Lycopene as a Therapeutic Agent against Aflatoxin B1-Related Toxicity: Mechanistic Insights and Future Directions
Abstract
:1. Introduction
2. An Overview of Lycopene’s Protections against Aflatoxin B1 Toxicity
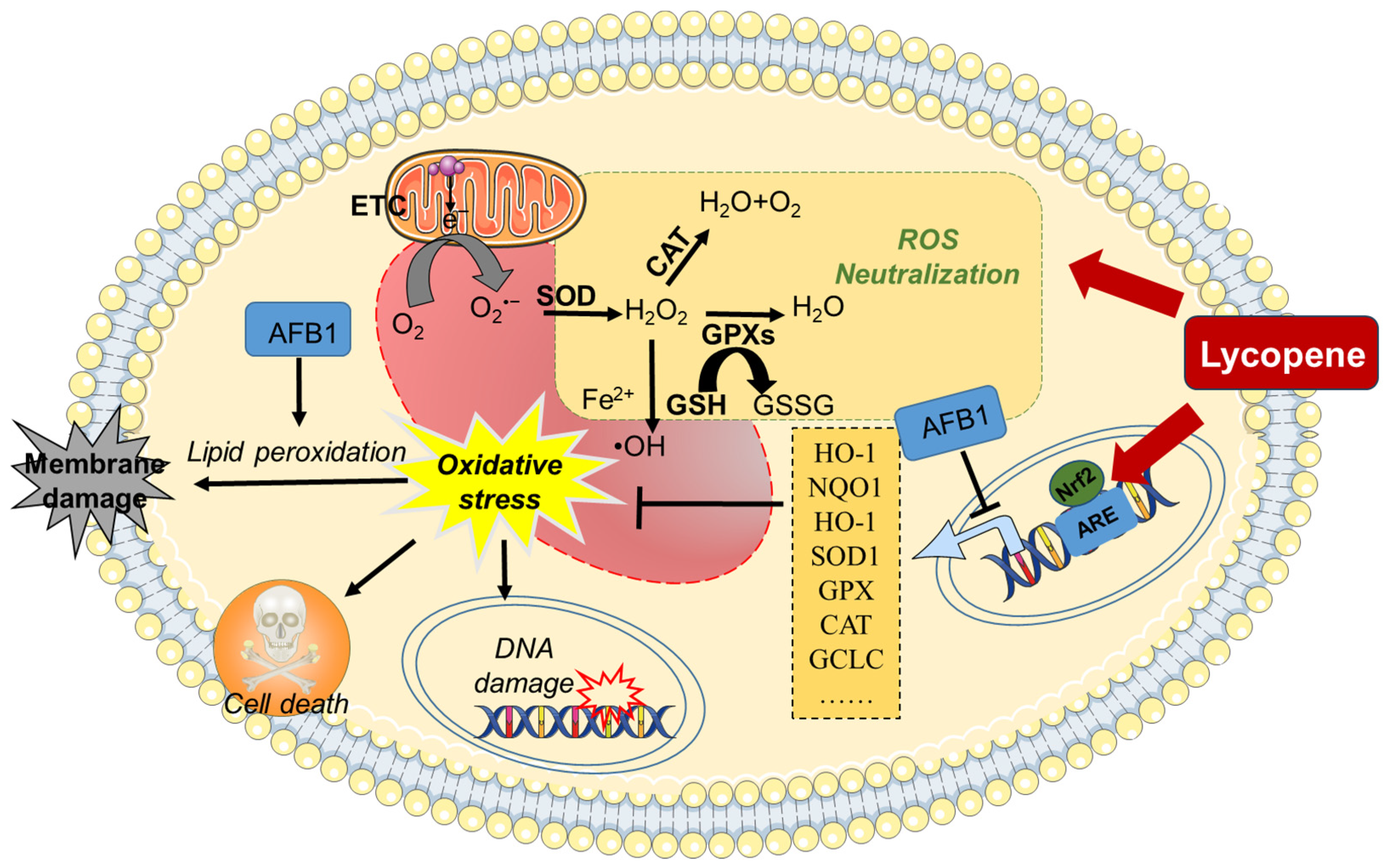
2.1. Inhibition of Oxidative Stress
2.2. Improvement in Inflammatory Response and Immune Function
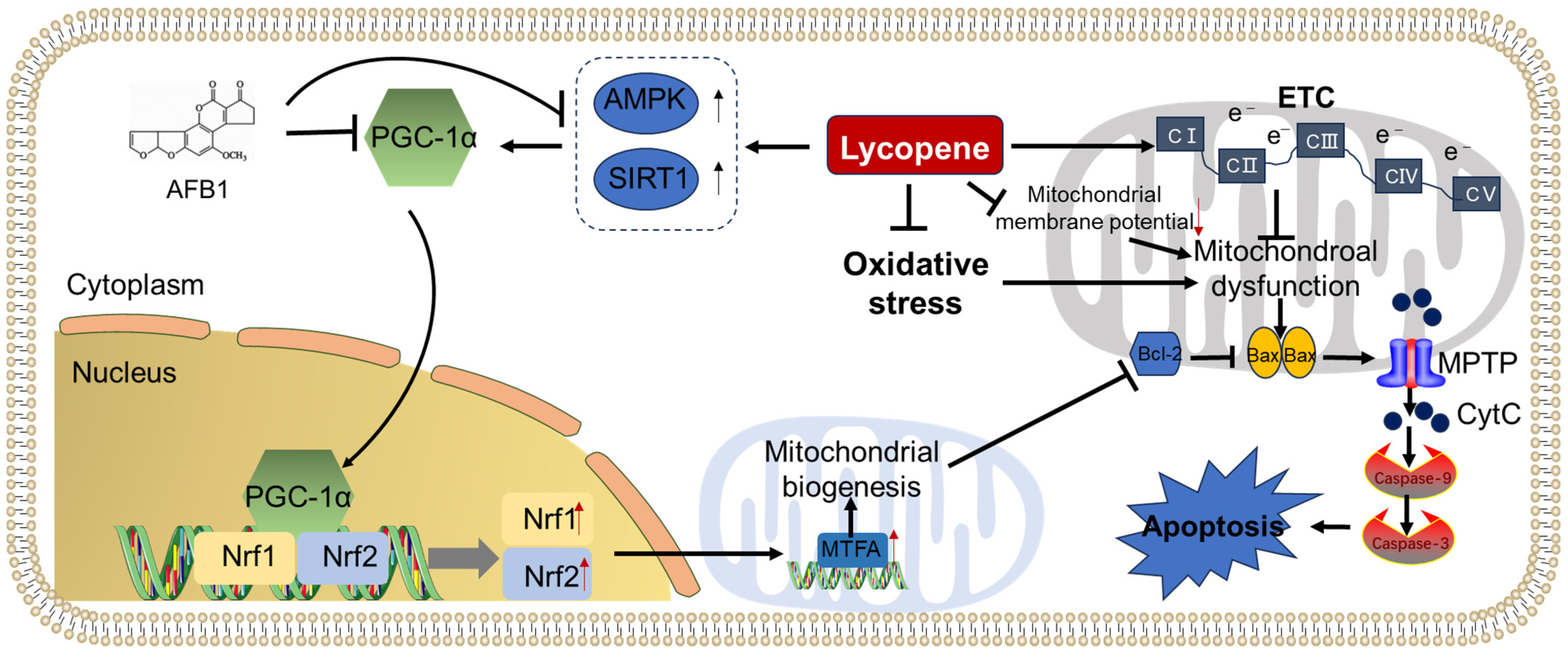
2.3. Inhibition of Mitochondrial Dysfunction and Apoptosis
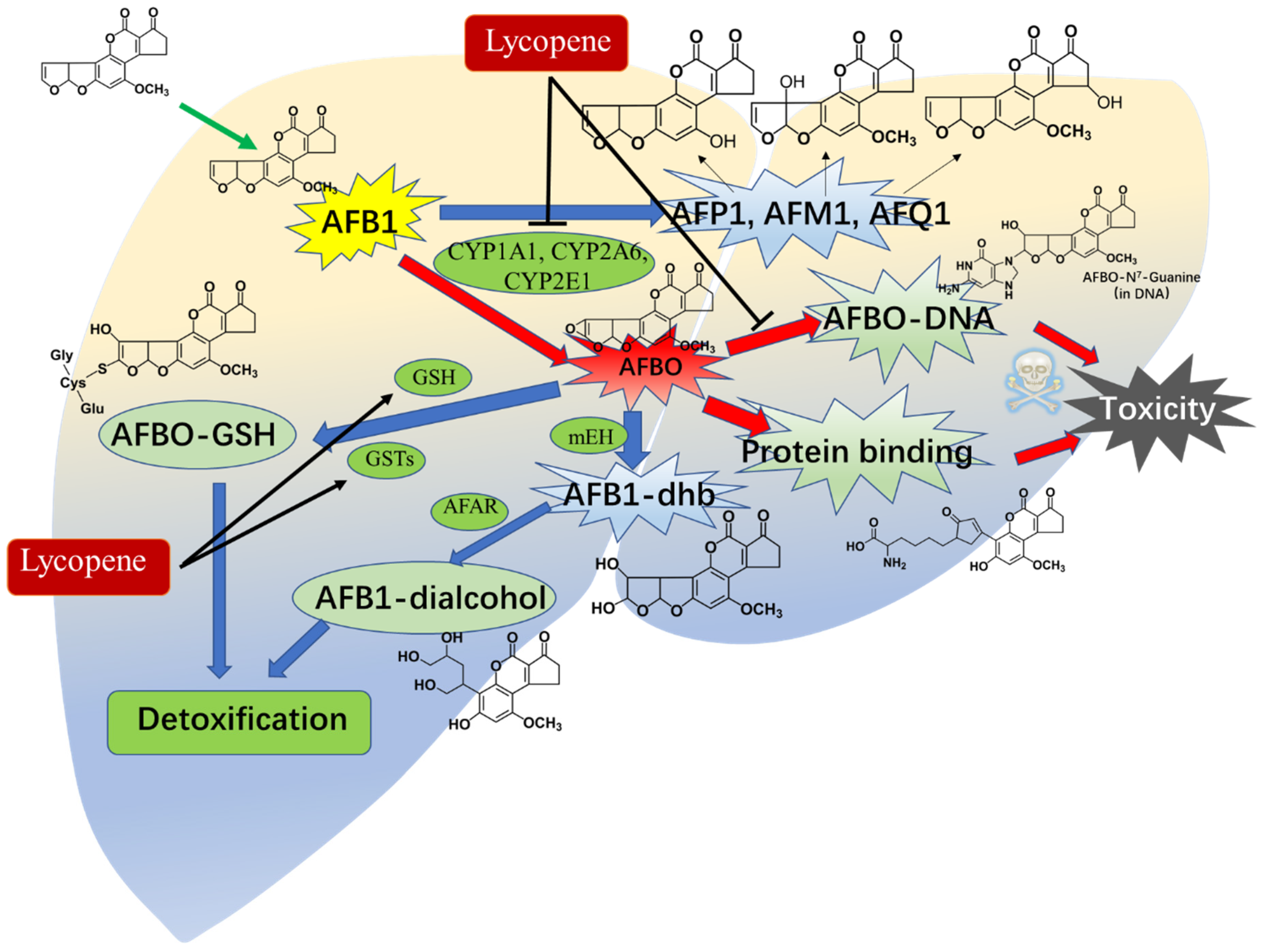
2.4. Metabolic Intervention
3. Safety of Lycopene and Its Clinical Application
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 toxicity and protective effects of curcumin: Molecular mechanisms and clinical implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- FAO Joint. Evaluation of certain mycotoxins in food. Fifty-sixth report of the joint FAO/WHO expert committee on food additives. World Health Organ. Tech. Rep. Ser. 2002, 906, i–viii. [Google Scholar]
- Ayelign, A.; De Saeger, S. Mycotoxins in ethiopia: Current status, implications to food safety and mitigation strategies. Food Control 2020, 113, 107163. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Ansar, S.; Bhat, Z.F.; Aadil, R.M.; Khaneghah, A.M. Mycotoxins and consumers’ awareness: Recent progress and future challenges. Toxicon 2023, 232, 107227. [Google Scholar] [CrossRef]
- Wei, G.; Guo, X.; Liang, Y.; Liu, C.; Zhang, G.; Liang, C.; Huang, Z.; Zheng, Y.; Chen, S.; Dong, L. Occurrence of fungi and mycotoxins in herbal medicines and rapid detection of toxin-producing fungi. Environ. Pollut. 2023, 333, 122082. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Li, F. Co-occurrence of multi-mycotoxins in wheat grains harvested in anhui province, china. Food Control 2019, 96, 180–185. [Google Scholar] [CrossRef]
- Assunção, R.; Viegas, S. Mycotoxin exposure and related diseases. Toxins 2020, 12, 172. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Sun, F.; Zhang, Y.; Hoyer, D.; Shen, J.; Tang, S.; Velkov, T. T-2 toxin neurotoxicity: Role of oxidative stress and mitochondrial dysfunction. Arch. Toxicol. 2019, 93, 3041–3056. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Stoloff, L. Carcinogenicity of aflatoxins. Science 1987, 237, 1283–1284. [Google Scholar] [CrossRef]
- Gourd, E. High concentrations of aflatoxin in ugandan grains sparks public health concern. Lancet Oncol. 2023, 24, 315. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Zhang, S.; Zhu, M.; Yang, Q.; Dong, J.; Zhang, Q.; Feng, P. Research progress related to aflatoxin contamination and prevention and control of soils. Toxins 2023, 15, 475. [Google Scholar] [CrossRef]
- Dai, C.; Sharma, G.; Liu, G.; Shen, J.; Shao, B.; Hao, Z. Therapeutic detoxification of quercetin for aflatoxin B1-related toxicity: Roles of oxidative stress, inflammation, and metabolic enzymes. Environ. Pollut. 2024, 345, 123474. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the iarc monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Wu, H.C.; Wang, Q.; Yang, H.I.; Ahsan, H.; Tsai, W.Y.; Wang, L.Y.; Chen, S.Y.; Chen, C.J.; Santella, R.M. Aflatoxin B1 exposure, hepatitis b virus infection, and hepatocellular carcinoma in taiwan. Cancer Epidemiol. Biomark. Prev. 2009, 18, 846–853. [Google Scholar] [CrossRef]
- Xiang, X.; Qin, H.G.; You, X.M.; Wang, Y.Y.; Qi, L.N.; Ma, L.; Xiang, B.D.; Zhong, J.H.; Li, L.Q. Expression of p62 in hepatocellular carcinoma involving hepatitis b virus infection and aflatoxin B1 exposure. Cancer Med. 2017, 6, 2357–2369. [Google Scholar] [CrossRef]
- Qiao, B.; He, Y.; Gao, X.; Liu, H.; Rao, G.; Su, Q.; Ruan, Z.; Tang, Z.; Hu, L. Curcumin attenuates AFB1-induced duck liver injury by inhibiting oxidative stress and lysosomal damage. Food Chem. Toxicol. 2023, 172, 113593. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, J.; Cao, Z.; Ji, Q.; Han, Y.; Song, M.; Shao, B.; Li, Y. Lycopene attenuates AFB1-induced renal injury with the activation of the nrf2 antioxidant signaling pathway in mice. Food Funct. 2018, 9, 6427–6434. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wu, Y.; Gao, S.; Zhou, M.; Liu, Z.; Xiong, Q.; Jiang, L.; Yuan, G.; Li, L.; Yang, L. Deciphering the hazardous effects of AFB1 and T-2 toxins: Unveiling toxicity and oxidative stress mechanisms in pk15 cells and mouse kidneys. Toxins 2023, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Abdin, M.Z.; Javed, S. Protective effect of esculin against prooxidant aflatoxin B1-induced nephrotoxicity in mice. Mycotoxin Res. 2014, 30, 25–32. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Wang, T.; Liao, G.; Gu, J.; Hou, R.; Qiu, J. Lipidomic profiling study on neurobehavior toxicity in zebrafish treated with aflatoxin B1. Sci. Total Environ. 2023, 898, 165553. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, P.; Yao, Q.; Shao, B.; Yu, H.; Yu, K.; Li, Y. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct. 2019, 10, 3868–3879. [Google Scholar] [CrossRef]
- Jin, X.; Li, Q.H.; Sun, J.; Zhang, M.; Xiang, Y.Q. Porcine β-defensin-2 alleviates AFB1-induced intestinal mucosal injury by inhibiting oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2023, 262, 115161. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Y.; Xu, Z.J.; Zhang, N.Y.; Zhang, W.P.; Zuo, G.; Khalil, M.M.; Sun, L.H. Selenium mitigated aflatoxin B1-induced cardiotoxicity with potential regulation of 4 selenoproteins and ferroptosis signaling in chicks. Food Chem. Toxicol. 2021, 154, 112320. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, Y.; Hu, X.; Ren, L.; Zhang, C.; Wang, X.; Wang, W.; Zhang, Z.; Hao, J.; Guo, M.; et al. Melatonin protects in vitro matured porcine oocytes from toxicity of aflatoxin B1. J. Pineal Res. 2019, 66, e12543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jia, F.; Guo, C.; Wang, Y.; Zhang, X.; Cui, Y.; Song, M.; Cao, Z.; Li, Y. Pink1/parkin-mediated mitophagy as a protective mechanism against AFB1-induced liver injury in mice. Food Chem. Toxicol. 2022, 164, 113043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the nlrp3 inflammasome and nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef]
- Liu, S.; Kang, W.; Mao, X.; Ge, L.; Du, H.; Li, J.; Hou, L.; Liu, D.; Yin, Y.; Liu, Y.; et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal fxr/liver tlr4 signaling axis in mice. J. Pineal Res. 2022, 73, e12812. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Chen, Y.; Ni, C.; Chen, X.; Zhang, L.; Xu, X.; Chen, M.; Ma, X.; Zhan, H.; et al. Aflatoxin B1 impairs leydig cells through inhibiting ampk/mtor-mediated autophagy flux pathway. Chemosphere 2019, 233, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cao, Z.; Zhang, J.; Ji, Q.; Li, Y. Aflatoxin B1 promotes autophagy associated with oxidative stress-related pi3k/akt/mtor signaling pathway in mice testis. Environ. Pollut. 2019, 255, 113317. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Feng, Y.; Wu, T.; Srinivas, S.; Yang, W.; Fan, J.; Yang, C.; Wang, S. Aflatoxin B1 negatively regulates wnt/β-catenin signaling pathway through activating mir-33a. PLoS ONE 2013, 8, e73004. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Sasaki, Y.; Terasaki, N.; Kawataki, T.; Takekawa, K.; Iwase, Y.; Shimizu, T.; Sanoh, S.; Ohta, S. Comparison of drug metabolism and its related hepatotoxic effects in heparg, cryopreserved human hepatocytes, and hepg2 cell cultures. Biol. Pharm. Bull. 2018, 41, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ban, F.; Peng, S.; Xu, D.; Li, H.; Mo, H.; Hu, L.; Zhou, X. Exogenous iron induces nadph oxidases-dependent ferroptosis in the conidia of aspergillus flavus. J. Agric. Food Chem. 2021, 69, 13608–13617. [Google Scholar] [CrossRef]
- Zhu, Q.; Ma, Y.; Liang, J.; Wei, Z.; Li, M.; Zhang, Y.; Liu, M.; He, H.; Qu, C.; Cai, J.; et al. Ahr mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 299. [Google Scholar] [CrossRef]
- El-Agamy, D.S. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats. Arch. Toxicol. 2010, 84, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Wei, G.; Guo, G.; Yu, H.; Zhang, Y.; Ishfaq, M.; Fazilani, S.A.; Zhang, X. Curcumin protects against aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 2021, 208, 111725. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Xia, S.; Wei, G.; Ishfaq, M.; Zhang, Y.; Zhang, X. Protective role of curcumin on aflatoxin B1-induced tlr4/ripk pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022, 233, 113319. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Chung, W.T.; Kwon, J.K.; Yu, J.Y.; Jang, Y.S.; Park, S.M.; Lee, S.Y.; Lee, J.C. Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice. Food Chem. Toxicol. 2010, 48, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Zhang, Y.; Li, P.; Yang, S.H.; Zhang, W.K.; Han, J.X.; Wang, Y.; He, J.B. Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by aflatoxin B1. Int. J. Mol. Sci. 2016, 17, 516. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.; Najophe, E.S.; Farombi, E.O.; Oyelere, A.K. Gallic acid protects against aflatoxin B1-induced oxidative and inflammatory stress damage in rats kidneys and liver. J. Food Biochem. 2020, 44, e13316. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Irozuru, C.E.; Arunsi, U.O.; Oyelere, A.K. Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B1. Toxicon 2022, 207, 1–12. [Google Scholar] [CrossRef]
- Aytekin Sahin, G.; Karabulut, D.; Unal, G.; Sayan, M.; Sahin, H. Effects of probiotic supplementation on very low dose AFB1-induced neurotoxicity in adult male rats. Life Sci. 2022, 306, 120798. [Google Scholar] [CrossRef]
- Huang, W.; Cao, Z.; Cui, Y.; Huo, S.; Shao, B.; Song, M.; Cheng, P.; Li, Y. Lycopene ameliorates aflatoxin B1-induced testicular lesion by attenuating oxidative stress and mitochondrial damage with nrf2 activation in mice. Ecotoxicol. Environ. Saf. 2023, 256, 114846. [Google Scholar] [CrossRef]
- Wan, X.L.; Li, N.; Chen, Y.J.; Chen, X.S.; Yang, Z.; Xu, L.; Yang, H.M.; Wang, Z.Y. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult. Sci. 2021, 100, 101441. [Google Scholar] [CrossRef]
- Tang, L.; Guan, H.; Ding, X.; Wang, J.S. Modulation of aflatoxin toxicity and biomarkers by lycopene in f344 rats. Toxicol. Appl. Pharmacol. 2007, 219, 10–17. [Google Scholar] [CrossRef]
- Hidayat, D.F.; Mahendra, M.Y.N.; Kamaludeen, J.; Pertiwi, H. Lycopene in feed as antioxidant and immuno-modulator improves broiler chicken’s performance under heat-stress conditions. Vet. Med. Int. 2023, 2023, 5418081. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.; Yilmaz, S.; Kaya, E.; Altun, S. The effect of lycopene on hepatotoxicity of aflatoxin B1 in rats. Arch. Physiol. Biochem. 2021, 127, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.T.; Wan, X.; Yang, H.; Wang, Z. Dietary lycopene supplementation could alleviate aflatoxin B1 induced intestinal damage through improving immune function and anti-oxidant capacity in broilers. Animals 2021, 11, 3165. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Kaya, E.; Karaca, A.; Karatas, O. Aflatoxin B1 induced renal and cardiac damage in rats: Protective effect of lycopene. Res. Vet. Sci. 2018, 119, 268–275. [Google Scholar] [CrossRef] [PubMed]
- El-Sheshtawy, S.M.; El-Zoghby, A.F.; Shawky, N.A.; Samak, D.H. Aflatoxicosis in pekin duckling and the effects of treatments with lycopene and silymarin. Vet. World 2021, 14, 788–793. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A potent antioxidant for the amelioration of type ii diabetes mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, S.; Tokarczyk, G. Lycopene in the prevention of cardiovascular diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Khongthaw, B.; Dulta, K.; Chauhan, P.K.; Kumar, V.; Ighalo, J.O. Lycopene: A therapeutic strategy against coronavirus disease 19 (COVID-19). Inflammopharmacology 2022, 30, 1955–1976. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Yang, C.H.; Sohn, D.W.; Kim, S.W.; Kang, S.H.; Cho, Y.H. Synergistic effect between lycopene and ciprofloxacin on a chronic bacterial prostatitis rat model. Int. J. Antimicrob. Agents 2008, 31 (Suppl. S1), S102–S107. [Google Scholar] [CrossRef]
- Tang, C.; Li, Q.; Lin, T. Lycopene attenuates staphylococcus aureus-induced inflammation via inhibiting α-hemolysin expression. Microbes Infect. 2021, 23, 104853. [Google Scholar] [CrossRef]
- Song, X.; Luo, Y.; Ma, L.; Hu, X.; Simal-Gandara, J.; Wang, L.S.; Bajpai, V.K.; Xiao, J.; Chen, F. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent. Semin. Cancer Biol. 2021, 73, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with nrf2 activation. J. Funct. Foods 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Wan, X.; Ji, H.; Ma, H.; Yang, Z.; Li, N.; Chen, X.; Chen, Y.; Yang, H.; Wang, Z. Lycopene alleviates aflatoxin B1 induced liver damage through inhibiting cytochrome 450 isozymes and improving detoxification and antioxidant systems in broiler chickens. Ital. J. Anim. Sci. 2022, 21, 31–40. [Google Scholar] [CrossRef]
- Sarker, M.T.; Wang, Z.Y.; Yang, H.; Wan, X.; Emmanuel, A. Evaluation of the protective effect of lycopene on growth performance, intestinal morphology, and digestive enzyme activities of aflatoxinB1 challenged broilers. Anim. Sci. J. 2021, 92, e13540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Xu, F.; Huang, W.; Ji, Q.; Han, Y.; Shao, B.; Li, Y. Protective effects of lycopene against AFB1-induced erythrocyte dysfunction and oxidative stress in mice. Res. Vet. Sci. 2020, 129, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; San, J.; Pang, H.; Du, Y.; Li, W.; Zhou, X.; Yang, X.; Hu, J.; Yang, J. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon 2022, 215, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of oral exposure to aflatoxin B1-induced renal dysfunction, oxidative stress, and cell apoptosis in mice kidney by curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.X.; Cao, Q.Q.; Zhang, C.D.; Xu, T.T.; Yue, K.; Li, Q.; Liu, F.; Wang, X.; Dong, H.J.; Huang, S.C.; et al. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicol. Environ. Saf. 2022, 232, 113225. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, T.; Li, P.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate aflatoxin B1-induced kidney oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 218, 112286. [Google Scholar] [CrossRef]
- Xu, D.; Peng, S.; Guo, R.; Yao, L.; Mo, H.; Li, H.; Song, H.; Hu, L. Egcg alleviates oxidative stress and inhibits aflatoxin B1 biosynthesis via mapk signaling pathway. Toxins 2021, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Li, Z.; Nabi, F.; Hu, Y.; Hu, Z.; Liu, J. Penthorum chinense pursh compound ameliorates AFB1-induced oxidative stress and apoptosis via modulation of mitochondrial pathways in broiler chicken kidneys. Front. Vet. Sci. 2021, 8, 750937. [Google Scholar] [CrossRef] [PubMed]
- Saad-Hussein, A.; Shahy, E.M.; Shaheen, W.; Ibrahim, K.S.; Mahdy-Abdallah, H.; Taha, M.M.; Hafez, S.F. Hepatotoxicity of aflatoxin B1 and its oxidative effects in wood dust egyptian exposed workers. Arch. Environ. Occup. Health 2021, 76, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Shaukat, A.; Wu, K.; Rajput, I.R.; Baloch, D.M.; Akhtar, R.W.; Raza, M.A.; Najda, A.; Rafał, P.; Albrakati, A.; et al. Luteolin alleviates aflatoxinB1-induced apoptosis and oxidative stress in the liver of mice through activation of nrf2 signaling pathway. Antioxidants 2021, 10, 1268. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Discovering the protective effects of resveratrol on aflatoxin B1-induced toxicity: A whole transcriptomic study in a bovine hepatocyte cell line. Antioxidants 2021, 10, 1225. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional profiling of aflatoxin B1-induced oxidative stress and inflammatory response in macrophages. Toxins 2021, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin B1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.E.; Burd, R. Quercetin as a systemic chemopreventative agent: Structural and functional mechanisms. Mini Rev. Med. Chem. 2011, 11, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Stinco, C.M.; Meléndez-Martínez, A.J. Free radical scavenging properties of phytofluene and phytoene isomers as compared to lycopene: A combined experimental and theoretical study. J. Phys. Chem. B 2014, 118, 9819–9825. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion complexes of lycopene and β-cyclodextrin: Preparation, characterization, stability and antioxidant activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zuo, C.; Liang, T.; Huang, Y.; Kang, P.; Xiao, K.; Liu, Y. Lycopene alleviates multiple-mycotoxin-induced toxicity by inhibiting mitochondrial damage and ferroptosis in the mouse jejunum. Food Funct. 2022, 13, 11532–11542. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. Nrf2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Takaku, M.; Egner, P.A.; Morita, M.; Kaneko, T.; Mashimo, T.; Kensler, T.W.; Yamamoto, M. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 toxicity. Toxicol. Sci. 2016, 152, 40–52. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Ma, J.; Xv, Q.; Gao, H.; Yin, H.; Yan, G.; Jiang, X.; Yu, W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the nrf2 antioxidant system. Redox Biol. 2022, 57, 102494. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Hassanein, E.H.M.; Binmahfouz, L.S.; Bagher, A.M.; Hareeri, R.H.; Algandaby, M.M.; Fadladdin, Y.A.J.; Aleya, L.; Abdel-Daim, M.M. Lycopene attenuates chlorpyrifos-induced hepatotoxicity in rats via activation of nrf2/ho-1 axis. Ecotoxicol. Environ. Saf. 2023, 262, 115122. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Deng, S.; Zhang, S.; Zhou, Y.; Velkov, T.; Li, J.; Xiao, X. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the nrf2/ho-1 pathway. Antimicrob. Agents Chemother. 2015, 59, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xia, J.; Zhao, H.S.; Hou, R.; Talukder, M.; Yu, L.; Guo, J.Y.; Li, J.L. Lycopene triggers nrf2-ampk cross talk to alleviate atrazine-induced nephrotoxicity in mice. J. Agric. Food Chem. 2018, 66, 12385–12394. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Zhao, Y.; Dai, X.Y.; Talukder, M.; Li, J.L. Lycopene ameliorates dehp exposure-induced renal pyroptosis through the nrf2/keap-1/nlrp3/caspase-1 axis. J. Nutr. Biochem. 2023, 113, 109266. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Tuzcu, M.; Sahin, N.; Ali, S.; Kucuk, O. Nrf2/ho-1 signaling pathway may be the prime target for chemoprevention of cisplatin-induced nephrotoxicity by lycopene. Food Chem. Toxicol. 2010, 48, 2670–2674. [Google Scholar] [CrossRef]
- Guo, W.; Huang, D.; Li, S. Lycopene alleviates oxidative stress-induced cell injury in human vascular endothelial cells by encouraging the sirt1/nrf2/ho-1 pathway. Clin. Exp. Hypertens. 2023, 45, 2205051. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Zhu, S.Y.; Chen, J.; Li, M.Z.; Zhao, Y.; Talukder, M.; Li, J.L. Lycopene alleviates di(2-ethylhexyl) phthalate-induced splenic injury by activating p62-keap1-nrf2 signaling. Food Chem. Toxicol. 2022, 168, 113324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, L.; Wang, J.S. Aflatoxin B1 induces gut-inflammation-associated fecal lipidome changes in f344 rats. Toxicol. Sci. 2021, 183, 363–377. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Tsim, K.W.K.; Shen, X.; Li, X.; Li, X.; Lei, H.; Liu, Y. Aflatoxin B1 induces inflammatory liver injury via gut microbiota in mice. J. Agric. Food Chem. 2023, 71, 10787–10797. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, Y.; Zhang, M.; Lan, H. Aflatoxin B1 exposure triggers inflammation and premature skin aging via ERMCS/Ca2+/ROS signaling cascade. Int. Immunopharmacol. 2023, 124, 110961. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The compromised intestinal barrier induced by mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Toshima, T.; Taketomi, A.; Taguchi, K.; Yoshizumi, T.; Uchiyama, H.; Harimoto, N.; Kajiyama, K.; Egashira, A.; Maehara, Y. Hepatic aflatoxin B1-DNA adducts and tp53 mutations in patients with hepatocellular carcinoma despite low exposure to aflatoxin B1 in southern japan. Liver Int. 2011, 31, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Yeh, S.H.; Chen, P.J. Androgen enhances aflatoxin-induced genotoxicity and inflammation to liver cancer in male hepatitis b patients. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, H.; Yi, L.; Shao, P.; Soulika, A.M.; Meng, X.; Xing, L.; Yan, X.; Zhang, X. Oral administration of aflatoxin g1 induces chronic alveolar inflammation associated with lung tumorigenesis. Toxicol. Lett. 2015, 232, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Gao, Y.T.; Dean, M.; Egner, P.; Nepal, C.; Jones, K.; Wang, B.; Rashid, A.; Luo, W.; Van Dyke, A.L.; et al. Association of aflatoxin and gallbladder cancer. Gastroenterology 2017, 153, 488–494.e1. [Google Scholar] [CrossRef]
- Xiang, X.; Hao, Y.; Cheng, C.; Hu, H.; Chen, H.; Tan, J.; Wang, Y.; Liu, X.; Peng, B.; Liao, J.; et al. A tgf-β-dominant chemoresistant phenotype of hepatoblastoma associated with aflatoxin exposure in children. Hepatology 2024, 79, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Lee, M.H.; Liu, J.; Wang, L.Y.; Lu, S.N.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of hepatocellular carcinoma associated with hepatitis c virus infection or alcohol consumption. Eur. J. Cancer 2018, 94, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, Y.; Wang, Y.; Wang, Q.; Huo, S.; Zhang, X.; Cao, Z.; Song, M.; Li, Y. Pink1/parkin-mediated mitophagy is activated to protect against AFB1-induced immunosuppression in mice spleen. Toxicol. Lett. 2022, 366, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Bai, S.; Ding, X.; Zhang, K. Pathological impairment, cell cycle arrest and apoptosis of thymus and bursa of fabricius induced by aflatoxin-contaminated corn in broilers. Int. J. Environ. Res. Public Health 2017, 14, 77. [Google Scholar] [CrossRef]
- Mehrzad, J.; Bahari, A.; Bassami, M.R.; Mahmoudi, M.; Dehghani, H. Immunobiologically relevant level of aflatoxin B1 alters transcription of key functional immune genes, phagocytosis and survival of human dendritic cells. Immunol. Lett. 2018, 197, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Feng, Y.; Theve, E.J.; Raczynski, A.R.; Fiala, J.L.; Doernte, A.L.; Williams, M.; McFaline, J.L.; Essigmann, J.M.; Schauer, D.B.; et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 2010, 59, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, D.; Zhang, J.; Tang, H.; Li, F.; Peng, Y.; Duan, X.; Meng, E.; Zhang, C.; Zeng, T.; et al. Role of macrophage ahr/tlr4/stat3 signaling axis in the colitis induced by non-canonical ahr ligand aflatoxin B1. J. Hazard. Mater. 2023, 452, 131262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Dai, X.; Liu, T.; Zhang, Y.; Wang, S.; Shi, H.; Yin, J.; Xu, T.; Zhu, R.; et al. Lycopene ameliorates islet function and down-regulates the tlr4/myd88/nf-κb pathway in diabetic mice and min6 cells. Food Funct. 2023, 14, 5090–5104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.W.; Guo, J.Y.; Lin, J.; Zhu, S.Y.; Dai, X.Y.; Saleem, M.A.U.; Zhao, Y.; Li, J.L. Mapk/nf-κb signaling mediates atrazine-induced cardiorenal syndrome and antagonism of lycopene. Sci. Total Environ. 2024, 922, 171015. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin attenuates colistin-induced neurotoxicity in n2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Mishra, S.K.; Tripathi, S.; de Alencar, M.; JMC, E.S.; Rolim, H.M.L.; de Medeiros, M.; Ferreira, P.M.P.; Rouf, R.; Uddin, S.J.; et al. Mycotoxin-assisted mitochondrial dysfunction and cytotoxicity: Unexploited tools against proliferative disorders. IUBMB Life 2018, 70, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Chen, S.; Shen, M.Y.; Huang, Q.Y.; Li, H.G.; Sun, S.C.; Wang, J.L.; Luo, X.Q. Aflatoxin B1 impairs porcine oocyte quality via disturbing intracellular membrane system and atp production. Ecotoxicol. Environ. Saf. 2023, 263, 115213. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, B.G.; Bhat, N.K.; Avadhani, N.G. Preferential attack of mitochondrial DNA by aflatoxin B1 during hepatocarcinogenesis. Science 1982, 215, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Katayama, H.; Oikawa, A.; Negishi, L.; Ichikawa, T.; Suzuki, M.; Murase, K.; Takayama, S.; Sakuda, S. Dioctatin activates clpp to degrade mitochondrial components and inhibits aflatoxin production. Cell Chem. Biol. 2020, 27, 1396–1409.e10. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Cao, Z.; Zhang, J.; Huang, W. AFB1-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition. Ecotoxicol. Environ. Saf. 2021, 216, 112213. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Van Nieuwerburgh, F.; Rajkovic, A. New insights into the combined toxicity of aflatoxin B1 and fumonisin B1 in Hepg2 cells using seahorse respirometry analysis and RNA transcriptome sequencing. Environ. Int. 2023, 175, 107945. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator pgc-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, H.X.; Luo, Y.; Cui, J.G.; Talukder, M.; Li, J.L. Lycopene mitigates dehp-induced hepatic mitochondrial quality control disorder via regulating sirt1/pink1/mitophagy axis and mitochondrial unfolded protein response. Environ. Pollut. 2022, 292, 118390. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Luo, Y.; Yan, H.; Yu, J. Lycopene increases the proportion of slow-twitch muscle fiber by ampk signaling to improve muscle anti-fatigue ability. J. Nutr. Biochem. 2021, 94, 108750. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective properties of berberine: Molecular mechanisms and clinical implications. Antioxidants 2023, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lv, Y.; Huang, K.; Luo, Y.; Xu, W. Zinc inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in human hepatocytes (hepg2 cells). Food Chem. Toxicol. 2016, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, W.R.; Tang, J.K.; Jin, X.; Xue, H.H.; Wang, T.; Zhang, L.W.; Sun, Q.Y.; Liang, Z.X. Dietary phillygenin supplementation ameliorates aflatoxin B1-induced oxidative stress, inflammation, and apoptosis in chicken liver. Ecotoxicol. Environ. Saf. 2022, 236, 113481. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Abdeen, A.; Jalouli, M.; Abdelkader, A.; Megahed, A.; Alkahtane, A.; Almeer, R.; Alhoshani, N.M.; Al-Johani, N.S.; Alkahtani, S.; et al. Fucoidan supplementation modulates hepato-renal oxidative stress and DNA damage induced by aflatoxin B1 intoxication in rats. Sci. Total Environ. 2021, 768, 144781. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ni, Y.; Guo, B.; Feng, X.; Jiang, Z. Lycpene antagonizes lead toxicity by reducing mitochondrial oxidative damage and mitochondria-mediatd apoptosis in cultured hippocampal neurons. MedComm 2020, 1, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene protects against t-bhp-induced neuronal oxidative damage and apoptosis via activation of the pi3k/akt pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Odhav, B.; Bhoola, K. Aflatoxin B1-induced toxicity in hepg2 cells inhibited by carotenoids: Morphology, apoptosis and DNA damage. Biol. Chem. 2006, 387, 87–93. [Google Scholar] [CrossRef]
- Pope, S.; Land, J.M.; Heales, S.J. Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? Biochim. Biophys. Acta 2008, 1777, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Dumanović, J.; Alomar, S.Y.; Resanović, R.; Milovanović, Z.; Nepovimova, E.; Wu, Q.; Franca, T.C.C.; Wu, W.; Kuča, K. Research update on aflatoxins toxicity, metabolism, distribution, and detection: A concise overview. Toxicology 2023, 492, 153549. [Google Scholar] [CrossRef] [PubMed]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, M.; Bordin, K.; Souto, P.; Corassin, C.; Oliveira, C. Comparative biotransformation of aflatoxin b 1 in swine, domestic fowls, and humans. Toxin Rev. 2015, 34, 142–150. [Google Scholar] [CrossRef]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M. Cytochrome p450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010, 89, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Johnson, W.W.; Shimada, T.; Ueng, Y.F.; Yamazaki, H.; Langouët, S. Activation and detoxication of aflatoxin B1. Mutat. Res. 1998, 402, 121–128. [Google Scholar] [CrossRef]
- Kamdem, L.K.; Meineke, I.; Gödtel-Armbrust, U.; Brockmöller, J.; Wojnowski, L. Dominant contribution of p450 3a4 to the hepatic carcinogenic activation of aflatoxin B1. Chem. Res. Toxicol. 2006, 19, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.W.; Guengerich, F.P. Reaction of aflatoxin B1 exo-8,9-epoxide with DNA: Kinetic analysis of covalent binding and DNA-induced hydrolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Song, C.; Ye, L.; Xu, J.; Guo, D.; Shi, Q. The effect of lycopene on cytochrome p450 isoenzymes and p-glycoprotein by using human liver microsomes and caco-2 cell monolayer model. Int. J. Food Sci. Nutr. 2018, 69, 835–841. [Google Scholar] [CrossRef]
- Nosková, K.; Dovrtělová, G.; Zendulka, O.; Strakošová, M.; Peš, O.; Juřica, J. Lycopene increases metabolic activity of rat liver cyp2b, cyp2d and cyp3a. Pharmacol. Rep. 2020, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liang, T.; Huang, Y.; Zuo, C.; Wang, D.; Liu, Y. Co-occurrence of mycotoxin-induced hepatotoxicity in mice inhibited by lycopene: Mitochondrial impairment and early hepatic fibrosis. Mol. Nutr. Food Res. 2023, 67, e2200671. [Google Scholar] [CrossRef] [PubMed]
- Scholl, P.F.; Musser, S.M.; Groopman, J.D. Synthesis and characterization of aflatoxin B1 mercapturic acids and their identification in rat urine. Chem. Res. Toxicol. 1997, 10, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of parkinson’s disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Karabelas, A.J. Natural origin lycopene and its “green” downstream processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 686–709. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves hdl functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.A.; Richmond, R.C.; Ferreira, D.L.S.; Ness, A.R.; May, M.; Smith, G.D.; Vincent, E.E.; Adams, C.; Ala-Korpela, M.; Würtz, P.; et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: The prodiet randomised controlled trial. Int. J. Cancer 2019, 144, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Biddle, M.J.; Lennie, T.A.; Bricker, G.V.; Kopec, R.E.; Schwartz, S.J.; Moser, D.K. Lycopene dietary intervention: A pilot study in patients with heart failure. J. Cardiovasc. Nurs. 2015, 30, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, T.; Li, M.; Fu, Z.; Chen, L.; Shi, D.; Qiu, F.; Tan, X. Lycopene attenuates oxidative stress-induced hepatic dysfunction of insulin signal transduction: Involvement of fgf21 and mitochondria. J. Nutr. Biochem. 2022, 110, 109144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, R.K.; Zhu, S.Y.; Talukder, M.; Cui, J.G.; Zhang, H.; Li, X.N.; Li, J.L. Lycopene prevents dehp-induced hepatic oxidative stress damage by crosstalk between ahr-nrf2 pathway. Environ. Pollut. 2021, 285, 117080. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Nagata, N.; Xu, L.; Yamamoto, S.; Fuke, N.; Ushida, Y.; Suganuma, H.; Kaneko, S.; et al. Lycopene prevents the progression of lipotoxicity-induced nonalcoholic steatohepatitis by decreasing oxidative stress in mice. Free Radic. Biol. Med. 2020, 152, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, H.; Wang, J.; Liu, P.; Tan, X.; Ren, B.; Liu, Z.; Liu, X. Lycopene supplementation attenuates oxidative stress, neuroinflammation, and cognitive impairment in aged cd-1 mice. J. Agric. Food Chem. 2018, 66, 3127–3136. [Google Scholar] [CrossRef]
- Milani, C.; Maccari, M.; Mosconi, P. Action of lycopene in the experimental gastric ulcer. Pharmacology 1970, 4, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Michael McClain, R.; Bausch, J. Summary of safety studies conducted with synthetic lycopene. Regul. Toxicol. Pharmacol. 2003, 37, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef] [PubMed]

| Animal Models | Treatments | Protective Effects of Lycopene | Refs. |
|---|---|---|---|
| Male Kunming mice | Mice were orally administrated with AFB1 at the dose of 0.75 mg/kg/day or cotreated with lycopene at the dose of 5 mg/kg/day. All mice were treated for consecutive 30 days. | Lycopene supplementation significantly attenuated AFB1 exposure-caused lesions of testicular microstructure and ultrastructure, and sperm abnormalities in mice. Meanwhile, lycopene supplementation significantly ameliorated AFB1 exposure-induced oxidative stress and the functional deficiency of mitochondrial biosynthesis, and significantly activated the Nrf2 pathway and the PGC-1α pathway in the testicular tissue of mice. | [47] |
| One-day-old Pekin ducklings | The ducklings were fed a ration contaminated with 30 ppb (equal to 30 μg/kg body weight) of AFTs (a mixture containing AFB1 and other AFTs) for 2 weeks and co-treated with or without lycopene, at the final dose of 100 mg/kg body weight. After AFT treatment, the ducklings were orally fed continually for an additional 10 days. | Lycopene supplementation markedly attenuated AFTs exposure-induced liver dysfunction. Lycopene supplementation also significantly increased the levels of total antioxidant capacity (TAC), catalase (CAT), and glutathione S-transferase (GST) activities, and significantly decreased the levels of malondialdehyde (MDA), finally effectively improving AFTs exposure-induced hepatic oxidative stress damage. Meanwhile, lycopene treatment significantly decreased the residues of AFTs in the liver tissue. | [54] |
| Male F344 rats | Rats were orally administrated with AFB1 at the final dose of 250 μg/kg body weight daily and co-treated with lycopene at the final dose of 100 mg/kg body weight daily. All rats were treated for 3 weeks (5 days per week). | Lycopene treatment markedly attenuated AFB1 exposure-induced toxic symptoms, including weakness, anorexia, bloody urine, ascites, and ataxia. In addition, gross necropsy and histopathological examination found lycopene treatment marked decreased AFB1 exposure-caused necrosis, hepatotropism, fatty infiltration, and bile duct epithelium hyperplasia in liver tissue. In addition, lycopene treatment greatly modulated AFB1 metabolism and metabolic activation, and significantly reduced formation of AFB1–DNA adducts. | [49] |
| One-day-old male Arbor Acres broiler chicks | Chicks were orally fed with a 100 µg/kg AFB1-contaminated basal diet and co-fed with or without lycopene (purity ≥ 80%) with a 200 mg/kg basal diet. All chicks were treated for 42 days. | Lycopene supplementation significantly improved the liver function of AFB1-treated chicks. It significantly decreased the levels of H2O2 and reactive oxygen species (ROS) levels, and significantly increased the levels of GSH and the activities of superoxide dismutase (SOD), thioredoxin peroxidase (TPX), and glutathione peroxidase (GPX) in AFB1-treated liver tissue. Meanwhile, lycopene supplementation significantly attenuated AFB1 exposure-induced mitochondrial dysfunction and the functional loss of mitochondrial biogenesis, increased the activities of mitochondrial electron transfer chain complexes, and activated the PGC-1α pathway. Lycopene supplementation decreased the intestinal villus height (VH) and crypt depth ratio (VCR) while increasing the crypt depth. Lycopene supplementation could also decrease the activities of cytochrome P450 (CYP450) isozymes (e.g., CYP1A1 and CYP2A6), then reduced the formation of AFB1–DNA in the liver tissue of chicks. | [48,64] |
| One-day-old male Arbor Acres broilers | Chicks were orally fed with a 100 µg/kg AFB1-contaminated basal diet and co-fed with or without lycopene (purity ≥ 80%) with a 200 mg/kg basal diet. All chicks were treated for 42 days. | Lycopene treatment significantly increased the levels of interleukin (IL)-10 protein and downregulated the expression of IL-1β mRNA, as well as attenuating the inflammatory response in the jejunum tissue of AFB1-treated chicks. Moreover, lycopene supplementation also significantly attenuated AFB1 exposure-induced oxidative damage in the jejunum tissue of chicks. | [52,65] |
| Male Wistar-Albino rats | Rats were orally administrated with AFB1 at a dose of 0.5 mg/kg/day for 7 days and lycopene at a dose of 5 mg/kg/day, for 15 days. | Lycopene supplementation markedly attenuated AFB1 exposure-induced liver dysfunction and liver oxidative damage through upregulating the levels of antioxidants and the activities of antioxidant enzymes. | [51] |
| Male Wistar-Albino rats | Rats were orally administrated with AFB1 at the dose of 0.5 mg/kg/day for 7 days or 1.5 mg/kg/day for 3 days, and all AFB1-treated rats were treated with or without lycopene at a dose of 5 mg/kg/day for 15 days. | Lycopene supplementation significantly attenuated AFB1 exposure-induced pathological changes in the kidney and heart tissues of rats. It also significantly inhibited AFB1 exposure-induced lipid peroxidation and upregulated antioxidant enzyme activities in the kidney and heart tissues of rats. | [53] |
| Male Kunming mice | Mice were orally administrated with AFB1 at the dose of 0.75 mg/kg body weight per day and co-treated orally with lycopene at the dose of 5 mg/kg body weight per day. All mice were treated for 30 days. | Lycopene supplementation significantly protected against AFB1-induced erythrocyte dysfunction and spleen toxicity via the inhibition of the inflammatory response, oxidative stress, and the mitochondrial apoptotic pathway and via the improvement in immune function in mice. | [26,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Tang, S.; Peng, X.; Sharma, G.; Yin, S.; Hao, Z.; Li, J.; Shen, J.; Dai, C. Lycopene as a Therapeutic Agent against Aflatoxin B1-Related Toxicity: Mechanistic Insights and Future Directions. Antioxidants 2024, 13, 452. https://doi.org/10.3390/antiox13040452
Li M, Tang S, Peng X, Sharma G, Yin S, Hao Z, Li J, Shen J, Dai C. Lycopene as a Therapeutic Agent against Aflatoxin B1-Related Toxicity: Mechanistic Insights and Future Directions. Antioxidants. 2024; 13(4):452. https://doi.org/10.3390/antiox13040452
Chicago/Turabian StyleLi, Meng, Shusheng Tang, Xinyan Peng, Gaurav Sharma, Shutao Yin, Zhihui Hao, Jichang Li, Jianzhong Shen, and Chongshan Dai. 2024. "Lycopene as a Therapeutic Agent against Aflatoxin B1-Related Toxicity: Mechanistic Insights and Future Directions" Antioxidants 13, no. 4: 452. https://doi.org/10.3390/antiox13040452






