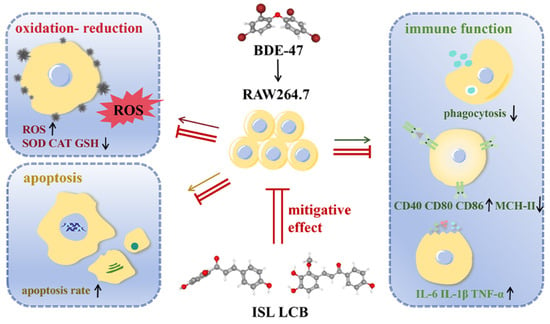
Protective Effects of Isoliquiritigenin and Licochalcone B on the Immunotoxicity of BDE-47: Antioxidant Effects Based on the Activation of the Nrf2 Pathway and Inhibition of the NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Experimental Design
2.3. Cell Viability Assay
2.4. Phagocytic Capacity of Cells
2.5. Enzyme-Linked Immunosorbent (ELISA) Assay
2.6. Observation of Nuclear Morphology
2.7. Apoptotic Rate Detection
2.8. Western Blotting Assay
2.9. Examination of ROS Levels
2.10. Analysis of Antioxidant Substance Activity
2.11. Quantitative Real-Time Polymerase Chain Reaction (QRT-PCR) Analysis
2.12. Statistical Analysis
3. Results
3.1. ISL and LCB Alleviate BDE-47’s Cytotoxic Effects on RAW264.7
3.2. ISL and LCB Ameliorate BDE-47-Induced Immune Impairment
3.3. ISL and LCB Alleviate BDE-47-Induced Apoptosis
3.4. ISL and LCB Alleviate BDE-47-Induced Oxidative Stress
3.5. ISL and LCB Activate the Nrf2 Pathway and Inhibit the NF-ĸB Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Liu, B.; Yu, Y.; Li, H.; Li, Q.; Cui, Y.; Ma, Y. Polybrominated diphenyl ethers (PBDEs) in household dust: A systematic review on spatio-temporal distribution, sources, and health risk assessment. Chemosphere 2023, 314, 137641. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Song, Q.; Yuan, W.; Ruan, J.; Duan, H.; Li, Y.; Li, J. Human exposure to PBDEs in e-waste areas: A review. Environ. Pollut. 2020, 267, 115634. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Polybrominated diphenyl ethers in the environmental systems: A review. J. Environ. Health Sci. Eng. 2021, 19, 1229–1247. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhou, Z.Y.; He, Y.N.; Dong, M.H.; Wang, Z.N.; Chen, H.M. BDE-47 Induces Immunotoxicity in RAW264.7 Macrophages through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway. Molecules 2023, 28, 2036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Lind, P.M.; Salihovic, S.; van Bavel, B.; Ekdahl, K.N.; Nilsson, B.; Lind, L.; Ingelsson, E. Influence of persistent organic pollutants on the complement system in a population-based human sample. Environ. Int. 2014, 71, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Schauer, J.; Pessah, I.N.; Van de Water, J. Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. J. Neuroimmunol. 2009, 208, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Ruiz, C.; Morghese, M.; Renda, G.; Gugliandolo, C.; Esteban, M.A.; Santulli, A.; Messina, C.M. Effects of BDE-47 exposure on immune-related parameters of Mytilus galloprovincialis. Aquat. Toxicol. 2019, 215, 105266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tang, X.; Sun, T.; Wang, Y. BDE-47 exposure changed the immune function of haemocytes in Mytilus edulis: An explanation based on ROS-mediated pathway. Aquat. Toxicol. 2017, 182, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, T.; Dong, S.; Chen, H. Immunotoxicity Evaluation of Tetrabromodiphenyl Ether (BDE-47) on Mice and RAW264.7 Macrophages. In Proceedings of the 9th International Congress of Asian Society of Toxicology (ASIATOX-IX) 8th CST Youth Forum of Science & Technology, Hangzhou, China, 20–23 October 2021; pp. 45–46. [Google Scholar]
- Longo, V.; Longo, A.; Di Sano, C.; Cigna, D.; Cibella, F.; Di Felice, G.; Colombo, P. In vitro exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) impairs innate inflammatory response. Chemosphere 2019, 219, 845–854. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, J. In vitro immunotoxicity and possible mechanisms of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) on Ruditapes philippinarum hemocytes. Fish Shellfish Immunol. 2022, 127, 386–395. [Google Scholar] [CrossRef]
- Arkoosh, M.R.; Van Gaest, A.L.; Strickland, S.A.; Hutchinson, G.P.; Krupkin, A.B.; Hicks, M.B.R.; Dietrich, J.P. Dietary exposure to a binary mixture of polybrominated diphenyl ethers alters innate immunity and disease susceptibility in juvenile Chinook salmon (Oncorhynchus tshawytscha). Ecotoxicol. Environ. Saf. 2018, 163, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Arkoosh, M.R.; Van Gaest, A.L.; Strickland, S.A.; Hutchinson, G.P.; Krupkin, A.B.; Dietrich, J.P. Dietary Exposure to Individual Polybrominated Diphenyl Ether Congeners BDE-47 and BDE-99 Alters Innate Immunity and Disease Susceptibility in Juvenile Chinook Salmon. Environ. Sci. Technol. 2015, 49, 6974–6981. [Google Scholar] [CrossRef] [PubMed]
- Lamkin, D.M.; Chen, S.; Bradshaw, K.P.; Xu, S.; Faull, K.F.; Sloan, E.K.; Cole, S.W. Low-dose exposure to PBDE disrupts genomic integrity and innate immunity in mammary tissue. Front. Genet. 2022, 13, 904607. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Wei, J.; Lu, W.; Xia, B.; Li, S.; Liu, D.; Liu, N.; Wang, X.; Lin, G. BDE-47 disturbs the immune response of lymphocytes to LPS by downregulating NF-κB pathway. Chemosphere 2022, 308 Pt 3, 136562. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Loch-Caruso, R. Protective effect of nuclear factor E2-related factor 2 on inflammatory cytokine response to brominated diphenyl ether-47 in the HTR-8/SVneo human first trimester extravillous trophoblast cell line. Toxicol. Appl. Pharmacol. 2014, 281, 67–77. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 signaling pathway by natural and synthetic chalcones: A therapeutic road map for oxidative stress. Expert. Rev. Clin. Pharmacol. 2021, 14, 465–480. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, W.; Yang, X.; Lu, T.; Liu, Y. Isoliquiritigenin, a potential therapeutic agent for treatment of inflammation-associated diseases. J. Ethnopharmacol. 2024, 318 Pt B, 117059. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; Hu, Y.; Zhu, J.; Xia, R.; Yu, Y.; Shen, J.; Zhang, Z.; Wang, S.L. Isoliquiritigenin as an antioxidant phytochemical ameliorates the developmental anomalies of zebrafish induced by 2,2′,4,4′-tetrabromodiphenyl ether. Sci. Total Environ. 2019, 666, 390–398. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, J.R.; Ku, S.K.; Cho, I.J.; Byun, S.H.; Kim, S.C.; Park, S.J.; Kim, Y.W. Isoliquiritigenin in licorice functions as a hepatic protectant by induction of antioxidant genes through extracellular signal-regulated kinase-mediated NF-E2-related factor-2 signaling pathway. Eur. J. Nutr. 2016, 55, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, H.; Zhang, B.; Zhang, L. Licochalcone B attenuates neuronal injury through anti-oxidant effect and enhancement of Nrf2 pathway in MCAO rat model of stroke. Int. Immunopharmacol. 2021, 100, 108073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, C.; Zhao, G.; Li, M.; Ma, D.; Meng, Q.; Tang, W.; Huang, Q.; Shi, P.; Li, Y.; et al. PM2.5 Synergizes with Pseudomonas aeruginosa to Suppress Alveolar Macrophage Function in Mice Through the mTOR Pathway. Front. Pharmacol. 2022, 13, 924242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.M.; Hu, Y.Y.; Yang, T.; Wu, N.; Wang, X.N. Reactive Oxygen Species and Oxidative Stress in Vascular-Related Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7906091. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xu, J.; Wu, J. The Promising Role of Chemokines in Vitiligo: From Oxidative Stress to the Autoimmune Response. Oxid. Med. Cell. Longev. 2022, 2022, 8796735. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Lian, X.; Liu, H.; Zhang, Y.; Li, Q.; Cai, Y.; Ma, H.; Yu, X. Understanding Diabetic Neuropathy: Focus on Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 9524635. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.A.M.; Al-Dolaimy, F.; Qasim Almajidi, Y.; Baymakov, S.; Kader, M.M.; Ullah, M.I.; Abbas, A.H.R.; Khlewee, I.H.; Bisht, Y.S.; Alsaalamy, A.H. Oxidative stress affects the beginning of the growth of cancer cells through a variety of routes. Pathol. Res. Pract. 2023, 249, 154664. [Google Scholar] [CrossRef]
- Jain, R.; Hussein, M.A.; Pierce, S.; Martens, C.; Shahagadkar, P.; Munirathinam, G. Oncopreventive and oncotherapeutic potential of licorice triterpenoid compound glycyrrhizin and its derivatives: Molecular insights. Pharmacol. Res. 2022, 178, 106138. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Nie, X.; Wu, Y.; Zhang, C.; Lee, S.M.; Lv, K.; Leung, G.P.; Fu, C.; Zhang, J.; et al. Natural compound glycyrrhetinic acid protects against doxorubicin-induced cardiotoxicity by activating the Nrf2/HO-1 signaling pathway. Phytominedicine 2022, 106, 154407. [Google Scholar] [CrossRef]
- Tang, S.; Liu, H.; Yin, H.; Liu, X.; Peng, H.; Lu, G.; Dang, Z.; He, C. Effect of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) and its metabolites on cell viability, oxidative stress, and apoptosis of HepG2. Chemosphere 2018, 193, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, Z.; Rao, Q.; Li, X.; Ruan, Z.; Yang, J. BDE-47 induces nephrotoxicity through ROS-dependent pathways of mitochondrial dynamics in PK15 cells. Ecotoxicol. Environ. Saf. 2021, 222, 112549. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Y.; Jiang, Y.; Cheng, J.; Xu, S.; Zhang, J. Cellular metabolomics reveals glutamate and pyrimidine metabolism pathway alterations induced by BDE-47 in human neuroblastoma SK-N-SH cells. Ecotoxicol. Environ. Saf. 2019, 182, 109427. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hu, B.; Zheng, H.; Qian, X.; Zhang, Y.; Zhu, J.; Xu, G.; Chen, D.; Jin, X.; Li, W.; et al. 2,2′,4,4′-Tetrabromodiphenyl ether (BDE-47) activates Aryl hydrocarbon receptor (AhR) mediated ROS and NLRP3 inflammasome/p38 MAPK pathway inducing necrosis in cochlear hair cells. Ecotoxicol. Environ. Saf. 2021, 221, 112423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, B.; Qian, X.; Xu, G.; Jin, X.; Chen, D.; Tang, J.; Xu, L. Transcriptomics-based analysis of co-exposure of cadmium (Cd) and 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) indicates mitochondrial dysfunction induces NLRP3 inflammasome and inflammatory cell death in renal tubular epithelial cells. Ecotoxicol. Environ. Saf. 2022, 241, 113790. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Yang, G.; Li, Y.; Fu, Y.; Zheng, Y.; Jiang, X. The licorice flavonoid isoliquiritigenin attenuates Mycobacterium tuberculosis-induced inflammation through Notch1/NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 2022, 294, 115368. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jun, J.G. Licochalcone B exhibits anti-inflammatory effects via modulation of NF-κB and AP-1. Biomed. Sci. Lett. 2015, 21, 218–226. [Google Scholar] [CrossRef]
- Wang, L.J.; He, L.; Hao, L.; Guo, H.L.; Zeng, X.P.; Bi, Y.W.; Lu, G.T.; Li, Z.S.; Hu, L.H. Isoliquiritigenin ameliorates caerulein-induced chronic pancreatitis by inhibiting the activation of PSCs and pancreatic infiltration of macrophages. J. Cell Mol. Med. 2020, 24, 9667–9681. [Google Scholar] [CrossRef]
- Luan, P.; Zhang, H.; Chen, X.; Zhu, Y.; Hu, G.; Cai, J.; Zhang, Z. Melatonin relieves 2,2,4,4-tetrabromodiphenyl ether (BDE-47)-induced apoptosis and mitochondrial dysfunction through the AMPK-Sirt1-PGC-1α axis in fish kidney cells (CIK). Ecotoxicol. Environ. Saf. 2022, 232, 113276. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, B.; Chen, H.; Lu, K.; Wang, Y. Oxidative stress activates the Nrf2-mediated antioxidant response and P38 MAPK pathway: A possible apoptotic mechanism induced by BDE-47 in rainbow trout (Oncorhynchus mykiss) gonadal RTG-2 cells. Environ. Pollut. 2021, 287, 117341. [Google Scholar] [CrossRef]
- Meng, S.; Chen, X.; Gyimah, E.; Xu, H.; Chen, J. Hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish following sub-chronic exposure to BDE-47 and BDE-153. Environ. Toxicol. 2020, 35, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kwak, I.S. Apoptotic p53 Gene Expression in the Regulation of Persistent Organic Pollutant (POP)-Induced Oxidative Stress in the Intertidal Crab Macrophthalmusjaponicus. Antioxidants 2022, 11, 771. [Google Scholar] [CrossRef]
- Zhuang, J.; Pan, Z.J.; Mengqiu, L.; Hong, F.S.; Zhu, C.K.; Wu, N.; Chang, G.; Wang, H.; Zhao, X.X. BDE-47 induced apoptosis in zebrafish embryos through mitochondrial ROS-mediated JNK signaling. Chemosphere 2020, 258, 127385. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Zhao, Y.; Ma, F.; Lin, Z.; Liu, Y.; Dong, Z.; Chen, G.; Liu, D. PBDEs disrupt homeostasis maintenance and regeneration of planarians due to DNA damage, proliferation and apoptosis anomaly. Ecotoxicol. Environ. Saf. 2022, 248, 114287. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.; You, X.; Zhou, B.; Wang, Y.; Zhou, Z. Purine Metabolism and Pyrimidine Metabolism Alteration Is a Potential Mechanism of BDE-47-Induced Apoptosis in Marine Rotifer Brachionus plicatilis. Int. J. Mol. Sci. 2023, 24, 12726. [Google Scholar] [CrossRef]
- Li, K.; Geng, Y.; Lin, B.; Xi, Z. Molecular mechanisms underlying mitochondrial damage, endoplasmic reticulum stress, and oxidative stress induced by environmental pollutants. Toxicol. Res. 2023, 12, 1014–1023. [Google Scholar] [CrossRef]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski. 2020, 48, 124–127. [Google Scholar] [PubMed]
- Yang, Z.; Min, Z.; Yu, B. Reactive oxygen species and immune regulation. Int. Rev. Immunol. 2020, 39, 292–298. [Google Scholar] [CrossRef]
- Lee, S.; Ko, E.; Lee, H.; Kim, K.T.; Choi, M.; Shin, S. Mixed Exposure of Persistent Organic Pollutants Alters Oxidative Stress Markers and Mitochondrial Function in the Tail of Zebrafish Depending on Sex. Int. J. Environ. Res. Public Health 2021, 18, 9539. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Xie, S.; Lai, Y.; Mo, C.; Zeng, T.; Kuang, S.; Zhou, C.; Zeng, Z.; Chen, Y.; et al. Isoliquiritigenin alleviates liver fibrosis through caveolin-1-mediated hepatic stellate cells ferroptosis in zebrafish and mice. Phytomedicine 2022, 101, 154117. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Nam, B.R.; Huh, J.W.; Lee, D.S. Isoliquiritigenin Reduces LPS-Induced Inflammation by Preventing Mitochondrial Fission in BV-2 Microglial Cells. Inflammation 2021, 44, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Min, J.S.; Lee, H.S.; Lee, D.S. Isoliquiritigenin attenuates glutamate-induced mitochondrial fission via calcineurin-mediated Drp1 dephosphorylation in HT22 hippocampal neuron cells. Neurotoxicology 2018, 68, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wu, J.; Tang, Y.; Yun, X.; Lo, H.H.; Yu, L.; Li, W.; Wu, A.; Law, B.Y.K. Licochalcone B, a Natural Autophagic Agent for Alleviating Oxidative Stress-Induced Cell Death in Neuronal Cells and Caenorhabditis elegans Models. Pharmaceuticals 2022, 15, 1052. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Qian, D.W.; Xie, Z.; Hui, H. Protective role of licochalcone B against ethanol-induced hepatotoxicity through regulation of Erk signaling. Iran. J. Basic Med. Sci. 2017, 20, 131–137. [Google Scholar] [CrossRef]
- Teng, H.; Chen, M.; Zou, A.; Jiang, H.; Han, J.; Sun, L.; Feng, C.; Liu, J. Hepatoprotective effects of licochalcone B on carbon tetrachloride-induced liver toxicity in mice. Iran. J. Basic Med. Sci. 2016, 19, 910–915. [Google Scholar] [PubMed]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Yan, Z.; Qi, W.; Zhan, J.; Lin, Z.; Lin, J.; Xue, X.; Pan, X.; Zhou, Y. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J. Cell. Mol. Med. 2020, 24, 13046–13057. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, J.; Li, M.; Zhao, Y.; Guo, Y.; Xu, J.; Liu, R.; Li, Z.; Ren, D.; Liu, P. Liquiritigenin Confers Liver Protection by Enhancing NRF2 Signaling through Both Canonical and Non-canonical Signaling Pathways. J. Med. Chem. 2023, 66, 11324–11334. [Google Scholar] [CrossRef]
- Ni, B.; Liu, Y.; Gao, X.; Cai, M.; Fu, J.; Yin, X.; Ni, J.; Dong, X. Isoliquiritigenin attenuates emodin-induced hepatotoxicity in vivo and in vitro through Nrf2 pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109430. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Qiao, S.; Ma, W.; Cai, J.; Zhang, X.; Zhang, Z. Melatonin administration alleviates 2,2,4,4-tetra-brominated diphenyl ether (PBDE-47)-induced necroptosis and secretion of inflammatory factors via miR-140-5p/TLR4/NF-κB axis in fish kidney cells. Fish Shellfish Immunol. 2022, 128, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Zhuang, J.; Zheng, G.; Zhang, Z.; Zhang, Y.; Lu, J.; Zheng, Y. Troxerutin Reduces Kidney Damage against BDE-47-Induced Apoptosis via Inhibiting NOX2 Activity and Increasing Nrf2 Activity. Oxid. Med. Cell. Longev. 2017, 2017, 6034692. [Google Scholar] [CrossRef]
- Shaoyong, W.; Liu, Y.; Xu, B.; Pan, B.; Xianmi, X.; Wang, Y.; Jin, M. Exposure to BDE-47 causes female infertility risk and induces oxidative stress and lipotoxicity-mediated ovarian hormone secretion disruption in mice. Sci. Total Environ. 2022, 842, 156885. [Google Scholar] [CrossRef] [PubMed]
- Matta, K.; Lefebvre, T.; Vigneau, E.; Cariou, V.; Marchand, P.; Guitton, Y.; Royer, A.L.; Ploteau, S.; Le Bizec, B.; Antignac, J.P.; et al. Associations between persistent organic pollutants and endometriosis: A multiblock approach integrating metabolic and cytokine profiling. Environ. Int. 2022, 158, 106926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Y.; Xiao, X.; Liu, A.; Wang, S.; Preston, R.J.S.; Zaytseva, Y.Y.; He, G.; Xiao, W.; Hennig, B.; et al. Inflammation and cardiometabolic diseases induced by persistent organic pollutants and nutritional interventions: Effects of multi-organ interactions. Environ. Pollut. 2023, 339, 122756. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, F.A. Hyperinflammation, apoptosis, and organ damage. Exp. Biol. Med. 2022, 247, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [CrossRef]
- Shan, Q.; Zheng, G.H.; Han, X.R.; Wen, X.; Wang, S.; Li, M.Q.; Zhuang, J.; Zhang, Z.F.; Hu, B.; Zhang, Y.; et al. Troxerutin Protects Kidney Tissue against BDE-47-Induced Inflammatory Damage through CXCR4-TXNIP/NLRP3 Signaling. Oxid. Med. Cell. Longev. 2018, 2018, 9865495. [Google Scholar] [CrossRef]
- Liao, Y.; Tan, R.Z.; Li, J.C.; Liu, T.T.; Zhong, X.; Yan, Y.; Yang, J.K.; Lin, X.; Fan, J.M.; Wang, L. Isoliquiritigenin Attenuates UUO-Induced Renal Inflammation and Fibrosis by Inhibiting Mincle/Syk/NF-Kappa B Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, Y.; Du, F.; Guo, L.; Wang, G.; Kim, S.C.; Lee, C.W.; Shen, L.; Zhao, R. Isoliquiritigenin Attenuates Monocrotaline-Induced Pulmonary Hypertension via Inhibition of the Inflammatory Response and PASMCs Proliferation. Evid. Based Complement. Altern. Med. 2019, 2019, 4568198. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Shi, Y.; Chen, X.; Sun, Z.; Luo, W.; Hu, X.; Jin, G.; You, S.; Qian, Y.; Wu, W.; et al. Isoliquiritigenin attenuates diabetic cardiomyopathy via inhibition of hyperglycemia-induced inflammatory response and oxidative stress. Phytomedicine 2020, 78, 153319. [Google Scholar] [CrossRef] [PubMed]







| Target Gene | Accession Number | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|---|
| CD40 | NM_011611 | ACCAGCAAGGATTGCGAGGCAT | GGATGACAGACGGTATCAGTGG |
| CD80 | NM_009855 | CCTCAAGTTTCCATGTCCAAGGC | GAGGAGAGTTGTAACGGCAAGG |
| CD86 | NM_019388 | ACGTATTGGAAGGAGATTACAGCT | TCTGTCAGCGTTACTATCCCGC |
| MHC-II | NM_207105 | GTGTGCAGACACAACTACGAGG | CTGTCACTGAGCAGACCAGAGT |
| CAT | NM_009804 | GCTCTCACATGGCTGCGAAGG | TCCTCAGGCTCGGCTTCACG |
| SOD | NM_011434 | AACCAGTTGTGTTGTCAGGAC | CCACCATGTTTCTTAGAGTGAGG |
| GSR | NM_010344 | CGGCGTGGAGGTGTTGAAGTTC | TGGTCGTGGTGGGCTTCCTAC |
| Nrf2 | NM_010902 | AAGCACAGCCAGCACATTCTCC | TGACCAGGACTCACGGGAACTTC |
| Keap1 | NM_001110305 | ATCCAGAGAGGAATGAGTGGCG | TCAACTGGTCCTGCCCATCGTA |
| NQO1 | NM_008706 | GCGAGAAGAGCCCTGATTGTACTG | AGCCTCTACAGCAGCCTCCTTC |
| HO-1 | NM_010442 | ACCGCCTTCCTGCTCAACATTG | CTCTGACGAAGTGACGCCATCTG |
| IKBKB | NM_010546 | GCAGACTGACATTGTGGACCTG | ATCTCCTGGCTGTCACCTTCTG |
| IκB-Alpha | NM_010907 | GCCAGGAATTGCTGAGGCACTT | GTCTGCGTCAAGACTGCTACAC |
| NF-κB p65 | NM_009045 | TCCTGTTCGAGTCTCCATGCAG | GGTCTCATAGGTCCTTTTGCGC |
| GAPDH | NM_008084 | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, M.; Yang, Z.; Gao, Q.; Deng, Q.; Li, L.; Chen, H. Protective Effects of Isoliquiritigenin and Licochalcone B on the Immunotoxicity of BDE-47: Antioxidant Effects Based on the Activation of the Nrf2 Pathway and Inhibition of the NF-κB Pathway. Antioxidants 2024, 13, 445. https://doi.org/10.3390/antiox13040445
Dong M, Yang Z, Gao Q, Deng Q, Li L, Chen H. Protective Effects of Isoliquiritigenin and Licochalcone B on the Immunotoxicity of BDE-47: Antioxidant Effects Based on the Activation of the Nrf2 Pathway and Inhibition of the NF-κB Pathway. Antioxidants. 2024; 13(4):445. https://doi.org/10.3390/antiox13040445
Chicago/Turabian StyleDong, Minghui, Ziying Yang, Qian Gao, Qingyuan Deng, Le Li, and Hongmei Chen. 2024. "Protective Effects of Isoliquiritigenin and Licochalcone B on the Immunotoxicity of BDE-47: Antioxidant Effects Based on the Activation of the Nrf2 Pathway and Inhibition of the NF-κB Pathway" Antioxidants 13, no. 4: 445. https://doi.org/10.3390/antiox13040445
APA StyleDong, M., Yang, Z., Gao, Q., Deng, Q., Li, L., & Chen, H. (2024). Protective Effects of Isoliquiritigenin and Licochalcone B on the Immunotoxicity of BDE-47: Antioxidant Effects Based on the Activation of the Nrf2 Pathway and Inhibition of the NF-κB Pathway. Antioxidants, 13(4), 445. https://doi.org/10.3390/antiox13040445