and Cellular Lipid Accumulation in Hepatocytes in Vitro. Oncotarget 2015, 6, 41464–41478. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.G.; Ehlers, R.A.; Trambly, C.S.; Clemens, D.L.; Fox, H.S.; Tuma, D.J.; Donohue, T.M. Multilevel Regulation of Autophagosome Content by Ethanol Oxidation in HepG2 Cells. Autophagy 2013, 9, 63–73. [Google Scholar] [CrossRef]
- Ding, W.; Li, M.; Chen, X.; Ni, H.; Lin, C.; Gao, W.; Lu, B.; Stolz, D.B.; Clemens, D.L.; Yin, X. Autophagy Reduces Acute Ethanol-Induced Hepatotoxicity and Steatosis in Mice. Gastroenterology 2010, 139, 1740–1752. [Google Scholar] [CrossRef]
- Guo, R.; Xu, X.; Babcock, S.A.; Zhang, Y.; Ren, J. Aldehyde Dedydrogenase-2 Plays a Beneficial Role in Ameliorating Chronic Alcohol-Induced Hepatic Steatosis and Inflammation through Regulation of Autophagy. J. Hepatol. 2015, 62, 647–656. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Zhou, R.; Yang, L.; Cederbaum, A.I. Alcohol Steatosis and Cytotoxicity: The Role of Cytochrome P4502E1 and Autophagy. Free Radic. Biol. Med. 2012, 53, 1346–1357. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Zhou, R.; Cederbaum, A. CYP2E1 Enhances Ethanol-Induced Lipid Accumulation but Impairs Autophagy in HepG2 E47 Cells. Biochem. Biophys. Res. Commun. 2010, 402, 116–122. [Google Scholar] [CrossRef]
- Samuvel, D.J.; Li, L.; Krishnasamy, Y.; Gooz, M.; Takemoto, K.; Woster, P.M.; Lemasters, J.J.; Zhong, Z. Mitochondrial Depolarization after Acute Ethanol Treatment Drives Mitophagy in Living Mice. Autophagy 2022, 18, 2671–2685. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhou, J.; Chen, X.; Dong, Z.; Yin, X.-M. Diverse Consequences in Liver Injury in Mice with Different Autophagy Functional Status Treated with Alcohol. Am. J. Pathol. 2019, 189, 1744–1762. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Ni, H.-M.; Ding, Y.; Ding, W.-X. Parkin Regulates Mitophagy and Mitochondrial Function to Protect against Alcohol-Induced Liver Injury and Steatosis in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G324–G340. [Google Scholar] [CrossRef] [PubMed]
- Manley, S.; Ni, H.-M.; Williams, J.A.; Kong, B.; DiTacchio, L.; Guo, G.; Ding, W.-X. Farnesoid X Receptor Regulates Forkhead Box O3a Activation in Ethanol-Induced Autophagy and Hepatotoxicity. Redox Biol. 2014, 2, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.-M.; Du, K.; You, M.; Ding, W.-X. Critical Role of FoxO3a in Alcohol-Induced Autophagy and Hepatotoxicity. Am. J. Pathol. 2013, 183, 1815–1825. [Google Scholar] [CrossRef]
- Lin, C.-W.; Zhang, H.; Li, M.; Xiong, X.; Chen, X.; Chen, X.; Dong, X.C.; Yin, X.-M. Pharmacological Promotion of Autophagy Alleviates Steatosis and Injury in Alcoholic and Non-Alcoholic Fatty Liver Conditions in Mice. J. Hepatol. 2013, 58, 993–999. [Google Scholar] [CrossRef]
- Yang, L.; Wu, D.; Wang, X.; Cederbaum, A.I. Cytochrome P4502E1, Oxidative Stress, JNK and Autophagy in Acute Alcohol-Induced Fatty Liver. Free Radic. Biol. Med. 2012, 53, 1170–1180. [Google Scholar] [CrossRef]
- Thomes, P.G.; Trambly, C.S.; Thiele, G.M.; Duryee, M.J.; Fox, H.S.; Haorah, J.; Donohue, T.M. Proteasome Activity and Autophagosome Content in Liver Are Reciprocally Regulated by Ethanol Treatment. Biochem. Biophys. Res. Commun. 2012, 417, 262–267. [Google Scholar] [CrossRef]
- Guo, W.; Zhong, W.; Hao, L.; Sun, X.; Zhou, Z. Activation of MTORC1 by Free Fatty Acids Suppresses LAMP2 and Autophagy Function via ER Stress in Alcohol-Related Liver Disease. Cells 2021, 10, 2730. [Google Scholar] [CrossRef]
- Guo, W.; Zhong, W.; Hao, L.; Dong, H.; Sun, X.; Yue, R.; Li, T.; Zhou, Z. Fatty Acids Inhibit LAMP2-Mediated Autophagy Flux via Activating ER Stress Pathway in Alcohol-Related Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1599–1615. [Google Scholar] [CrossRef] [PubMed]
- Babuta, M.; Furi, I.; Bala, S.; Bukong, T.N.; Lowe, P.; Catalano, D.; Calenda, C.; Kodys, K.; Szabo, G. Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease. Hepatology 2019, 70, 2123–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, P.; Wang, J.; Toan, S.; Ren, J. DNA-PKcs Promotes Alcohol-Related Liver Disease by Activating Drp1-Related Mitochondrial Fission and Repressing FUNDC1-Required Mitophagy. Signal Transduct. Target. Ther. 2019, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Menk, M.; Graw, J.A.; Poyraz, D.; Möbius, N.; Spies, C.D.; von Haefen, C. Chronic Alcohol Consumption Inhibits Autophagy and Promotes Apoptosis in the Liver. Int. J. Med. Sci. 2018, 15, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Wang, S.; Zhao, K.; Li, Y.; Williams, J.A.; Li, T.; Chavan, H.; Krishnamurthy, P.; He, X.C.; Li, L.; et al. Impaired TFEB-Mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-Induced Liver Injury and Steatosis in Mice. Gastroenterology 2018, 155, 865–879.e12. [Google Scholar] [CrossRef]
- Kong, X.; Yang, Y.; Ren, L.; Shao, T.; Li, F.; Zhao, C.; Liu, L.; Zhang, H.; McClain, C.J.; Feng, W. Activation of Autophagy Attenuates EtOH-LPS-Induced Hepatic Steatosis and Injury through MD2 Associated TLR4 Signaling. Sci. Rep. 2017, 7, 9292. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Autophagy Protects against CYP2E1/Chronic Ethanol-Induced Hepatotoxicity. Biomolecules 2015, 5, 2659–2674. [Google Scholar] [CrossRef]
- King, A.L.; Swain, T.M.; Mao, Z.; Udoh, U.S.; Oliva, C.R.; Betancourt, A.M.; Griguer, C.E.; Crowe, D.R.; Lesort, M.; Bailey, S.M. Involvement of the Mitochondrial Permeability Transition Pore in Chronic Ethanol-Mediated Liver Injury in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G265–G277. [Google Scholar] [CrossRef]
- Tan, T.C.H.; Crawford, D.H.G.; Jaskowski, L.A.; Subramaniam, V.N.; Clouston, A.D.; Crane, D.I.; Bridle, K.R.; Anderson, G.J.; Fletcher, L.M. Excess Iron Modulates Endoplasmic Reticulum Stress-Associated Pathways in a Mouse Model of Alcohol and High-Fat Diet-Induced Liver Injury. Lab. Investig. 2013, 93, 1295–1312. [Google Scholar] [CrossRef]
- Liang, S.; Zhong, Z.; Kim, S.Y.; Uchiyama, R.; Roh, Y.S.; Matsushita, H.; Gottlieb, R.A.; Seki, E. Murine Macrophage Autophagy Protects against Alcohol-Induced Liver Injury by Degrading Interferon Regulatory Factor 1 (IRF1) and Removing Damaged Mitochondria. J. Biol. Chem. 2019, 294, 12359–12369. [Google Scholar] [CrossRef]
- Ilyas, G.; Cingolani, F.; Zhao, E.; Tanaka, K.; Czaja, M.J. Decreased Macrophage Autophagy Promotes Liver Injury and Inflammation from Alcohol. Alcohol. Clin. Exp. Res. 2019, 43, 1403–1413. [Google Scholar] [CrossRef]
- Xie, Z.-Y.; Xiao, Z.-H.; Wang, F.-F. Inhibition of Autophagy Reverses Alcohol-Induced Hepatic Stellate Cells Activation through Activation of Nrf2-Keap1-ARE Signaling Pathway. Biochimie 2018, 147, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Denaës, T.; Lodder, J.; Chobert, M.-N.; Ruiz, I.; Pawlotsky, J.-M.; Lotersztajn, S.; Teixeira-Clerc, F. The Cannabinoid Receptor 2 Protects Against Alcoholic Liver Disease Via a Macrophage Autophagy-Dependent Pathway. Sci. Rep. 2016, 6, 28806. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gea, V.; Hilscher, M.; Rozenfeld, R.; Lim, M.P.; Nieto, N.; Werner, S.; Devi, L.A.; Friedman, S.L. Endoplasmic Reticulum Stress Induces Fibrogenic Activity in Hepatic Stellate Cells through Autophagy. J. Hepatol. 2013, 59, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Jo, E.; Han, J.-H.; Jung, S.-H.; Lee, D.-H.; Park, I.; Heo, K.-S.; Na, M.; Myung, C.-S. Hepatoprotective Effects of an Acer Tegmentosum Maxim Extract through Antioxidant Activity and the Regulation of Autophagy. J. Ethnopharmacol. 2019, 239, 111912. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Li, L.; Hu, W.; Qu, Y.; Ding, Y.; Meng, L.; Teng, L.; Wang, D. Hepatoprotective Effects of Antrodia cinnamomea: The Modulation of Oxidative Stress Signaling in a Mouse Model of Alcohol-Induced Acute Liver Injury. Oxid. Med. Cell. Longev. 2017, 2017, e7841823. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, Z.; Liang, L.; Yang, X.; Sheng, D.; Zeng, J.; Li, X.; Shi, R.; Han, Z.; Wei, L. Babao Dan Attenuates Acute Ethanol-Induced Liver Injury via Nrf2 Activation and Autophagy. Cell Biosci. 2019, 9, 80. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Hwang, S.H.; Jia, Y.; Seo, W.-D.; Lee, S.-J. Barley Sprout Extracts Reduce Hepatic Lipid Accumulation in Ethanol-Fed Mice by Activating Hepatic AMP-Activated Protein Kinase. Food Res. Int. 2017, 101, 209–217. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, Y.; You, K.; Zhang, J.; Yang, F.; Li, Y. Calcitriol Alleviates Ethanol-Induced Hepatotoxicity via AMPK/MTOR-Mediated Autophagy. Arch. Biochem. Biophys. 2021, 697, 108694. [Google Scholar] [CrossRef]
- Yang, L.; Rozenfeld, R.; Wu, D.; Devi, L.A.; Zhang, Z.; Cederbaum, A. Cannabidiol Protects Liver from Binge Alcohol-Induced Steatosis by Mechanisms Including Inhibition of Oxidative Stress and Increase in Autophagy. Free Radic. Biol. Med. 2014, 68, 260–267. [Google Scholar] [CrossRef]
- Khan, I.; Bhardwaj, M.; Shukla, S.; Min, S.-H.; Choi, D.K.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Carvacrol Inhibits Cytochrome P450 and Protects against Binge Alcohol-Induced Liver Toxicity. Food Chem. Toxicol. 2019, 131, 110582. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, C.-L.; Song, F.-Y.; Zhao, X.-L.; Xie, K.-Q. CMZ Reversed Chronic Ethanol-Induced Disturbance of PPAR-α Possibly by Suppressing Oxidative Stress and PGC-1α Acetylation, and Activating the MAPK and GSK3β Pathway. PLoS ONE 2014, 9, e98658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Zhang, C.-L.; Zhao, X.-L.; Xie, K.-Q.; Zeng, T. Inhibition of Cytochrome P4502E1 by Chlormethiazole Attenuated Acute Ethanol-Induced Fatty Liver. Chem. Biol. Interact. 2014, 222, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shu, M.-S.; Kim, J.-Y.; Kim, Y.-H.; Sim, K.H.; Sung, W.J.; Eun, J.R. Cilostazol Protects Hepatocytes against Alcohol-Induced Apoptosis via Activation of AMPK Pathway. PLoS ONE 2019, 14, e0211415. [Google Scholar] [CrossRef]
- Guo, X.; Cui, R.; Zhao, J.; Mo, R.; Peng, L.; Yan, M. Corosolic Acid Protects Hepatocytes against Ethanol-Induced Damage by Modulating Mitogen-Activated Protein Kinases and Activating Autophagy. Eur. J. Pharmacol. 2016, 791, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Dong, Y.; Li, B.; Kang, X.; Gu, C.; Zhu, T.; Luo, Y.; Pang, M.; Du, W.; Ge, W. Dihydromyricetin Modulates P62 and Autophagy Crosstalk with the Keap-1/Nrf2 Pathway to Alleviate Ethanol-Induced Hepatic Injury. Toxicol. Lett. 2017, 274, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, Q.; Li, X.; Jiang, M.; Cui, B.-W.; Xia, K.-L.; Wu, Y.-L.; Lian, L.-H.; Nan, J.-X. Amelioration of Alcoholic Liver Steatosis by Dihydroquercetin through the Modulation of AMPK-Dependent Lipogenesis Mediated by P2X7R–NLRP3-Inflammasome Activation. J. Agric. Food Chem. 2018, 66, 4862–4871. [Google Scholar] [CrossRef]
- Zhou, Z.-S.; Kong, C.-F.; Sun, J.-R.; Qu, X.-K.; Sun, J.-H.; Sun, A.-T. Fisetin Ameliorates Alcohol-Induced Liver Injury through Regulating SIRT1 and SphK1 Pathway. Am. J. Chin. Med. 2022, 50, 2171–2184. [Google Scholar] [CrossRef]
- Xue, M.; Liang, H.; Zhou, Z.; Liu, Y.; He, X.; Zhang, Z.; Sun, T.; Yang, J.; Qin, Y.; Qin, K. Effect of Fucoidan on Ethanol-Induced Liver Injury and Steatosis in Mice and the Underlying Mechanism. Food Nutr. Res. 2021, 65, 5384. [Google Scholar] [CrossRef]
- Xue, M.; Tian, Y.; Sui, Y.; Zhao, H.; Gao, H.; Liang, H.; Qiu, X.; Sun, Z.; Zhang, Y.; Qin, Y. Protective Effect of Fucoidan against Iron Overload and Ferroptosis-Induced Liver Injury in Rats Exposed to Alcohol. Biomed. Pharmacother. 2022, 153, 113402. [Google Scholar] [CrossRef]
- Song, X.; Yin, S.; Huo, Y.; Liang, M.; Fan, L.; Ye, M.; Hu, H. Glycycoumarin Ameliorates Alcohol-Induced Hepatotoxicity via Activation of Nrf2 and Autophagy. Free Radic. Biol. Med. 2015, 89, 135–146. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Yang, X.-Q.; Yu, D.-K.; Xiao, H.-Y.; Du, J.-R. Nrf2 Signalling Pathway and Autophagy Impact on the Preventive Effect of Green Tea Extract against Alcohol-Induced Liver Injury. J. Pharm. Pharmacol. 2021, 73, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhuo, H.; Yang, L.; Ouyang, H.; Chen, J.; Liu, B.; Huang, H. A Peptide HEPFYGNEGALR from Apostichopus Japonicus Alleviates Acute Alcoholic Liver Injury by Enhancing Antioxidant Response in Male C57BL/6J Mice. Molecules 2022, 27, 5839. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, X.; Fu, Z.; Yin, J.; Wang, Y.; Sun, W.; Ren, H.; Zhang, Y. Kinsenoside Alleviates Alcoholic Liver Injury by Reducing Oxidative Stress, Inhibiting Endoplasmic Reticulum Stress, and Regulating AMPK-Dependent Autophagy. Front. Pharmacol. 2022, 12, 747325. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, L.; Qin, Y.; Zhou, Y.; Liu, W.; Li, Y.; Chen, Y.; Xu, Y. Protective Effects of Rare Earth Lanthanum on Acute Ethanol-Induced Oxidative Stress in Mice via Keap 1/Nrf2/P62 Activation. Sci. Total Environ. 2021, 758, 143626. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Tkachenko, H.; Lukash, O. Melatonin Modulates Oxidative Phosphorylation, Hepatic and Kidney Autophagy-Caused Subclinical Endotoxemia and Acute Ethanol-Induced Oxidative Stress. Chronobiol. Int. 2020, 37, 1709–1724. [Google Scholar] [CrossRef]
- Lin, G.-S.; Zhao, M.-M.; Fu, Q.-C.; Zhao, S.-Y.; Ba, T.-T.; Yu, H.-X. Palmatine Attenuates Hepatocyte Injury by Promoting Autophagy via the AMPK/MTOR Pathway after Alcoholic Liver Disease. Drug Dev. Res. 2022, 83, 1613–1622. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, M.; Deng, Y.; Guo, C.; Liao, L.; He, L.; Peng, C.; Li, Y. Functional Teas from Penthorum Chinense Pursh Alleviates Ethanol-Induced Hepatic Oxidative Stress and Autophagy Impairment in Zebrafish via Modulating the AMPK/P62/Nrf2/MTOR Signaling Axis. Plant Foods Hum. Nutr. 2022, 77, 514–520. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, Y.; Huang, Z.; Sullivan, M.A.; He, Z.; Wang, J.; Chen, Z.; Hu, H.; Wang, K. The Preventative Effects of Procyanidin on Binge Ethanol-Induced Lipid Accumulation and ROS Overproduction via the Promotion of Hepatic Autophagy. Mol. Nutr. Food Res. 2019, 63, 1801255. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Zhang, S.; Sun, J.; Liu, P.; Xiao, L.; Tang, Y.; Liu, L.; Yao, P. Quercetin Attenuates Chronic Ethanol-Induced Hepatic Mitochondrial Damage through Enhanced Mitophagy. Nutrients 2016, 8, 27. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, X.; Wang, J.; Dai, S.; Peng, C.; Li, Y. Quercetin Alleviates Ethanol-Induced Hepatic Steatosis in L02 Cells by Activating TFEB Translocation to Compensate for Inadequate Autophagy. Phytother. Res. 2023, 37, 62–76. [Google Scholar] [CrossRef]
- Lin, H.; Guo, X.; Liu, J.; Liu, P.; Mei, G.; Li, H.; Li, D.; Chen, H.; Chen, L.; Zhao, Y.; et al. Improving Lipophagy by Restoring Rab7 Cycle: Protective Effects of Quercetin on Ethanol-Induced Liver Steatosis. Nutrients 2022, 14, 658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, X.; Zhao, J. Quercetin Prevents Alcohol-induced Liver Injury through Targeting of PI3K/Akt/Nuclear Factor-κB and STAT3 Signaling Pathway. Exp. Ther. Med. 2017, 14, 6169–6175. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, X.; Zhou, F.; Xiao, L.; Liu, J.; Jiang, C.; Xing, M.; Yao, P. Quercetin Alleviates Ethanol-Induced Liver Steatosis Associated with Improvement of Lipophagy. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 125, 21–28. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, L.; Wang, C.; Liu, M.; Hu, N.; Dai, X.; Peng, C.; Li, Y. Quercetin Mitigates Ethanol-Induced Hepatic Steatosis in Zebrafish via P2X7R-Mediated PI3K/ Keap1/Nrf2 Signaling Pathway. J. Ethnopharmacol. 2021, 268, 113569. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, F.; Fang, Z.; Hu, C. Resveratrol Ameliorates Alcoholic Fatty Liver by Inducing Autophagy. Am. J. Chin. Med. 2016, 44, 1207–1220. [Google Scholar] [CrossRef]
- You, M.; Liang, X.; Ajmo, J.M.; Ness, G.C. Involvement of Mammalian Sirtuin 1 in the Action of Ethanol in the Liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G892–G898. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, R.; Zhao, Y.; Fu, R.; Wang, R.; Zhao, H.; Wang, Z.; Tang, F.; Zhang, N.; Tian, X.; et al. Promotion of Autophagosome–Lysosome Fusion via Salvianolic Acid A-Mediated SIRT1 up-Regulation Ameliorates Alcoholic Liver Disease. RSC Adv. 2018, 8, 20411–20422. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, Z.; Hu, F.; Zhang, Y.; Li, C.; Yan, P.; Feng, L.; Shen, J.; Huang, B. The Protective Effects of Selenium-Enriched Spirulina Platensis on Chronic Alcohol-Induced Liver Injury in Mice. Food Funct. 2018, 9, 3155–3165. [Google Scholar] [CrossRef]
- Song, X.-Y.; Liu, P.-C.; Liu, W.-W.; Zhou, J.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Silibinin Inhibits Ethanol- or Acetaldehyde-Induced Ferroptosis in Liver Cell Lines. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2022, 82, 105388. [Google Scholar] [CrossRef]
- Atef, M.M.; Hafez, Y.M.; Alshenawy, H.A.; Emam, M.N. Ameliorative Effects of Autophagy Inducer, Simvastatin on Alcohol-Induced Liver Disease in a Rat Model. J. Cell. Biochem. 2019, 120, 7679–7688. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, J.; Wu, D. Sulforaphane Induces Nrf2 and Protects against CYP2E1-Dependent Binge Alcohol-Induced Liver Steatosis. Biochim. Biophys. Acta 2014, 1840, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, Y.; Yuan, F.; Peng, L.; Qiu, C. Tangeretin Protects Mice from Alcohol-Induced Fatty Liver by Activating Mitophagy through the AMPK–ULK1 Pathway. J. Agric. Food Chem. 2022, 70, 11236–11244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, R.; Wang, X.; Jiang, Y.; Xu, W.; Shao, Y.; Yue, C.; Shi, W.; Jin, H.; Ge, T.; et al. Activation of UQCRC2-Dependent Mitophagy by Tetramethylpyrazine Inhibits MLKL-Mediated Hepatocyte Necroptosis in Alcoholic Liver Disease. Free Radic. Biol. Med. 2022, 179, 301–316. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Kang, S.A.; Chang, J.W.; Liu, Q.; Zhang, J.; Gao, Y.; Reichling, L.J.; Sim, T.; Sabatini, D.M.; Gray, N.S. An ATP-Competitive Mammalian Target of Rapamycin Inhibitor Reveals Rapamycin-Resistant Functions of MTORC1. J. Biol. Chem. 2009, 284, 8023–8032. [Google Scholar] [CrossRef]
- Wu, W.-B.; Chen, Y.-Y.; Zhu, B.; Peng, X.-M.; Zhang, S.-W.; Zhou, M.-L. Excessive Bile Acid Activated NF-Kappa B and Promoted the Development of Alcoholic Steatohepatitis in Farnesoid X Receptor Deficient Mice. Biochimie 2015, 115, 86–92. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Yoo, C. Role of Zinc in the Regulation of Autophagy During Ethanol Exposure in Human Hepatoma Cells. Biol. Trace Elem. Res. 2013, 156, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cederbaum, A.I. Oxidative Stress and Alcoholic Liver Disease. Semin. Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Misra, H.; Li, Y.R. Oxidative Stress and Redox Signaling Mechanisms of Alcoholic Liver Disease: Updated Experimental and Clinical Evidence. J. Dig. Dis. 2012, 13, 133–142. [Google Scholar] [CrossRef]
- Balbo, S.; Hashibe, M.; Gundy, S.; Brennan, P.; Canova, C.; Simonato, L.; Merletti, F.; Richiardi, L.; Agudo, A.; Castellsagué, X.; et al. N2-Ethyldeoxyguanosine as a Potential Biomarker for Assessing Effects of Alcohol Consumption on DNA. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 3026–3032. [Google Scholar] [CrossRef]
- Lucey, M.R.; Mathurin, P.; Morgan, T.R. Alcoholic Hepatitis. N. Engl. J. Med. 2009, 360, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative Stress in Alcohol-Related Liver Disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between Oxidative Stress and Inflammatory Liver Injury in the Pathogenesis of Alcoholic Liver Disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of Oxidative Stress in Alcohol-Induced Liver Injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Galli, A.; Pinaire, J.; Fischer, M.; Dorris, R.; Crabb, D.W. The Transcriptional and DNA Binding Activity of Peroxisome Proliferator-Activated Receptor Alpha Is Inhibited by Ethanol Metabolism. A Novel Mechanism for the Development of Ethanol-Induced Fatty Liver. J. Biol. Chem. 2001, 276, 68–75. [Google Scholar] [CrossRef]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol Induces Fatty Acid Synthesis Pathways by Activation of Sterol Regulatory Element-Binding Protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef]
- Donohue, T.M.; Osna, N.A.; Trambly, C.S.; Whitaker, N.P.; Thomes, P.G.; Todero, S.L.; Davis, J.S. Early Growth Response-1 Contributes to Steatosis Development after Acute Ethanol Administration. Alcohol. Clin. Exp. Res. 2012, 36, 759–767. [Google Scholar] [CrossRef]
- Sacitharan, P.K.; Bou-Gharios, G.; Edwards, J.R. SIRT1 Directly Activates Autophagy in Human Chondrocytes. Cell Death Discov. 2020, 6, 41. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Stickel, F. Risk Factors and Mechanisms of Hepatocarcinogenesis with Special Emphasis on Alcohol and Oxidative Stress. Biol. Chem. 2006, 387, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and Oxidative Liver Injury by Alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Ambade, A.; Mandrekar, P. Oxidative Stress and Inflammation: Essential Partners in Alcoholic Liver Disease. Int. J. Hepatol. 2012, 2012, 853175. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Chen, Y.; Wang, J.; Hao, L.; Huang, C.; Griffiths, A.; Sun, Z.; Zhou, Z.; Song, Z. ER Stress-Induced Upregulation of NNMT Contributes to Alcohol-Related Fatty Liver Development. J. Hepatol. 2020, 73, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Ma, Y.; Lai, S.; Dou, X.; Li, S. NNMT Aggravates Hepatic Steatosis, but Alleviates Liver Injury in Alcoholic Liver Disease. J. Hepatol. 2021, 74, 1248–1250. [Google Scholar] [CrossRef]
- Campagna, R.; Vignini, A. NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, C.W.; Yoon, G.; Hong, S.M.; Choi, K.Y. NNMT Depletion Contributes to Liver Cancer Cell Survival by Enhancing Autophagy under Nutrient Starvation. Oncogenesis 2018, 7, 58. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, X.; Wang, Y.; Huang, X.; Yang, J.; Zeng, J.; Li, G.; Xie, X.; Zhang, J. Nicotinamide N-Methyltransferase Inhibits Autophagy Induced by Oxidative Stress through Suppressing the AMPK Pathway in Breast Cancer Cells. Cancer Cell Int. 2020, 20, 191. [Google Scholar] [CrossRef]
- Campagna, R.; Mateuszuk, Ł.; Wojnar-Lason, K.; Kaczara, P.; Tworzydło, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-Methyltransferase in Endothelium Protects against Oxidant Stress-Induced Endothelial Injury. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef]
- Guarino, M.; Dufour, J.-F. Nicotinamide and NAFLD: Is There Nothing New Under the Sun? Metabolites 2019, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Rotilio, G.; Ciriolo, M.R. Disulfide Relays and Phosphorylative Cascades: Partners in Redox-Mediated Signaling Pathways. Cell Death Differ. 2005, 12, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Jiang, J.; Zhan, M.; Zhang, H.; Wang, Q.-T.; Sun, S.-N.; Guo, X.-K.; Yin, H.; Wei, Y.; Liu, J.O.; et al. Targeting Neoantigens in Hepatocellular Carcinoma for Immunotherapy: A Futile Strategy? Hepatology 2021, 73, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zapotoczny, B.; Braet, F.; Kus, E.; Ginda-Mäkelä, K.; Klejevskaja, B.; Campagna, R.; Chlopicki, S.; Szymonski, M. Actin-spectrin Scaffold Supports Open Fenestrae in Liver Sinusoidal Endothelial Cells. Traffic Cph. Den. 2019, 20, 932–942. [Google Scholar] [CrossRef]
- Kimata, Y.; Kohno, K. Endoplasmic Reticulum Stress-Sensing Mechanisms in Yeast and Mammalian Cells. Curr. Opin. Cell Biol. 2011, 23, 135–142. [Google Scholar] [CrossRef]
- Bailey, S.M. A Review of the Role of Reactive Oxygen and Nitrogen Species in Alcohol-Induced Mitochondrial Dysfunction. Free Radic. Res. 2003, 37, 585–596. [Google Scholar] [CrossRef]
- Haldar, S.M.; Stamler, J.S. S-Nitrosylation at the Interface of Autophagy and Disease. Mol. Cell 2011, 43, 1–3. [Google Scholar] [CrossRef]
- Rizza, S.; Cardaci, S.; Montagna, C.; Di Giacomo, G.; De Zio, D.; Bordi, M.; Maiani, E.; Campello, S.; Borreca, A.; Puca, A.A.; et al. S-Nitrosylation Drives Cell Senescence and Aging in Mammals by Controlling Mitochondrial Dynamics and Mitophagy. Proc. Natl. Acad. Sci. USA 2018, 115, E3388–E3397. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell Death and Diseases Related to Oxidative Stress: 4-Hydroxynonenal (HNE) in the Balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Linhart, K.; Bartsch, H.; Seitz, H.K. The Role of Reactive Oxygen Species (ROS) and Cytochrome P-450 2E1 in the Generation of Carcinogenic Etheno-DNA Adducts. Redox Biol. 2014, 3, 56–62. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Choi, Y.; Ha, S.-K.; Song, B.-J. Cytochrome P450-2E1 Promotes Aging-Related Hepatic Steatosis, Apoptosis and Fibrosis through Increased Nitroxidative Stress. Free Radic. Biol. Med. 2016, 91, 188–202. [Google Scholar] [CrossRef]
- Nair, J.; Srivatanakul, P.; Haas, C.; Jedpiyawongse, A.; Khuhaprema, T.; Seitz, H.K.; Bartsch, H. High Urinary Excretion of Lipid Peroxidation-Derived DNA Damage in Patients with Cancer-Prone Liver Diseases. Mutat. Res. 2010, 683, 23–28. [Google Scholar] [CrossRef]
- Neeley, W.L.; Essigmann, J.M. Mechanisms of Formation, Genotoxicity, and Mutation of Guanine Oxidation Products. Chem. Res. Toxicol. 2006, 19, 491–505. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of Mitochondria in Alcoholic Liver Disease. World J. Gastroenterol. 2014, 20, 2136–2142. [Google Scholar] [CrossRef]
- Grattagliano, I.; Russmann, S.; Diogo, C.; Bonfrate, L.; Oliveira, P.J.; Wang, D.Q.-H.; Portincasa, P. Mitochondria in Chronic Liver Disease. Curr. Drug Targets 2011, 12, 879–893. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Kaplowitz, N.; Fernandez-Checa, J.C. Role of Mitochondria in Alcoholic Liver Disease. Curr. Pathobiol. Rep. 2013, 1, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; Salvesen, G.S. Regulation of the Apaf-1–Caspase-9 Apoptosome. J. Cell Sci. 2010, 123, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive Oxygen Species at the Crossroads of Inflammasome and Inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Brenner, D.A.; Karin, M. A Liver Full of JNK: Signaling in Regulation of Cell Function and Disease Pathogenesis, and Clinical Approaches. Gastroenterology 2012, 143, 307–320. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Shulga, N.; Hoek, J.B. TNF-α-Induced Cell Death in Ethanol-Exposed Cells Depends on P38 MAPK Signaling but Is Independent of Bid and Caspase-8. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G503–G516. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA Mutations in Disease and Aging. Environ. Mol. Mutagen. 2010, 51, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G. Mitochondria and Reactive Oxygen Species. Which Role in Physiology and Pathology? Adv. Exp. Med. Biol. 2012, 942, 93–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lemasters, J.J. Mitophagy Selectively Degrades Individual Damaged Mitochondria after Photoirradiation. Antioxid. Redox Signal. 2011, 14, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, E.; Cavallini, G.; Donati, A.; Gori, Z. The Anti-Ageing Effects of Caloric Restriction May Involve Stimulation of Macroautophagy and Lysosomal Degradation, and Can Be Intensified Pharmacologically. Biomed. Pharmacother. 2003, 57, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Dolganiuc, A.; Thomes, P.G.; Ding, W.-X.; Lemasters, J.J.; Donohue, T.M. Autophagy in Alcohol-Induced Liver Diseases. Alcohol. Clin. Exp. Res. 2012, 36, 1301–1308. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A. Glutathione Depletion in CYP2E1-Expressing Liver Cells Induces Toxicity Due to the Activation of P38 Mitogen-Activated Protein Kinase and Reduction of Nuclear Factor-ΚB DNA Binding Activity. Mol. Pharmacol. 2004, 66, 749–760. [Google Scholar] [CrossRef]
- Albano, E. Oxidative Mechanisms in the Pathogenesis of Alcoholic Liver Disease. Mol. Aspects Med. 2008, 29, 9–16. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Morgan, M.J.; Choksi, S.; Liu, Z.-G. TNF-Induced Activation of the Nox1 NADPH Oxidase and Its Role in the Induction of Necrotic Cell Death. Mol. Cell 2007, 26, 675–687. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wang, H.; Xie, Q.; Xu, Y. Notch1-Nuclear Factor ΚB Involves in Oxidative Stress-Induced Alcoholic Steatohepatitis. Alcohol Alcohol. 2014, 49, 10–16. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and Inflammasomes in Alcoholic Liver Disease/Acute Alcoholic Hepatitis and Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.; Schroder, K. NLRP3 Inflammasome Activation: The Convergence of Multiple Signalling Pathways on ROS Production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, W.; Zhong, W.; Sun, X.; Zhou, Z. Pharmacological Inhibition of NOX4 Ameliorates Alcohol-Induced Liver Injury in Mice through Improving Oxidative Stress and Mitochondrial Function. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2912–2921. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rusyn, I.; Yin, M.; Gäbele, E.; Yamashina, S.; Dikalova, A.; Kadiiska, M.B.; Connor, H.D.; Mason, R.P.; Segal, B.H.; et al. NADPH Oxidase-Derived Free Radicals Are Key Oxidants in Alcohol-Induced Liver Disease. J. Clin. Investig. 2000, 106, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Pritchard, M.T.; McMullen, M.R.; Wang, Q.; Nagy, L.E. Chronic Ethanol Feeding Increases Activation of NADPH Oxidase by Lipopolysaccharide in Rat Kupffer Cells: Role of Increased Reactive Oxygen in LPS-Stimulated ERK1/2 Activation and TNF-Alpha Production. J. Leukoc. Biol. 2006, 79, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Milic, S.; Mikolasevic, I.; Orlic, L.; Devcic, E.; Starcevic-Cizmarevic, N.; Stimac, D.; Kapovic, M.; Ristic, S. The Role of Iron and Iron Overload in Chronic Liver Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2144–2151. [Google Scholar] [CrossRef]
- Purohit, V.; Russo, D.; Salin, M. Role of Iron in Alcoholic Liver Disease: Introduction and Summary of the Symposium. Alcohol Fayettev. N 2003, 30, 93–97. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The Release and Activity of HMGB1 in Ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Choi, D.W.; Kim, S.Y.; Kim, S.K.; Kim, Y.C. Factors Involved in Hepatic Glutathione Depletion Induced by Acute Ethanol Administration. J. Toxicol. Environ. Health A 2000, 60, 459–469. [Google Scholar] [CrossRef]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Landar, A.; Darley-Usmar, V.; Bailey, S.M. Novel Interactions of Mitochondria and Reactive Oxygen/Nitrogen Species in Alcohol Mediated Liver Disease. World J. Gastroenterol. 2007, 13, 4967–4973. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS Signaling under Metabolic Stress: Cross-Talk between AMPK and AKT Pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Meijer, A.J.; Codogno, P. Regulation and Role of Autophagy in Mammalian Cells. Int. J. Biochem. Cell Biol. 2004, 36, 2445–2462. [Google Scholar] [CrossRef]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. Microautophagy in Mammalian Cells: Revisiting a 40-Year-Old Conundrum. Autophagy 2011, 7, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. Chaperone-Mediated Autophagy: A Unique Way to Enter the Lysosome World. Trends Cell Biol. 2012, 22, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Ding, W.-X. Mechanisms, Pathophysiological Roles and Methods for Analyzing Mitophagy—Recent Insights. Biol. Chem. 2018, 399, 147–178. [Google Scholar] [CrossRef] [PubMed]
- Gatica, D.; Lahiri, V.; Klionsky, D.J. Cargo Recognition and Degradation by Selective Autophagy. Nat. Cell Biol. 2018, 20, 233–242. [Google Scholar] [CrossRef]
- Garcia-Macia, M.; Santos-Ledo, A.; Leslie, J.; Paish, H.L.; Collins, A.L.; Scott, R.S.; Watson, A.; Burgoyne, R.A.; White, S.; French, J.; et al. A Mammalian Target of Rapamycin-Perilipin 3 (MTORC1-Plin3) Pathway Is Essential to Activate Lipophagy and Protects Against Hepatosteatosis. Hepatology 2021, 74, 3441–3459. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Levine, B. To Be or Not to Be? How Selective Autophagy and Cell Death Govern Cell Fate. Cell 2014, 157, 65–75. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor P62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Mizushima, N. The Role of the Atg1/ULK1 Complex in Autophagy Regulation. Curr. Opin. Cell Biol. 2010, 22, 132–139. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and MTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Jäättelä, M. AMP-Activated Protein Kinase: A Universal Regulator of Autophagy? Autophagy 2007, 3, 381–383. [Google Scholar] [CrossRef]
- Liang, J.; Shao, S.H.; Xu, Z.-X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The Energy Sensing LKB1-AMPK Pathway Regulates P27(Kip1) Phosphorylation Mediating the Decision to Enter Autophagy or Apoptosis. Nat. Cell Biol. 2007, 9, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes Form at ER–Mitochondria Contact Sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef]
- Itakura, E.; Kishi, C.; Inoue, K.; Mizushima, N. Beclin 1 Forms Two Distinct Phosphatidylinositol 3-Kinase Complexes with Mammalian Atg14 and UVRAG. Mol. Biol. Cell 2008, 19, 5360–5372. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Saitoh, T.; Tabata, K.; Omori, H.; Satoh, T.; Kurotori, N.; Maejima, I.; Shirahama-Noda, K.; Ichimura, T.; Isobe, T.; et al. Two Beclin 1-Binding Proteins, Atg14L and Rubicon, Reciprocally Regulate Autophagy at Different Stages. Nat. Cell Biol. 2009, 11, 385–396. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.-Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.-L. ULK1 Induces Autophagy by Phosphorylating Beclin-1 and Activating VPS34 Lipid Kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef]
- Obara, K.; Ohsumi, Y. Dynamics and Function of PtdIns(3)P in Autophagy. Autophagy 2008, 4, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Polson, H.E.J.; de Lartigue, J.; Rigden, D.J.; Reedijk, M.; Urbé, S.; Clague, M.J.; Tooze, S.A. Mammalian Atg18 (WIPI2) Localizes to Omegasome-Anchored Phagophores and Positively Regulates LC3 Lipidation. Autophagy 2010, 6, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Molecular Dissection of Autophagy: Two Ubiquitin-like Systems. Nat. Rev. Mol. Cell Biol. 2001, 2, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Yoshimori, T.; Tooze, S.A. The Autophagosome: Origins Unknown, Biogenesis Complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef]
- Tan, C.T.; Soh, N.J.H.; Chang, H.-C.; Yu, V.C. P62/SQSTM1 in Liver Diseases: The Usual Suspect with Multifarious Identities. FEBS J. 2021, 290, 892–912. [Google Scholar] [CrossRef]
- Takahashi, Y.; He, H.; Tang, Z.; Hattori, T.; Liu, Y.; Young, M.M.; Serfass, J.M.; Chen, L.; Gebru, M.; Chen, C.; et al. An Autophagy Assay Reveals the ESCRT-III Component CHMP2A as a Regulator of Phagophore Closure. Nat. Commun. 2018, 9, 2855. [Google Scholar] [CrossRef]
- Takahashi, Y.; Liang, X.; Hattori, T.; Tang, Z.; He, H.; Chen, H.; Liu, X.; Abraham, T.; Imamura-Kawasawa, Y.; Buchkovich, N.J.; et al. VPS37A Directs ESCRT Recruitment for Phagophore Closure. J. Cell Biol. 2019, 218, 3336–3354. [Google Scholar] [CrossRef]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The Hairpin-Type Tail-Anchored SNARE Syntaxin 17 Targets to Autophagosomes for Fusion with Endosomes/Lysosomes. Cell 2012, 151, 1256–1269. [Google Scholar] [CrossRef]
- Shen, Q.; Shi, Y.; Liu, J.; Su, H.; Huang, J.; Zhang, Y.; Peng, C.; Zhou, T.; Sun, Q.; Wan, W.; et al. Acetylation of STX17 (Syntaxin 17) Controls Autophagosome Maturation. Autophagy 2021, 17, 1157–1169. [Google Scholar] [CrossRef]
- Hubert, V.; Peschel, A.; Langer, B.; Gröger, M.; Rees, A.; Kain, R. LAMP-2 Is Required for Incorporating Syntaxin-17 into Autophagosomes and for Their Fusion with Lysosomes. Biol. Open 2016, 5, 1516–1529. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Mitophagy: Molecular Mechanisms, New Concepts on Parkin Activation and the Emerging Role of AMPK/ULK1 Axis. Cells 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-Mediated Mitophagy Is Dependent on VDAC1 and P62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Narendra, D.P. Mechanisms of Mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.M.; Lazarou, M.; Wang, C.; Kane, L.A.; Narendra, D.P.; Youle, R.J. Mitochondrial Membrane Potential Regulates PINK1 Import and Proteolytic Destabilization by PARL. J. Cell Biol. 2010, 191, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Kujuro, Y.; Okatsu, K.; Koyano, F.; Kosako, H.; Kimura, M.; Suzuki, N.; Uchiyama, S.; Tanaka, K.; Matsuda, N. Parkin-Catalyzed Ubiquitin-Ester Transfer Is Triggered by PINK1-Dependent Phosphorylation. J. Biol. Chem. 2013, 288, 22019–22032. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Johansen, T. P62/SQSTM1: A Missing Link between Protein Aggregates and the Autophagy Machinery. Autophagy 2006, 2, 138–139. [Google Scholar] [CrossRef]
- Ichimura, Y.; Kominami, E.; Tanaka, K.; Komatsu, M. Selective Turnover of P62/A170/SQSTM1 by Autophagy. Autophagy 2008, 4, 1063–1066. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Dunfield, J.; Roderique, T.; Ni, H.-M. Liver-Adipose Tissue Crosstalk in Alcohol-Associated Liver Disease: The Role of MTOR. Liver Res. 2022, 6, 227–237. [Google Scholar] [CrossRef]
- Li, Y.; Chao, X.; Wang, S.; Williams, J.A.; Ni, H.-M.; Ding, W.-X. Role of Mechanistic Target of Rapamycin and Autophagy in Alcohol-Induced Adipose Atrophy and Liver Injury. Am. J. Pathol. 2020, 190, 158–175. [Google Scholar] [CrossRef]
- Sakane, S.; Hikita, H.; Shirai, K.; Myojin, Y.; Sasaki, Y.; Kudo, S.; Fukumoto, K.; Mizutani, N.; Tahata, Y.; Makino, Y.; et al. White Adipose Tissue Autophagy and Adipose-Liver Crosstalk Exacerbate Nonalcoholic Fatty Liver Disease in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1683–1699. [Google Scholar] [CrossRef] [PubMed]
- Sid, B.; Verrax, J.; Calderon, P.B. Role of AMPK Activation in Oxidative Cell Damage: Implications for Alcohol-Induced Liver Disease. Biochem. Pharmacol. 2013, 86, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhu, M.; Zhou, S.; Feng, W.; Chen, H. Rapamycin-Loaded MPEG-PLGA Nanoparticles Ameliorate Hepatic Steatosis and Liver Injury in Non-Alcoholic Fatty Liver Disease. Front. Chem. 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, X.; Wang, C.; Wang, J.; Peng, C.; Li, Y. Emerging Roles of Sirtuins in Alleviating Alcoholic Liver Disease: A Comprehensive Review. Int. Immunopharmacol. 2022, 108, 108712. [Google Scholar] [CrossRef]
- Rafiee, S.; Mohammadi, H.; Ghavami, A.; Sadeghi, E.; Safari, Z.; Askari, G. Efficacy of Resveratrol Supplementation in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Clinical Trials. Complement. Ther. Clin. Pract. 2021, 42, 101281. [Google Scholar] [CrossRef]
- Nguyen-Khac, E.; Thevenot, T.; Piquet, M.-A.; Benferhat, S.; Goria, O.; Chatelain, D.; Tramier, B.; Dewaele, F.; Ghrib, S.; Rudler, M.; et al. Glucocorticoids plus N -Acetylcysteine in Severe Alcoholic Hepatitis. N. Engl. J. Med. 2011, 365, 1781–1789. [Google Scholar] [CrossRef]
- Morley, K.C.; Baillie, A.; Van Den Brink, W.; Chitty, K.E.; Brady, K.; Back, S.E.; Seth, D.; Sutherland, G.; Leggio, L.; Haber, P.S. N-Acetyl Cysteine in the Treatment of Alcohol Use Disorder in Patients with Liver Disease: Rationale for Further Research. Expert Opin. Investig. Drugs 2018, 27, 667–675. [Google Scholar] [CrossRef]
- Ni, Y.-H.; Huo, L.-J.; Li, T.-T. Antioxidant Axis Nrf2-Keap1-ARE in Inhibition of Alcoholic Liver Fibrosis by IL-22. World J. Gastroenterol. 2017, 23, 2002–2011. [Google Scholar] [CrossRef]
- Lu, C.; Xu, W.; Zhang, F.; Shao, J.; Zheng, S. Nrf2 Knockdown Disrupts the Protective Effect of Curcumin on Alcohol-Induced Hepatocyte Necroptosis. Mol. Pharm. 2016, 13, 4043–4053. [Google Scholar] [CrossRef]
- Ospina, R.; Marmolejo-Ramos, F. Performance of Some Estimators of Relative Variability. Front. Appl. Math. Stat. 2019, 5, 43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).