Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective
Abstract
:1. Introduction
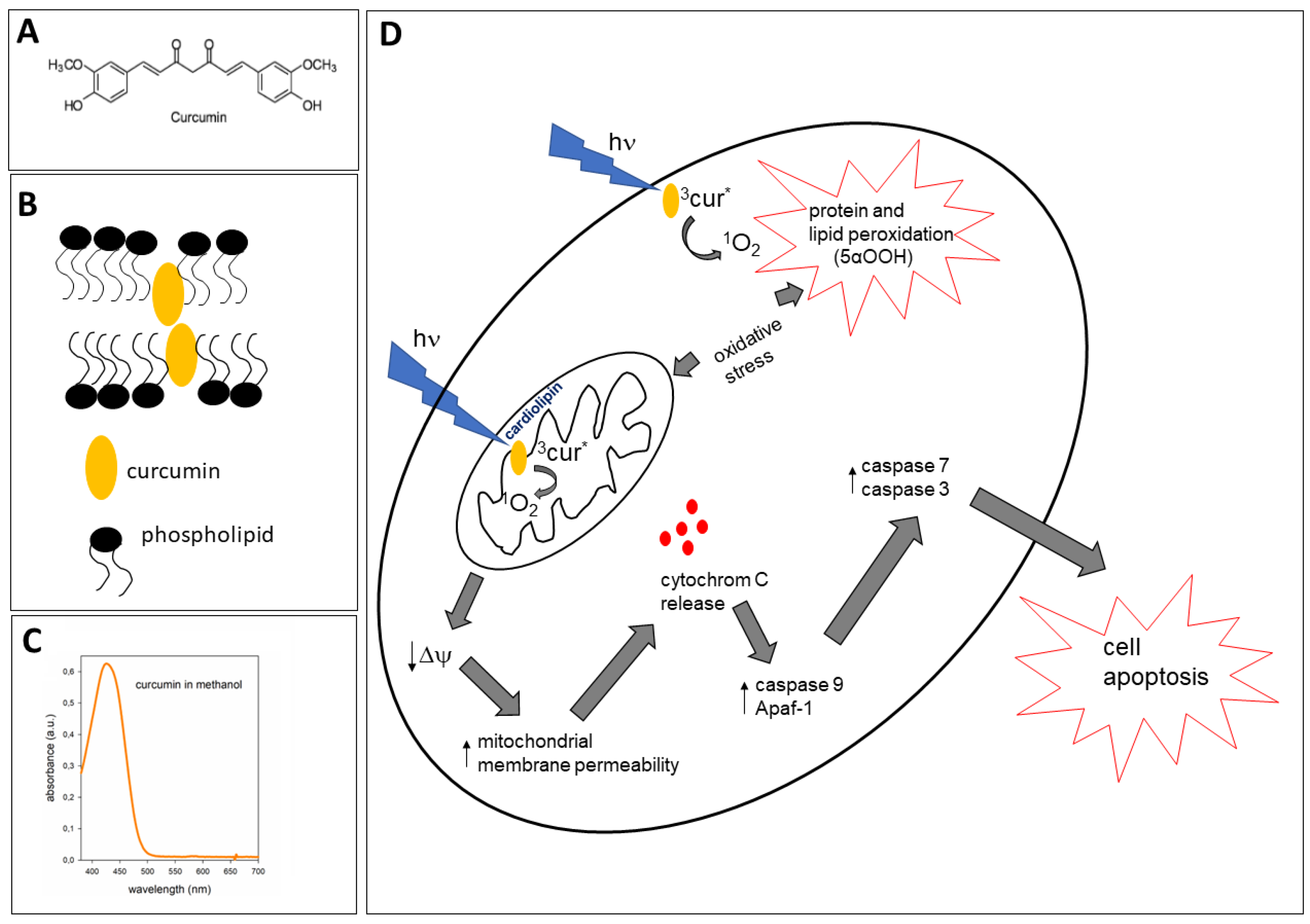
2. Curcumin–Membrane Interaction and Its Relevance to Protective and Pro-Oxidant Activity
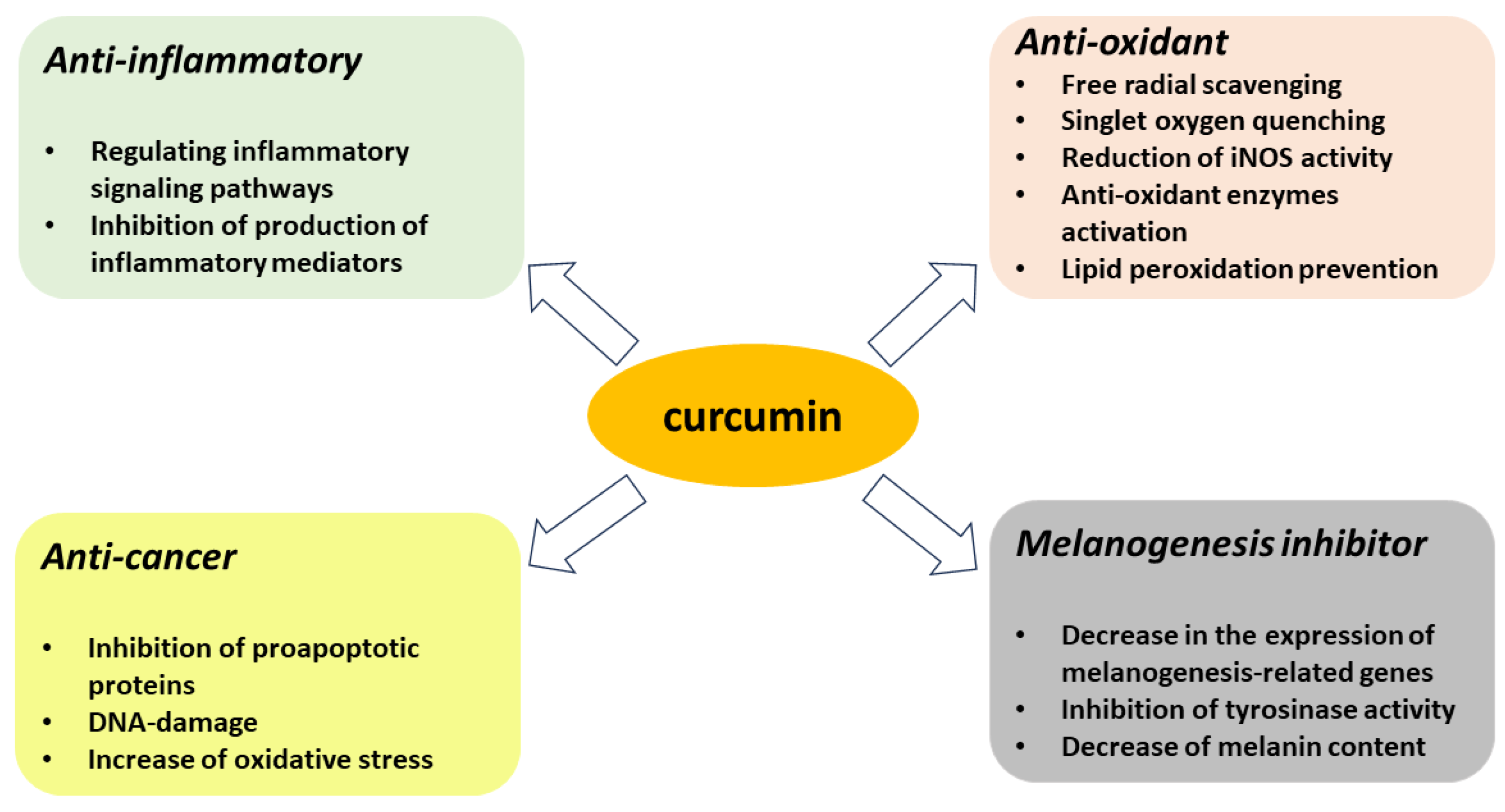
3. Antioxidant Properties of Curcumin
3.1. Curcumin as Reactive Oxygen and Nitrogen Species Scavenger/Quencher
3.2. Inhibitory Effect of Curcumin on Melanogenesis
4. Proapoptotic Effects of Curcumin
5. Pro-Oxidant Properties of Curcumin Induced by Light
5.1. Photogeneration of Singlet Oxygen (1O2) by Curcumin
5.2. Phototoxicity and Lipophilicity of Curcumin as a Base for Its Use in PDT
5.3. Antimicrobial Photodynamic Activity of Curcumin
| Type of Micro-Organisms | Type of Light/Curcumin Formula Used | Ref. |
|---|---|---|
| Bacteria | ||
| Streptococcus mutans | 405 nm LED, curcumin and Curcuma xanthorrhiza extract | [9] |
| “oral” bacteria | 455 nm LED, curcumin solution | [86] |
| Staphylococcus aureus and E. coli | 405 nm LED, curcumin@Ag core/shell structure fiber membrane | [83] |
| Staphylococcus aureus | Blue light, curcumin solution | [88] |
| Staphylococcus aureus | Biotable® device 450 nm, curcumin solution | [89] |
| Methicilin-resistant Staphylococcus aureus biofilm | 450 nm LED, curcumin solution | [90] |
| Propionibacterium acnes | 462 nm LED, curcumin solution | [91] |
| Vibrio parahaemolyticus | 470 nm LED, curcumin solution | [92] |
| Enterococcus faecalis | Blue LED, curcumin solution | [93] |
| Aggregatibacter actinomycetemcomitans | 420-480 nm LED, curcumin solution | [94] |
| Fungi | ||
| Candida albicans and other candidas | 455 nm LED, curcumin solution | [79,80,95] |
| Spores and cells of Aspergillus niger, Aspergillus flavus, Penicillium griseofulvum, Penicillium chrysogenum, Fusarium oxysporum, Candida albicans and Zygosaccharomyces bailii | 500-Watt Xenon arc lamp, 370–680 nm, curcumin solution (propylene glycol and water) | [96] |
| Trichophyton rubrum | 420 nm LED, curcumin solution | [97] |
5.4. Photodynamic Activity of Curcumin in Skin and Cancer Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dujic, J.; Kippenberger, S.; Ramirez-Bosca, A.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A.; Hofmann, M. Curcumin in Combination with Visible Light Inhibits Tumor Growth in a Xenograft Tumor Model. Int. J. Cancer 2009, 124, 1422–1428. [Google Scholar] [CrossRef]
- Shanmugam, M.; Rane, G.; Kanchi, M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.; Tan, B.; Kumar, A.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Dahll, T.A.; Bilski, P.; Reszka, K.J.; Chignell, C.F. Photocytotoxicity of Curcumin. Photochem. Photobiol. 1994, 59, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Fazlolahzadeh, O.; Atkin, S.L.; Majeed, M.; Butler, A.E.; Johnston, T.P.; Sahebkar, A. Evidence of Curcumin and Curcumin Analogue Effects in Skin Diseases: A Narrative Review. J. Cell Physiol. 2019, 234, 1165–1178. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Yang, H.; Ge, Y.; Xin, Y. Effects of Demethoxycurcumin on the Viability and Apoptosis of Skin Cancer Cells. Mol. Med. Rep. 2017, 16, 539–546. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Sousa Gonzaga, H.F.; Souza, G.A.; Alvares Goulart, R.; Sousa Gonzaga, M.L.; Alvarez Rezende, B. Dermatological Effects of Curcuma Species: A Systematic Review. Clin. Exp. Dermatol. 2021, 46, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and Neurodegenerative Diseases. BioFactors 2013, 39, 122–132. [Google Scholar] [CrossRef]
- Nabavi, S.; Thiagarajan, R.; Rastrelli, L.; Daglia, M.; Sobarzo-Sanchez, E.; Alinezhad, H.; Nabavi, S. Curcumin: A Natural Product for Diabetes and Its Complications. Curr. Top. Med. Chem. 2015, 15, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kang, S.-M.; Jeong, S.-H.; Chung, K.-H.; Kim, B.-I. Antibacterial Photodynamic Therapy with Curcumin and Curcuma xanthorrhiza Extract against Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2017, 20, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Bhavya, M.L.; Umesh Hebbar, H. Efficacy of Blue LED in Microbial Inactivation: Effect of Photosensitization and Process Parameters. Int. J. Food Microbiol. 2019, 290, 296–304. [Google Scholar] [CrossRef]
- Carmello, J.C.; Pavarina, A.C.; Oliveira, R.; Johansson, B. Genotoxic Effect of Photodynamic Therapy Mediated by Curcumin on Candida Albicans. FEMS Yeast Res. 2015, 15, fov018. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Delcanale, P.; Montali, C.; Tognolini, M.; Giorgio, C.; Corrado, M.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. Enhanced Photosensitizing Properties of Protein Bound Curcumin. Life Sci. 2019, 233, 116710. [Google Scholar] [CrossRef]
- Wolnicka-Glubisz, A.; Olchawa, M.; Duda, M.; Pabisz, P.; Wisniewska-Becker, A. The Role of Singlet Oxygen in Photoreactivity and Phototoxicity of Curcumin. Photochem. Photobiol. 2023, 99, 57–67. [Google Scholar] [CrossRef]
- Dujic, J.; Kippenberger, S.; Hoffmann, S.; Ramirez-Bosca, A.; Miquel, J.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A. Low Concentrations of Curcumin Induce Growth Arrest and Apoptosis in Skin Keratinocytes Only in Combination with UVA or Visible Light. J. Investig. Dermatol. 2007, 127, 1992–2000. [Google Scholar] [CrossRef]
- Ingolfsson, H.I.; Koeppe, R.E.; Andersen, O.S. Curcumin Is a Modulator of Bilayer Material Properties. Biochemistry 2007, 46, 10384–10391. [Google Scholar] [CrossRef]
- Duda, M.; Cygan, K.; Wisniewska-Becker, A. Effects of Curcumin on Lipid Membranes: An EPR Spin-Label Study. Cell Biochem. Biophys. 2020, 78, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-C.; Chen, F.-Y.; Lee, C.-C.; Sun, Y.; Lee, M.-T.; Huang, H.W. Membrane-Thinning Effect of Curcumin. Biophys. J. 2008, 94, 4331–4338. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, C.-C.; Hung, W.-C.; Chen, F.-Y.; Lee, M.-T.; Huang, H.W. The Bound States of Amphipathic Drugs in Lipid Bilayers: Study of Curcumin. Biophys. J. 2008, 95, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Alsop, R.J.; Dhaliwal, A.; Rheinstädter, M.C. Curcumin Protects Membranes through a Carpet or Insertion Model Depending on Hydration. Langmuir 2017, 33, 8516–8524. [Google Scholar] [CrossRef]
- Ben-Zichri, S.; Kolusheva, S.; Danilenko, M.; Ossikbayeva, S.; Stabbert, W.J.; Poggio, J.L.; Stein, D.E.; Orynbayeva, Z.; Jelinek, R. Cardiolipin Mediates Curcumin Interactions with Mitochondrial Membranes. Biochim. Biophys. Acta BBA—Biomembr. 2019, 1861, 75–82. [Google Scholar] [CrossRef]
- Sharma, V.K.; Gupta, J.; Srinivasan, H.; Bhatt, H.; García Sakai, V.; Mitra, S. Curcumin Accelerates the Lateral Motion of DPPC Membranes. Langmuir 2022, 38, 9649–9659. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lara, A.; Ausili, A.; Aranda, F.J.; de Godos, A.; Torrecillas, A.; Corbalán-García, S.; Gómez-Fernández, J.C. Curcumin Disorders 1,2-Dipalmitoyl-Sn-Glycero-3-Phosphocholine Membranes and Favors the Formation of Nonlamellar Structures by 1,2-Dielaidoyl-Sn-Glycero-3-Phosphoethanolamine. J. Phys. Chem. B 2010, 114, 9778–9786. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.S.; Lee, D.-K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef]
- Kotenkov, S.A.; Gnezdilov, O.I.; Khaliullina, A.V.; Antzutkin, O.N.; Gimatdinov, R.S.; Filippov, A.V. Effect of Cholesterol and Curcumin on Ordering of DMPC Bilayers. Appl. Magn. Reason. 2019, 50, 511–520. [Google Scholar] [CrossRef]
- Varshney, G.K.; Kintali, S.R.; Gupta, P.K.; Das, K. Effect of Bilayer Partitioning of Curcumin on the Adsorption and Transport of a Cationic Dye Across POPG Liposomes Probed by Second-Harmonic Spectroscopy. Langmuir 2016, 32, 10415–10421. [Google Scholar] [CrossRef]
- Ausili, A.; Gómez-Murcia, V.; Candel, A.M.; Beltrán, A.; Torrecillas, A.; He, L.; Jiang, Y.; Zhang, S.; Teruel, J.A.; Gómez-Fernández, J.C. A Comparison of the Location in Membranes of Curcumin and Curcumin-Derived Bivalent Compounds with Potential Neuroprotective Capacity for Alzheimer’s Disease. Colloids Surf. B. Biointerfaces 2021, 199, 111525. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Xiang, N.; Mondal, J.; Zhu, X.; Narsimhan, G. Characterization of Interactions between Curcumin and Different Types of Lipid Bilayers by Molecular Dynamics Simulation. J. Phys. Chem. B. 2018, 122, 2341–2354. [Google Scholar] [CrossRef]
- Schlame, M.; Brody, S.; Hostetler, K.Y. Mitochondrial Cardiolipin in Diverse Eukaryotes. Comparison of Biosynthetic Reactions and Molecular Acyl Species. Eur. J. Biochem. 1993, 212, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Jouhet, J. Importance of the Hexagonal Lipid Phase in Biological Membrane Organization. Front. Plant Sci. 2013, 4, 494. [Google Scholar] [CrossRef]
- Mileykovskaya, E.; Dowhan, W. Cardiolipin Membrane Domains in Prokaryotes and Eukaryotes. Biochim. Biophys. Acta BBA—Biomembr. 2009, 1788, 2084–2091. [Google Scholar] [CrossRef]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin Restores Mitochondrial Functions and Decreases Lipid Peroxidation in Liver and Kidneys of Diabetic Db/Db Mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-J.; Chang, H.-H.; Tsai, T.-H.; Lee, T.-Y. Positive Effect of Curcumin on Inflammation and Mitochondrial Dysfunction in Obese Mice with Liver Steatosis. Int. J. Mol. Med. 2012, 30, 673–679. [Google Scholar] [CrossRef]
- Pesakhov, S.; Khanin, M.; Studzinski, G.P.; Danilenko, M. Distinct Combinatorial Effects of the Plant Polyphenols Curcumin, Carnosic Acid, and Silibinin on Proliferation and Apoptosis in Acute Myeloid Leukemia Cells. Nutr. Cancer 2010, 62, 811–824. [Google Scholar] [CrossRef]
- Sen, S.; Sharma, H.; Singh, N. Curcumin Enhances Vinorelbine Mediated Apoptosis in NSCLC Cells by the Mitochondrial Pathway. Biochem. Biophys. Res. Commun. 2005, 331, 1245–1252. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Zheng, S.; Wu, T. Curcumin Induces Apoptosis through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B. 2009, 10, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, H.; Abraham, P.; Isaac, B. Mitochondrial Dysfunction and Electron Transport Chain Complex Defect in a Rat Model of Tenofovir Disoproxil Fumarate Nephrotoxicity. J. Biochem. Mol. Toxicol. 2014, 28, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Kuroda, K.; Ramamoorthy, A.; Yasuhara, K. Modulation of Raft Domains in a Lipid Bilayer by Boundary-Active Curcumin. Chem. Commun. 2014, 50, 3427. [Google Scholar] [CrossRef]
- Singh, U.; Barik, A.; Singh, B.G.; Priyadarsini, K.I. Reactions of Reactive Oxygen Species (ROS) with Curcumin Analogues: Structure–Activity Relationship. Free Radic. Res. 2011, 45, 317–325. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Ślifirski, P. Curcumin and Curcuminoids in Quest for Medicinal Status. Acta Biochim. Pol. 2012, 59, 201–212. [Google Scholar] [CrossRef]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Joe, B.; Lokesh, B.R. Role of Capsaicin, Curcumin and Dietary n − 3 Fatty Acids in Lowering the Generation of Reactive Oxygen Species in Rat Peritoneal Macrophages. Biochim. Biophys. Acta BBA—Mol. Cell Res. 1994, 1224, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Das, C.K. Curcumin (Diferuloylmethane), a Singlet Oxygen (1O2) Quencher. Biochem. Biophys. Res. Commun. 2002, 295, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Sreejayan; Rao, M.N.A. Curcuminoids as Potent Inhibitors of Lipid Peroxidation. J. Pharm. Pharmacol. 2011, 46, 1013–1016. [Google Scholar] [CrossRef]
- Masuda, T.; Maekawa, T.; Hidaka, K.; Bando, H.; Takeda, Y.; Yamaguchi, H. Chemical Studies on Antioxidant Mechanism of Curcumin: Analysis of Oxidative Coupling Products from Curcumin and Linoleate. J. Agric. Food Chem. 2001, 49, 2539–2547. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in Cancer Chemoprevention: Molecular Targets, Pharmacokinetics, Bioavailability, and Clinical Trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Dai, C.; Li, D.; Gong, L.; Xiao, X.; Tang, S. Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway. Molecules 2016, 21, 1061. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory Effect of Curcumin on Nitric Oxide Production from Lipopolysaccharide-Activated Primary Microglia. Life Sci. 2006, 79, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, H.; Qian, L.; Chen, G.; Buzby, J.S. Curcumin Protects Pre-Oligodendrocytes from Activated Microglia in Vitro and in Vivo. Brain Res. 2010, 1339, 60–69. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Chan, W.-H.; Wu, H.-J. Anti-Apoptotic Effects of Curcumin on Photosensitized Human Epidermal Carcinoma A431 Cells. J. Cell. Biochem. 2004, 92, 200–212. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of Melanogenesis—Controversies and New Concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A.; et al. Melanoma Induction by Ultraviolet A but Not Ultraviolet B Radiation Requires Melanin Pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef]
- Abdel-Malek, Z.A.; Knittel, J.; Kadekaro, A.L.; Swope, V.B.; Starner, R. The Melanocortin 1 Receptor and the UV Response of Human Melanocytes—A Shift in Paradigm. Photochem. Photobiol. 2008, 84, 501–508. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 42496. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jang, J.-Y.; Park, C.; Kim, B.-W.; Choi, Y.-H.; Choi, B.-T. Curcumin Suppresses α-Melanocyte Stimulating Hormone-Stimulated Melanogenesis in B16F10 Cells. Int. J. Mol. Med. 2010, 26, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.-J. Aromatic-Turmerone Inhibits α-MSH and IBMX-Induced Melanogenesis by Inactivating CREB and MITF Signaling Pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, K.; Kim, C.; Lee, S.-E. Antimelanogenic Effects of Curcumin and Its Dimethoxy Derivatives: Mechanistic Investigation Using B16F10 Melanoma Cells and Zebrafish (Danio Rerio) Embryos. Foods 2023, 12, 926. [Google Scholar] [CrossRef]
- Tu, C.-X.; Lin, M.; Lu, S.-S.; Qi, X.-Y.; Zhang, R.-X.; Zhang, Y.-Y. Curcumin Inhibits Melanogenesis in Human Melanocytes. Phytother. Res. 2012, 26, 174–179. [Google Scholar] [CrossRef]
- Nebrisi, E. El Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef]
- El-Shamarka, M.E.-S.; Abdel-Salam, O.M.; Shafee, N.; Zeidan, H.M. Curcumin Modulation of L-Dopa and Rasagiline-Induced Neuroprotection in Rotenone Model of Parkinson’s Disease. Iran. J. Basic Med. Sci. 2023, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wolnicka-Glubisz, A.; Nogal, K.; Żądło, A.; Płonka, P.M. Curcumin Does Not Switch Melanin Synthesis towards Pheomelanin in B16F10 Cells. Arch. Dermatol. Res. 2015, 307, 89–98. [Google Scholar] [CrossRef]
- Mastore, M.; Kohler, L.; Nappi, A.J. Production and Utilization of Hydrogen Peroxide Associated with Melanogenesis and Tyrosinase-Mediated Oxidations of DOPA and Dopamine. FEBS J. 2005, 272, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cervantes, C.; Martínez-Esparza, M.; Pérez, C.; Daum, N.; Solano, F.; García-Borrón, J.C. Inhibition of Melanogenesis in Response to Oxidative Stress: Transient Downregulation of Melanocyte Differentiation Markers and Possible Involvement of Microphthalmia Transcription Factor. J. Cell Sci. 2001, 114, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells Selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Kanai, M. Therapeutic Applications of Curcumin for Patients with Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 9384–9391. [Google Scholar]
- Crosby, N.M.; Ghosh, M.; Su, B.; Beckstead, J.A.; Kamei, A.; Simonsen, J.B.; Luo, B.; Gordon, L.I.; Forte, T.M.; Ryan, R.O. Anti-CD20 Single Chain Variable Antibody Fragment–Apolipoprotein A-I Chimera Containing Nanodisks Promote Targeted Bioactive Agent Delivery to CD20-Positive Lymphomas. Biochem. Cell Biol. 2015, 93, 343–350. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilski, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and Photochemical Properties of Curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Khopde, S.M.; Indira Priyadarsini, K.; Palit, D.K.; Mukherjee, T. Effect of Solvent on the Excited-State Photophysical Properties of Curcumin. Photochem. Photobiol. 2000, 72, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Korytowski, W.; Bachowski, G.J.; Girotti, A.W. Analysis of Cholesterol and Phospholipid Hydroperoxides by High-Performance Liquid Chromatography with Mercury Drop Electrochemical Detection. Anal. Biochem. 1993, 213, 111–119. [Google Scholar] [CrossRef]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals 2021, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Barolet, D. Light-Emitting Diodes (LEDs) in Dermatology. Semin. Cutan. Med. Surg. 2008, 27, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of Wavelength and Beam Width on Penetration in Light-Tissue Interaction Using Computational Methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; Machado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of Clinical Isolates of Candida to Photodynamic Effects of Curcumin. Lasers Surg. Med. 2011, 43, 927–934. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Carmello, J.C.; de Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-Mediated Photodynamic Inactivation of Candida albicans in a Murine Model of Oral Candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K.; Calandra, T.F.; Edwards, J.E.; Filler, S.G.; Fisher, J.F.; Kullberg, B.-J.; Zeichner, L.O.; et al. Clinical Practice Guidelines for the Management Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Perezous, L.F.; Flaitz, C.M.; Goldschmidt, M.E.; Engelmeier, R.L. Colonization of Candida Species in Denture Wearers with Emphasis on HIV Infection: A Literature Review. J. Prosthet. Dent. 2005, 93, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.; Lu, W.; Zhang, P. Fabrication of Curcumin@Ag Loaded Core/Shell Nanofiber Membrane and Its Synergistic Antibacterial Properties. Front. Chem. 2022, 10, 870666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aslan, K.; Previte, M.J.R.; Geddes, C.D. Plasmonic Engineering of Singlet Oxygen Generation. Proc. Natl. Acad. Sci. USA 2008, 105, 1798–1802. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and Silver Nanoparticles Carried out from Polysaccharide-Based Hydrogels Improved the Photodynamic Properties of Curcumin through Metal-Enhanced Singlet Oxygen Effect. Mater. Sci. Eng. C. 2020, 112, 110853. [Google Scholar] [CrossRef]
- Leite, D.P.V.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of Photodynamic Therapy with Blue Light and Curcumin as Mouth Rinse for Oral Disinfection: A Randomized Controlled Trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef]
- Paschoal, M.A.; Moura, C.M.Z.; Jeremias, F.; Souza, J.F.; Bagnato, V.S.; Giusti, J.S.M.; Santos-Pinto, L. Longitudinal Effect of Curcumin-Photodynamic Antimicrobial Chemotherapy in Adolescents during Fixed Orthodontic Treatment: A Single-Blind Randomized Clinical Trial Study. Lasers Med. Sci. 2015, 30, 2059–2065. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic Action of LED-Activated Curcumin against Staphylococcus aureus Involving Intracellular ROS Increase and Membrane Damage. Int. J. Photoenergy 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Dias, L.D.; Aguiar, A.S.N.; de Melo, N.J.; Inada, N.M.; Borges, L.L.; de Aquino, G.L.B.; Camargo, A.J.; Bagnato, V.S.; Napolitano, H.B. Structural Basis of Antibacterial Photodynamic Action of Curcumin against S. aureus. Photodiagnosis Photodyn. Ther. 2023, 43, 103654. [Google Scholar] [CrossRef]
- de Paula Ribeiro, I.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial Photodynamic Therapy with Curcumin on Methicillin-Resistant Staphylococcus aureus biofilm. Photodiagnosis Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Hu, A. Low-Dose Blue Light Irradiation Enhances the Antimicrobial Activities of Curcumin against Propionibacterium acnes. J. Photochem. Photobiol. B 2018, 189, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mou, H.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q.-J. Photodynamic Effect of Curcumin on Vibrio parahaemolyticus. Photodiagnosis Photodyn. Ther. 2016, 15, 34–39. [Google Scholar] [CrossRef] [PubMed]
- da Frota, M.F.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Bagnato, V.S.; Espir, C.G.; Berbert, F.L.C.V. Photodynamic Therapy in Root Canals Contaminated with Enterococcus faecalis Using Curcumin as Photosensitizer. Lasers Med. Sci. 2015, 30, 1867–1872. [Google Scholar] [CrossRef]
- Najafi, S.; Khayamzadeh, M.; Paknejad, M.; Poursepanj, G.; Kharazi Fard, M.J.; Bahador, A. An In Vitro Comparison of Antimicrobial Effects of Curcumin-Based Photodynamic Therapy and Chlorhexidine, on Aggregatibacter actinomycetemcomitans. J. Lasers Med. Sci. 2016, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.d.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the Photodynamic Effects of Curcumin Against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A Novel Photosensitization Treatment for the Inactivation of Fungal Spores and Cells Mediated by Curcumin. J. Photochem. Photobiol. B. 2017, 173, 301–306. [Google Scholar] [CrossRef]
- Brasch, J.; Beck-Jendroschek, V.; Mahn, V. Photochemical Inhibition of Trichophyton rubrum by Different Compoundings of Curcumin. Mycoses 2018, 61, 393–399. [Google Scholar] [CrossRef]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine Signaling in the Skin with a Special Focus on the Epidermal Neuropeptides. Am. J. Physiol.-Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Nogueira, L.; Tracey, A.T.; Alvim, R.; Reisz, P.; Scherz, A.; Coleman, J.A.; Kim, K. Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies. Molecules 2020, 25, 5417. [Google Scholar] [CrossRef]
- Beyer, K.; Nikfarjam, F.; Butting, M.; Meissner, M.; König, A.; Ramirez Bosca, A.; Kaufmann, R.; Heidemann, D.; Bernd, A.; Kippenberger, S.; et al. Photodynamic Treatment of Oral Squamous Cell Carcinoma Cells with Low Curcumin Concentrations. J. Cancer 2017, 8, 1271–1283. [Google Scholar] [CrossRef]
- Abdel Fadeel, D.A.; Kamel, R.; Fadel, M. PEGylated Lipid Nanocarrier for Enhancing Photodynamic Therapy of Skin Carcinoma Using Curcumin: In-Vitro/in-Vivo Studies and Histopathological Examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.; Dobra, J.; Goerg, K.; Hoffmann, S.; Kippenberger, S.; Kaufmann, R.; Hofmann, M.; Bernd, A. Visible Light Is a Better Co-Inducer of Apoptosis for Curcumin-Treated Human Melanoma Cells than UVA. PLoS ONE 2013, 8, e79748. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Tian, Y.; Mei, Z.; Guo, G. Inhibition of Autophagy Enhances Curcumin United Light Irradiation-Induced Oxidative Stress and Tumor Growth Suppression in Human Melanoma Cells. Sci. Rep. 2016, 6, 31383. [Google Scholar] [CrossRef]
- Szlasa, W.; Supplitt, S.; Drąg-Zalesińska, M.; Przystupski, D.; Kotowski, K.; Szewczyk, A.; Kasperkiewicz, P.; Saczko, J.; Kulbacka, J. Effects of Curcumin Based PDT on the Viability and the Organization of Actin in Melanotic (A375) and Amelanotic Melanoma (C32)—In Vitro Studies. Biomed. Pharmacother. 2020, 132, 110883. [Google Scholar] [CrossRef] [PubMed]
- Roos, F.; Binder, K.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Juengel, E.; Blaheta, R.A. The Antitumor Effect of Curcumin in Urothelial Cancer Cells Is Enhanced by Light Exposure In Vitro. Evid.-Based Complement. Altern. Med. 2019, 2019, 6374940. [Google Scholar] [CrossRef]
- Mani, J.; Fleger, J.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Relja, B.; Juengel, E.; et al. Curcumin Combined with Exposure to Visible Light Blocks Bladder Cancer Cell Adhesion and Migration by an Integrin Dependent Mechanism. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10564–10574. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, L.; Feng, C.; Gong, R.; Idiiatullina, E.; Huang, Q.; He, M.; Guo, S.; Yang, F.; Li, Y.; et al. Blue Light Emitting Diodes Irradiation Causes Cell Death in Colorectal Cancer by Inducing ROS Production and DNA Damage. Int. J. Biochem. Cell Biol. 2018, 103, 81–88. [Google Scholar] [CrossRef]
- Şueki, F.; Ruhi, M.K.; Gülsoy, M. The Effect of Curcumin in Antitumor Photodynamic Therapy: In Vitro Experiments with Caco-2 and PC-3 Cancer Lines. Photodiagnosis Photodyn. Ther. 2019, 27, 95–99. [Google Scholar] [CrossRef]
- Vetha, B.S.S.; Kim, E.-M.; Oh, P.-S.; Kim, S.H.; Lim, S.T.; Sohn, M.-H.; Jeong, H.-J. Curcumin Encapsulated Micellar Nanoplatform for Blue Light Emitting Diode Induced Apoptosis as a New Class of Cancer Therapy. Macromol. Res. 2019, 27, 1179–1184. [Google Scholar] [CrossRef]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin Derivatives as Photosensitizers in Photodynamic Therapy: Photophysical Properties and in Vitro Studies with Prostate Cancer Cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.; Maxeiner, S.; Juengel, E.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Blaheta, R.A. Growth and Proliferation of Renal Cell Carcinoma Cells Is Blocked by Low Curcumin Concentrations Combined with Visible Light Irradiation. Int. J. Mol. Sci. 2019, 20, 1464. [Google Scholar] [CrossRef] [PubMed]
- Rutz, J.; Maxeiner, S.; Justin, S.; Bachmeier, B.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Blaheta, R.A. Low Dosed Curcumin Combined with Visible Light Exposure Inhibits Renal Cell Carcinoma Metastatic Behavior in Vitros. Cancers 2020, 12, 302. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, S.; Li, K.; Wang, M.; Zhu, R.; Sun, X.; Wang, Q.; Wang, S. The Triplet State of Tanshinone I and Its Synergic Effect on the Phototherapy of Cancer Cells with Curcumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ellerkamp, V.; Bortel, N.; Schmid, E.; Kirchner, B.; Armeanu-Ebinger, S.; Fuchs, J. Photodynamic Therapy Potentiates the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells. Anticancer Res. 2016, 36, 3363–3372. [Google Scholar]
- Banerjee, S.; Prasad, P.; Hussain, A.; Khan, I.; Kondaiah, P.; Chakravarty, A.R. Remarkable Photocytotoxicity of Curcumin in HeLa Cells in Visible Light and Arresting Its Degradation on Oxovanadium(Iv) Complex Formation. Chem. Commun. 2012, 48, 7702. [Google Scholar] [CrossRef]
- He, G.; Mu, T.; Yuan, Y.; Yang, W.; Zhang, Y.; Chen, Q.; Bian, M.; Pan, Y.; Xiang, Q.; Chen, Z.; et al. Effects of Notch Signaling Pathway in Cervical Cancer by Curcumin Mediated Photodynamic Therapy and Its Possible Mechanisms in Vitro and in Vivo. J. Cancer 2019, 10, 4114–4122. [Google Scholar] [CrossRef]
- de Matos, R.P.A.; Calmon, M.F.; Amantino, C.F.; Villa, L.L.; Primo, F.L.; Tedesco, A.C.; Rahal, P. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Cervical Carcinoma Cell Lines. BioMed Res. Int. 2018, 2018, 4057959. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, R.; He, X.; Wang, J.; Wang, M.; Qian, Y.; Wang, S. Enhanced Photocytotoxicity of Curcumin Delivered by Solid Lipid Nanoparticles. Int. J. Nanomed. 2016, 12, 167–178. [Google Scholar] [CrossRef]
- Baghdan, E.; Duse, L.; Schüer, J.J.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Development of Inhalable Curcumin Loaded Nano-In-Microparticles for Bronchoscopic Photodynamic Therapy. Eur. J. Pharm. Sci. 2019, 132, 63–71. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef]
- Rahimi-Moghaddam, F.; Sattarahmady, N.; Azarpira, N. Gold-Curcumin Nanostructure in Photothermal Therapy on Breast Cancer Cell Line: 650 and 808 Nm Diode Lasers as Light Sources. J. Biomed. Phys. Eng. 2018, 9, 473–782. [Google Scholar] [CrossRef]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-Loaded Nanostructured Lipid Carriers Prepared Using PeceolTM and Olive Oil in Photodynamic Therapy: Development and Application in Breast Cancer Cell Line. Int. J. Nanomed. 2019, 14, 5073–5085. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.C.; Adum de Matos, R.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Breast Adenocarcinoma Cell Line. Bioorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Shahidi, F.K. Photodynamic Treatment with Anionic Nanoclays Containing Curcumin on Human Triple-negative Breast Cancer Cells: Cellular and Biochemical Studies. J. Cell Biochem. 2019, 120, 4998–5009. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Y.; He, Y.; Xiong, M.; Huang, H.; Pei, S.; Liao, J.; Wang, Y.; Shao, D. Green Synthesis of Carrier-Free Curcumin Nanodrugs for Light-Activated Breast Cancer Photodynamic Therapy. Colloids Surf. B. Biointerfaces 2019, 180, 313–318. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Yu, K.-H.; Huang, Y.-C.; Lee, C.-I. EGFR-Targeted Photodynamic Therapy by Curcumin-Encapsulated Chitosan/TPP Nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef]
- Szewczyk, G.; Zadlo, A.; Sarna, M.; Ito, S.; Wakamatsu, K.; Sarna, T. Aerobic Photoreactivity of Synthetic Eumelanins and Pheomelanins: Generation of Singlet Oxygen and Superoxide Anion. Pigment Cell Melanoma Res. 2016, 29, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Zbyradowski, M.; Duda, M.; Wisniewska-Becker, A.; Heriyanto; Rajwa, W.; Fiedor, J.; Cvetkovic, D.; Pilch, M.; Fiedor, L. Triplet-Driven Chemical Reactivity of β-Carotene and Its Biological Implications. Nat. Commun. 2022, 13, 2474. [Google Scholar] [CrossRef]
- Makhneva, Z.K.; Ashikhmin, A.A.; Bolshakov, M.A.; Moskalenko, A.A. Carotenoids Are Probably Involved in Singlet Oxygen Generation in the Membranes of Purple Photosynthetic Bacteria under Light Irradiation. Microbiology 2020, 89, 164–173. [Google Scholar] [CrossRef]
- Yoshii, H.; Yoshii, Y.; Asai, T.; Furukawa, T.; Takaichi, S.; Fujibayashi, Y. Photo-Excitation of Carotenoids Causes Cytotoxicity via Singlet Oxygen Production. Biochem. Biophys. Res. Commun. 2012, 417, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, N.C.; Mordorski, B.; Nosanchuk, J.; Friedman, J.M.; Friedman, A.J. Curcumin Nanoparticles as a Photoprotective Adjuvant. Exp. Dermatol. 2021, 30, 705–709. [Google Scholar] [CrossRef] [PubMed]
| Type of Cancer Cells | Type of Light and Formula Used | Ref |
|---|---|---|
| Oral cancer | ||
| HN cells | VIS, 5500 lx, UVA, curcumin solution | [101] |
| Skin cancer cells | ||
| A431/xenograft | VIS, 5500 lx, UVA, curcumin solution | [1] |
| A431 | VIS, curcumin solution | |
| A431 | VIS/PEGylated lipid nanocarrier in vitro | [102] |
| SCC | 380–550 nm, Lip-cur | [74] |
| Melanoma | ||
| G-361, A375 | VIS, 5500 lx, UVA, curcumin solution | [103] |
| A375 | combination 630 nm + 405 nm polarized blue light, solution DMSO | [104] |
| A735, C32 | [105] | |
| MugMel2 | VIS (380–550 nm), Lip-cur | [74] |
| Bladder cancer | ||
| RT112,UMUC3, TCCSUP | VIS, 5500 lx, curcumin solution | [106] |
| RT112,UMUC3, TCCSUP | VIS, 5500 lx, curcumin solution | [107] |
| Colon cancer | ||
| SW620, HT29 | 470 nm, curcumin solution | [108] |
| CaCo2 | 5-ALA + 635 nm diode laser system/solution DMSO | [109] |
| CaCo2 | 424 nm, CAgNPs | [85] |
| mouse colorectal-CT26 | 450 nm, F127-curcumin micelles | [110] |
| Prostate cancer | ||
| PC3 | 5-ALA + 635 nm diode laser system/solution DMSO | [109] |
| LNCaP | 430 nm LED, curcumin solution | [111] |
| Kidney cancer | ||
| A498, Caki1, KTCTL-26 | VIS, 5500 lx, curcumin solution | [112] |
| A498, Caki1, KTCTL-26 | VIS, 5500 lx, curcumin solution | [113] |
| Liver cancer | ||
| SMMC-7721 | 430 nm, curcumin solution | [114] |
| HuH6, HepT1,Hep-G2, HC-AFW1 | 390–440 nm, curcumin solution | [115] |
| Cervical cancer | ||
| HeLa | VIS (400–700 nm), curcumin solution | [116] |
| Me180 | 445 nm laser, Cur-LDH | [117] |
| SiHa, CasKi | 447 nm LED, nano-emulsion | [118] |
| Lung cancer | ||
| A549 | 430 nm LED, Cur-SLN | [119] |
| A549 | 457 nm LED, PLGA nanoparticles | [120] |
| Ovarian cancer | ||
| SK-OV3 | 457 nm, 620 nm LED), Cur-NP | [121] |
| Breast cancer | ||
| MCF-7 | 430 nm, Cur-NLCs | [122] |
| MCF-7 | 440 nm LED, nano-emulsion | [123] |
| MDA-MB-231 | curcumin-LDH nanoparticles | [124] |
| mouse 4T1 | Cur NDs | [125] |
| mouse 4T1 | Photothermal/Au-Cur nanostructure | [126] |
| Gastric cancer | ||
| MKN45 | 460 nm LED, nano-encapsulated curcumin with EGF | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolnicka-Glubisz, A.; Wisniewska-Becker, A. Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective. Antioxidants 2023, 12, 1725. https://doi.org/10.3390/antiox12091725
Wolnicka-Glubisz A, Wisniewska-Becker A. Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective. Antioxidants. 2023; 12(9):1725. https://doi.org/10.3390/antiox12091725
Chicago/Turabian StyleWolnicka-Glubisz, Agnieszka, and Anna Wisniewska-Becker. 2023. "Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective" Antioxidants 12, no. 9: 1725. https://doi.org/10.3390/antiox12091725









