Protein–Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications
Abstract
:1. Introduction
2. An Overview of CGA
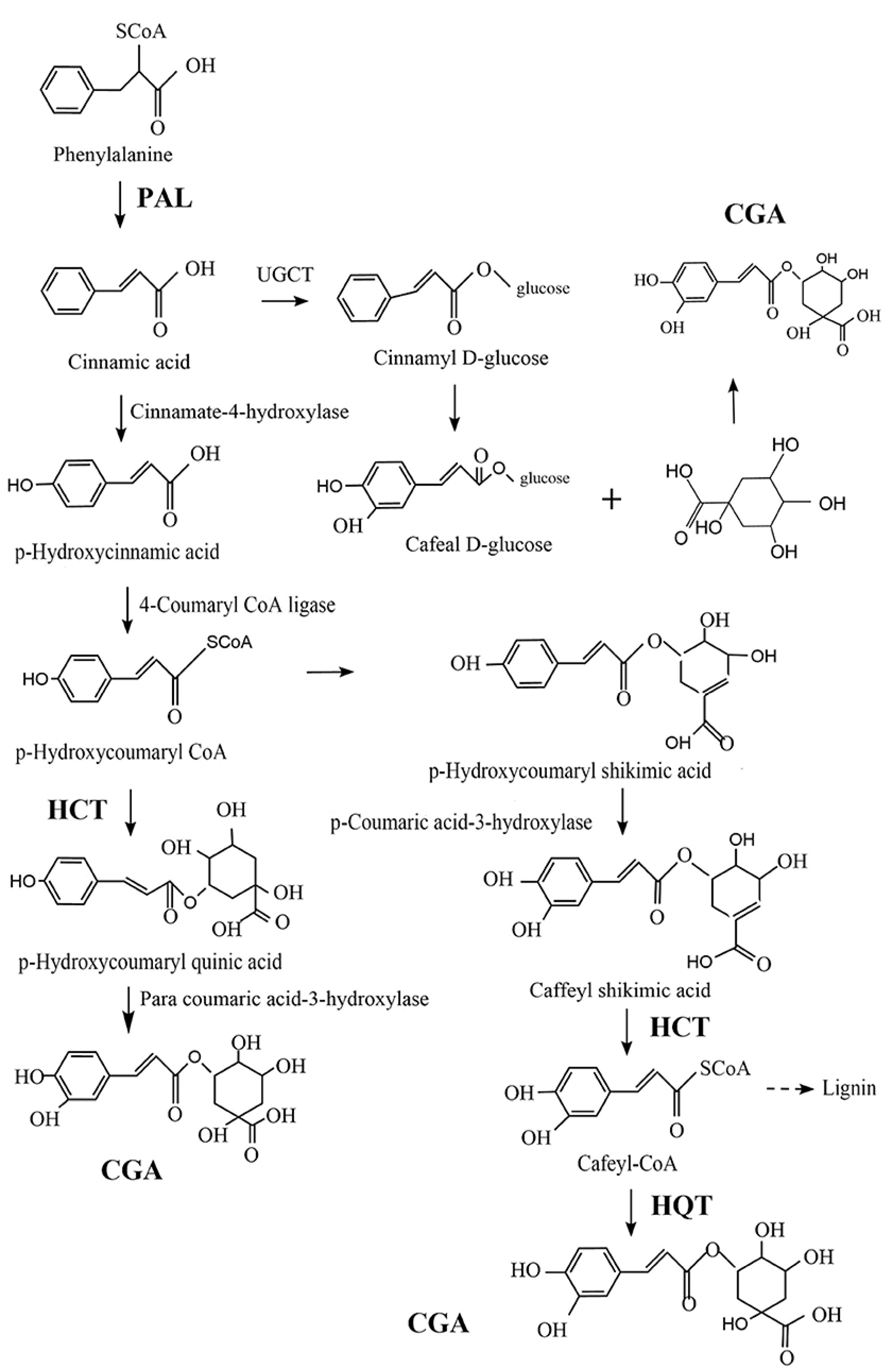
2.1. History, Chemistry, and Resources
2.2. Biological Properties
3. The Mechanism of Protein–CGA Interactions
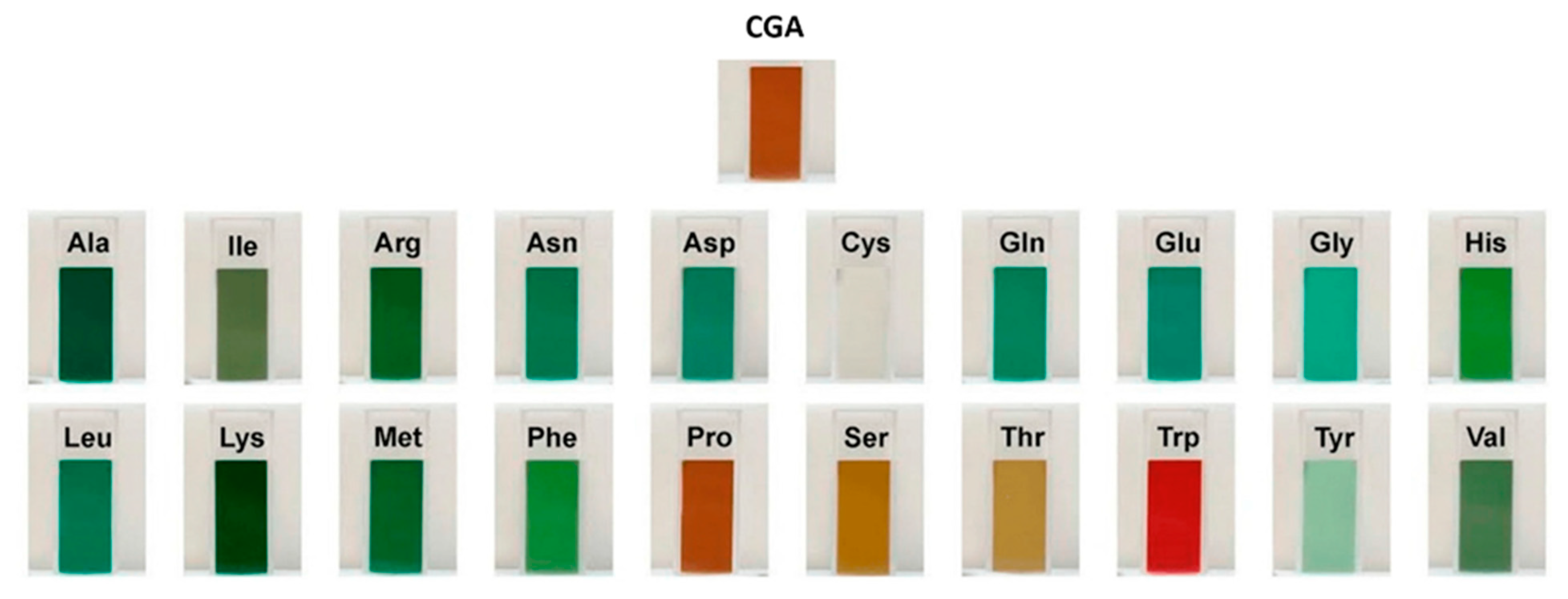
3.1. Covalent Interactions (Conjugates)
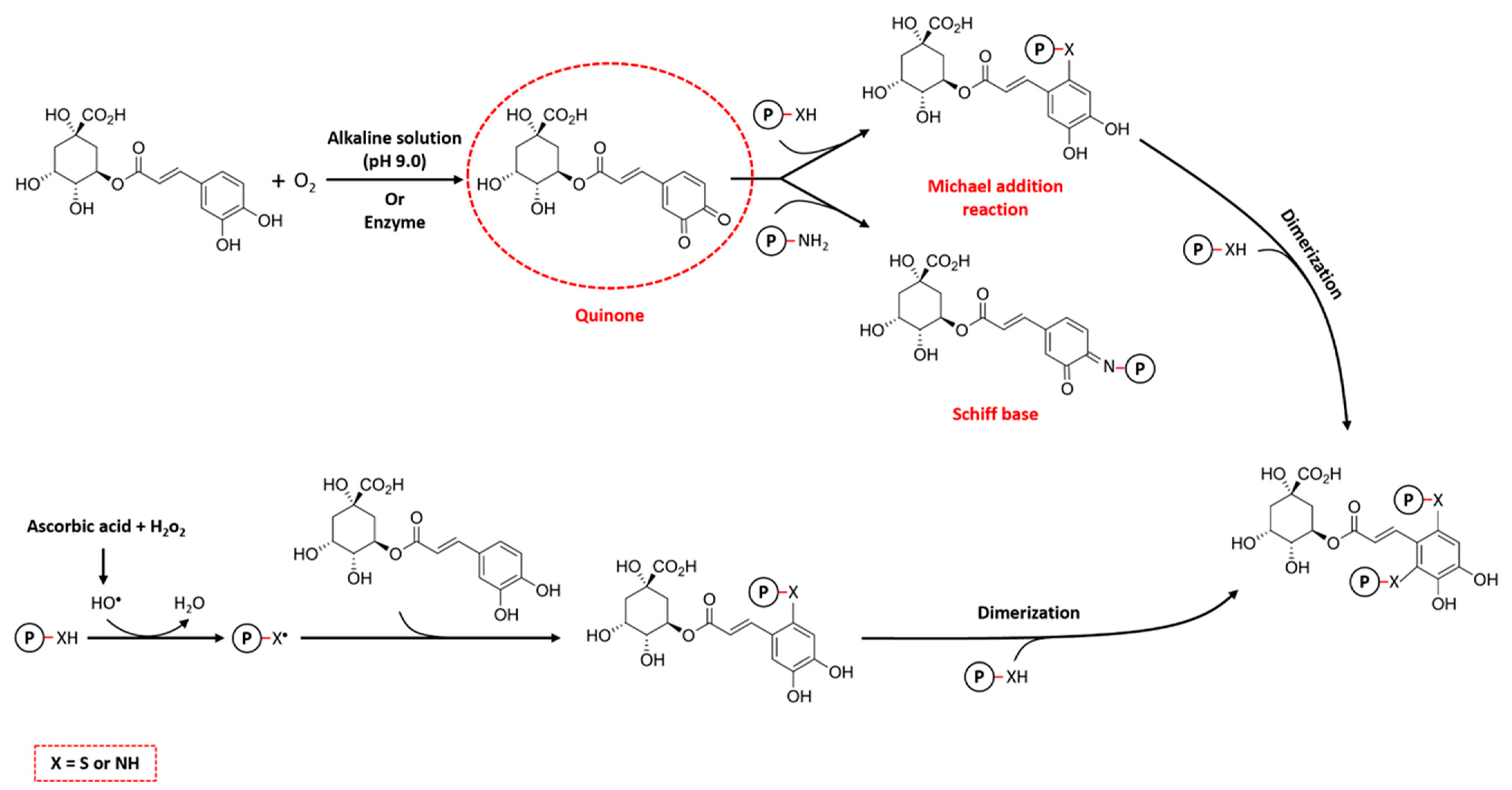
3.1.1. Alkaline Method
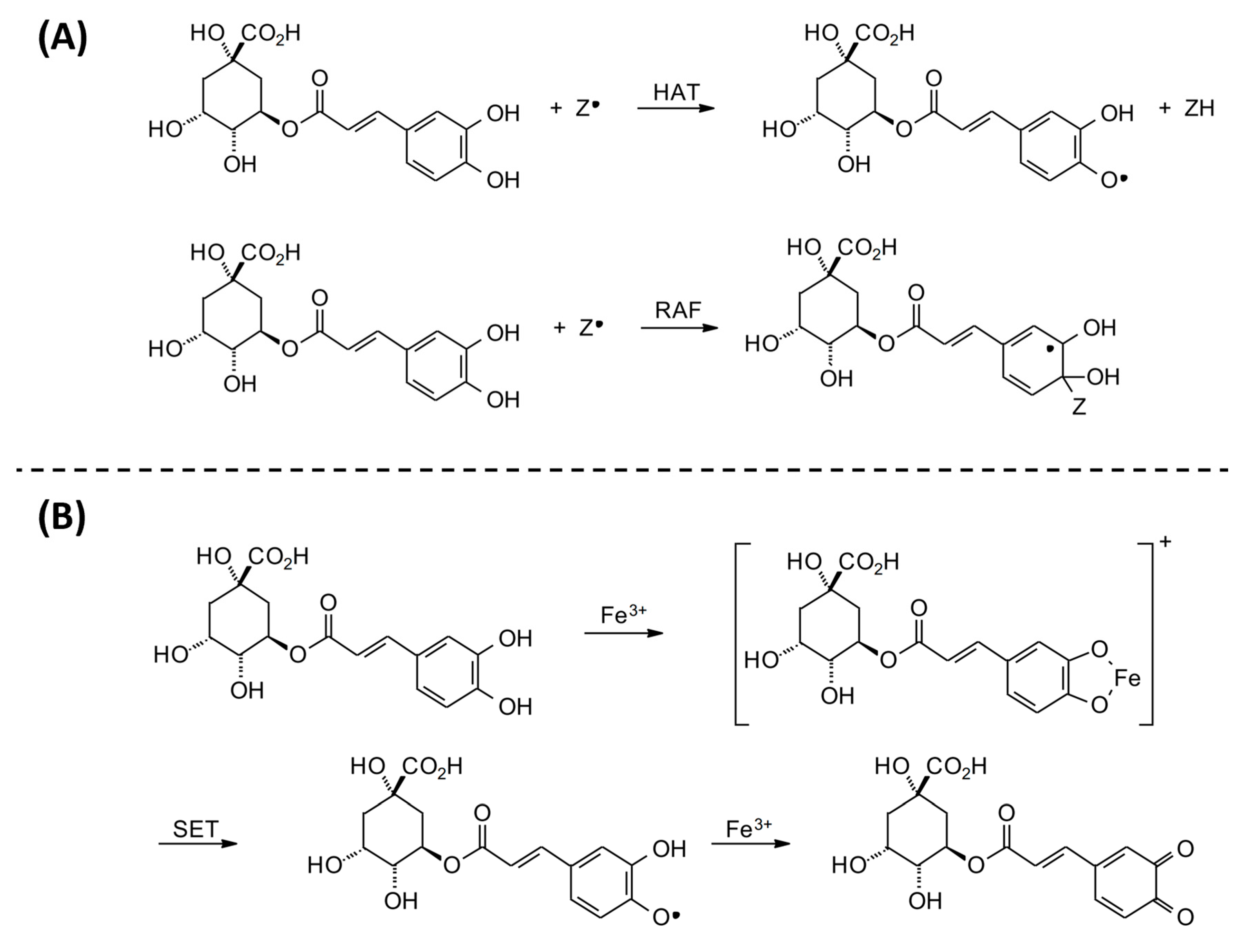
3.1.2. Free Radical Method
3.1.3. Enzymatic Method
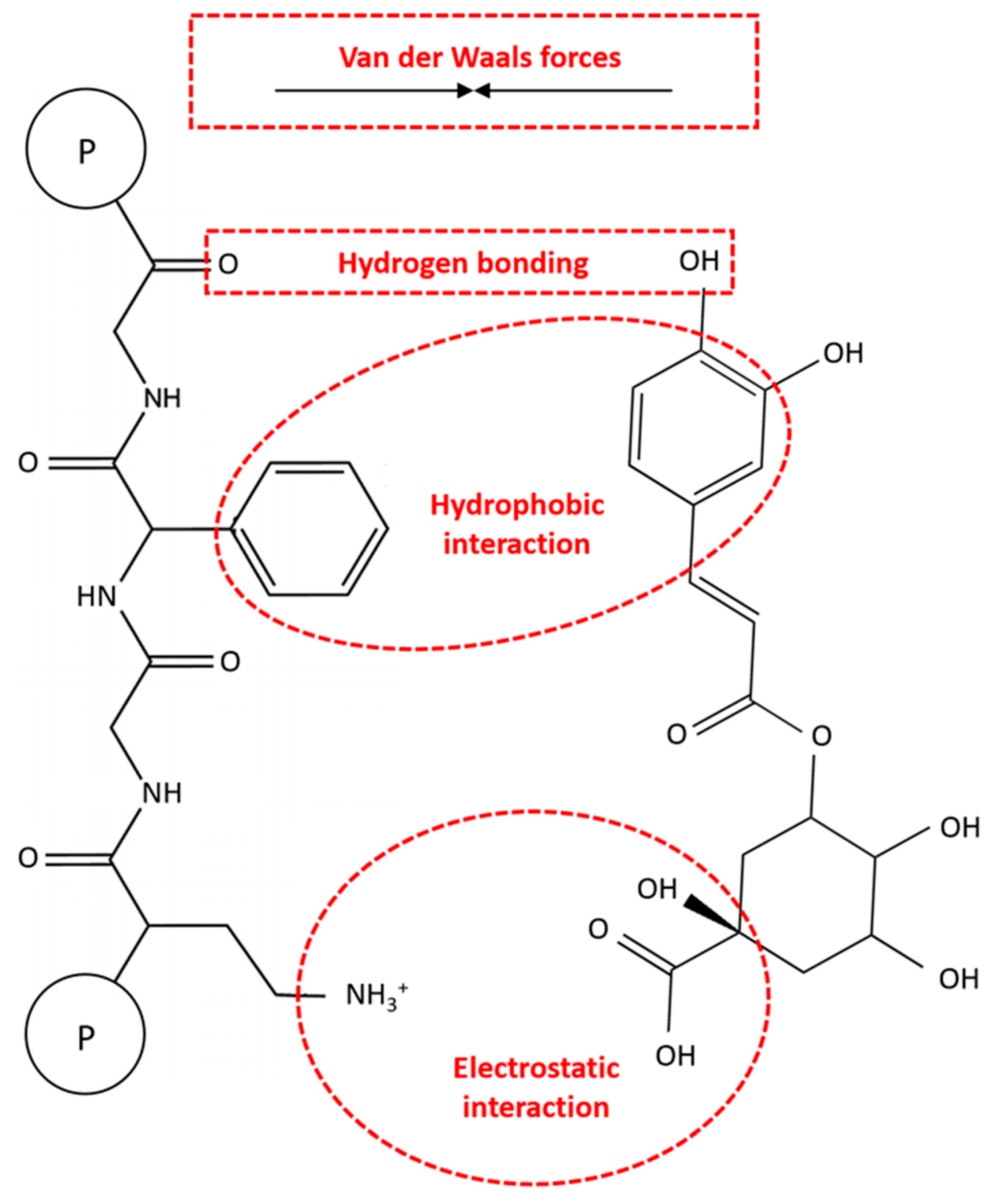
3.2. Non-Covalent Interactions (Complexes)
4. Changes in Protein Characteristics
4.1. Animal Protein–CGA Interactions
4.1.1. Milk Proteins
Whey Proteins
Casein Proteins
4.1.2. Myofibrillar Proteins
4.1.3. Human and Bovine Serum Albumins
4.1.4. Egg White Proteins
4.1.5. Others
| Protein Type | Binding Type | Preparation Method | CGA Concentration | Main Results | References |
|---|---|---|---|---|---|
| WPI | Non-covalent | Mixing in phosphate buffer | 500 μM |
| [77] |
| Covalent | Alkaline method | NS |
| [62] | |
| Covalent | Free radical method | 0.5 g |
| [78] | |
| α-LA and β-LG | Non-covalent (i.e., H-bond and hydrophobic) | NS | 2, 4, 8, 16, 32, and 64 µM |
| [70] |
| β-LG | Covalent and non-covalent | Mixing in phosphate buffer | 1.5 mM/150 μM Pr |
| [79] |
| CN | Non-covalent | Mixing in phosphate buffer | 20, 120, and 240 µM/g Pr |
| [83] |
| β-CN | Non-covalent (i.e., hydrophobic) | Mixing in phosphate buffer | 5, 10, 20, 30, 40, and 60 μM |
| [82] |
| Coregonus peled MP | Covalent and non-covalent | Mixing in Tris-HCl buffer | 6, 30, and 150 µM/g Pr |
| [85] |
| Pork MP | Covalent and non-covalent | Mixing in PIPES buffer | 6, 30, and 150 µM/g Pr |
| [86] |
| Covalent | Enzymatic method | 5 and 20 µM/g Pr |
| [87] | |
| Non-covalent (i.e., H-bond and hydrophobic) | Mixing in PIPES buffer | 150 µM/g Pr |
| [72] | |
| Non-covalent | Mixing in PIPES buffer | 2.65 mg/10 mg Pr |
| [88] | |
| HSA | Non-covalent (i.e., H-bond and electrostatic) | NS | NS |
| [92,93] |
| BSA | Covalent | Alkaline method | CGA/Pr (w/w) ratios of 1:2, 1:3, 1:5, 1:7, and 1:10 |
| [94] |
| EWP | Covalent | Free radical method | 0.1 g/100 mL Pr |
| [68] |
| Covalent | Free radical method + ultrasound treatment | 0.1 g/100 mL Pr |
| [97] | |
| OVA | Covalent | Free radical method | 0 0.08 g/0.2 g Pr |
| [71] |
| Non-covalent (i.e., H-bond and electrostatic) | Mixing in phosphate buffer | 5 mL, 5 × 10−4 M dm−3/3 mL, 5 × 10−6 M dm−3 Pr |
| [98] | |
| PPPH | Covalent | Mixing in phosphate buffer | 0.1, 0.5, 1, and 1.5%/40 mg Pr |
| [99,100] |
| Gelatin | Non-covalent | Mixing in phosphate buffer | CGA/Pr M ratios of 6:1, 4:1, 2:1, 1:1, 1:2, and 1:4 |
| [101] |
4.2. Plant Protein–CGA Interactions
4.2.1. Soy Protein
4.2.2. Zein
4.2.3. Wheat Protein
4.2.4. Rice Protein
4.2.5. Sunflower Protein
4.2.6. Others
| Protein Type | Binding Type | Preparation Method | CGA Concentration | Main Results | References |
|---|---|---|---|---|---|
| SPH | Non-covalent (i.e., H-bond and hydrophobic) | Mixing in phosphate buffer | 0.015 g/0.10 g Pr |
| [104] |
| SPI | Covalent | Alkaline method | 20, 40, 60, 80, 100 μM/g Pr |
| [105] |
| Soybean 7s globulin | Covalent | Alkaline method | 0.5 mM/100 mg Pr |
| [63] |
| Non-covalent (i.e., H-bond and van der Waals) | Mixing in phosphate buffer | 0–12 µM (Pr 10 µM) |
| [74] | |
| Zein | Non-covalent (i.e., H-bond and electrostatic) | Mixing in aqueous ethanol solution | 5–40 µM (Pr 0.2 mg/mL) |
| [75] |
| Covalent | Alkaline method | 0.25 mM/0.2 g Pr |
| [60] | |
| WGH | Covalent | Free radical method | 5 mM/2 g Pr |
| [67] |
| WG | Covalent | Alkaline method | 0.35 mM/10 mg/mL Pr |
| [107] |
| RBP | Non-covalent | Ultrasound treatment + mixing in phosphate buffer | 0.1 g/100 mL CGA/1 g/100 mL Pr |
| [110] |
| RPH | Covalent | Alkaline method | 0–0.125/2.5% (w/v) Pr |
| [61] |
| Covalent | Alkaline, enzyme, and free radical methods | 10 mg (fixed in all methods) |
| [17] | |
| SFP | Covalent | Alkaline method | 0.005–1 g/0.1 g Pr |
| [112] |
| Covalent and non-covalent | Alkaline or neutral method (pH 9 or 7) | CGA/Pr M ratios of 1:10, 1:5, 1:1, 5:1, and 10:1 |
| [64] | |
| HP | Non-covalent | Ultrasound treatment + mixing | 0.1% |
| [113] |
| QPH | Non-covalent | Mixing in phosphate buffer | 0, 20, 120, and 240 μM/g Pr |
| [114] |
| FP | Covalent | Alkaline method | CGA/Pr mass ratios 0.35%, 0.70%, 1.05% and 1.40% |
| [115] |
| PPI | Non-covalent (mainly electrostatic) | Mixing in phosphoric acid buffer | 25, 50, 100, 200, and 250 μM/g Pr |
| [116] |
5. Protein–CGA Interactions in Food Systems
5.1. Beverages
5.2. Films and Coatings
5.3. Emulsion-Based Delivery Systems
5.4. Natural Food Colorants
6. Research Gaps and Future Perspectives
7. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, C.; Zhang, J.; Yao, H.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Advances in renewable plant-derived protein source: The structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochem. 2019, 53, 83–98. [Google Scholar] [CrossRef]
- Baba, W.N.; McClements, D.J.; Maqsood, S. Whey protein–polyphenol conjugates and complexes: Production, characterization, and applications. Food Chem. 2021, 365, 130455. [Google Scholar] [CrossRef]
- Tarahi, M.; Abdolalizadeh, L.; Hedayati, S. Mung bean protein isolate: Extraction, structure, physicochemical properties, modifications, and food applications. Food Chem. 2024, 444, 138626. [Google Scholar] [CrossRef]
- Tarahi, M.; Ahmed, J. Recent advances in legume protein-based colloidal systems. Legume Sci. 2023, 5, e185. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Masoumi, B.; Tabibiazar, M.; Golchinfar, Z.; Mohammadifar, M.; Hamishehkar, H. A review of protein-phenolic acid interaction: Reaction mechanisms and applications. Crit. Rev. Food Sci. Nutr. 2022, 64, 3539–3555. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825–35840. [Google Scholar] [CrossRef]
- Tarahi, M.; Tahmouzi, S.; Kianiani, M.R.; Ezzati, S.; Hedayati, S.; Niakousari, M. Current Innovations in the Development of Functional Gummy Candies. Foods 2023, 13, 76. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Li, Z.; Niu, L.; Chen, Y.; Qiu, X.; Du, T.; Zhu, M.; Wang, M.; Mo, H.; Xiao, S. Recent advance in the biological activity of chlorogenic acid and its application in food industry. Int. J. Food Sci. Technol. 2023, 58, 4931–4947. [Google Scholar] [CrossRef]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A review of the protective effects of chlorogenic acid against different chemicals. J. Food Biochem. 2022, 46, e14254. [Google Scholar] [CrossRef]
- Manzoor, M.; Tchameni, Z.F.N.; Bhat, Z.F.; Jaiswal, A.K.; Jaglan, S. Recent insights on the conformational changes, functionality, and physiological properties of plant-based protein–polyphenol conjugates. Food Bioprocess Technol. 2023, 1–24. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Pan, X.; Fan, F.; Ding, J.; Li, P.; Sun, X.; Zhong, L.; Fang, Y. Altering functional properties of rice protein hydrolysates by covalent conjugation with chlorogenic acid. Food Chem. X 2022, 14, 100352. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Bender, O.; Atalay, A. Polyphenol chlorogenic acid, antioxidant profile, and breast cancer. In Cancer; Elsevier: Amsterdam, The Netherlands, 2021; pp. 311–321. [Google Scholar]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Levsh, O.; Chiang, Y.-C.; Tung, C.F.; Noel, J.P.; Wang, Y.; Weng, J.-K. Dynamic conformational states dictate selectivity toward the native substrate in a substrate-permissive acyltransferase. Biochemistry 2016, 55, 6314–6326. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Bai, M.; He, H.; Wang, H.; Wu, H. Correlation of the temporal and spatial expression patterns of HQT with the biosynthesis and accumulation of chlorogenic acid in Lonicera japonica flowers. Hortic. Res. 2019, 6, 73. [Google Scholar] [CrossRef]
- Chen, X.; Cai, W.; Xia, J.; Yu, H.; Wang, Q.; Pang, F.; Zhao, M. Metabolomic and transcriptomic analyses reveal that blue light promotes chlorogenic acid synthesis in strawberry. J. Agric. Food Chem. 2020, 68, 12485–12492. [Google Scholar] [PubMed]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids—Occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013, 141, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, X.; Tang, J.; Kong, Y.; Wang, X.; Wang, S. Chlorogenic acid protects against aluminum toxicity via MAPK/Akt signaling pathway in murine RAW264. 7 macrophages. J. Inorg. Biochem. 2019, 190, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic acid and mental diseases: From chemistry to medicine. Curr. Neuropharmacol. 2017, 15, 471. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Amelioration of oxidative stress in Caco-2 cells treated with pro-inflammatory proteins by chlorogenic acid isomers via activation of the Nrf2–Keap1–ARE-signaling pathway. J. Agric. Food Chem. 2018, 66, 11008–11017. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-M.; Mao, L.-Q.; Wu, C.-Y.; Ye, W.; Wang, X. Chlorogenic acid improves intestinal barrier function by downregulating CD14 to inhibit the NF-κB signaling pathway. J. Funct. Foods 2021, 85, 104640. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef]
- Nwafor, E.-O.; Lu, P.; Zhang, Y.; Liu, R.; Peng, H.; Xing, B.; Liu, Y.; Li, Z.; Zhang, K.; Zhang, Y. Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol. 2022, 15, 101294. [Google Scholar] [CrossRef]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, X.c.; Zhong, Y.l.; He, W.y.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Kozuma, K.; Tsuchiya, S.; Kohori, J.; Hase, T.; Tokimitsu, I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens. Res. 2005, 28, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Fujii, A.; Yamamoto, N.; Yamamoto, M.; Ohminami, H.; Kameyama, A.; Shibuya, Y.; Nishizawa, Y.; Tokimitsu, I.; Saito, I. Improvement of hypertension and vascular dysfunction by hydroxyhydroquinone-free coffee in a genetic model of hypertension. FEBS Lett. 2006, 580, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Rocha da Silva, C.; Sá, L.G.d.A.V.; Dos Santos, E.V.; Ferreira, T.L.; Coutinho, T.d.N.P.; Moreira, L.E.A.; de Sousa Campos, R.; de Andrade, C.R.; Barbosa da Silva, W.M.; de Sá Carneiro, I. Evaluation of the antifungal effect of chlorogenic acid against strains of Candida spp. resistant to fluconazole: Apoptosis induction and in silico analysis of the possible mechanisms of action. J. Med. Microbiol. 2022, 71, 001526. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Luo, T.; Wu, F.; Mei, Y.-W.; Peng, J.; Liu, H.; Li, H.-R.; Zhang, S.-L.; Dong, J.-H.; Fang, Y. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia. Life Sci. 2015, 127, 12–18. [Google Scholar] [CrossRef]
- Tamayose, C.; Torres, P.; Roque, N.; Ferreira, M. HIV-1 reverse transcriptase inhibitory activity of flavones and chlorogenic acid derivatives from Moquiniastrum floribundum (Asteraceae). S. Afr. J. Bot. 2019, 123, 142–146. [Google Scholar] [CrossRef]
- Mirmojarabian, S.; Karimi, A.; Lorigooini, Z.; Javadi-Farsani, F.; Soltani, A.; Moradi, M.-T. Phytochemical properties and antiviral effect of green tea (Camellia sinensis) extract on adenovirus in vitro. J. Shahrekord Univ. Med. Sci. 2022, 24, 104–110. [Google Scholar] [CrossRef]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of chlorogenic acid intake on cognitive function in the elderly: A pilot study. Evid.-Based Complement. Altern. Med. 2018, 2018, 8608497. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective effect of chlorogenic acid on mitochondrial dysfunction-mediated apoptotic death of DA neurons in a Parkinsonian mouse model. Oxidative Med. Cell. Longev. 2020, 2020, 6571484. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zeng, Z.; McClements, D.J.; Gong, X.; Yu, P.; Xia, J.; Gong, D. A review of the structure, function, and application of plant-based protein–phenolic conjugates and complexes. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1312–1336. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Z.; Xue, C.; Huang, Q. Covalent modification of zein with polyphenols: A feasible strategy to improve antioxidant activity and solubility. J. Food Sci. 2022, 87, 2965–2979. [Google Scholar] [CrossRef]
- Pan, X.; Fang, Y.; Wang, L.; Shi, Y.; Xie, M.; Xia, J.; Pei, F.; Li, P.; Xiong, W.; Shen, X. Covalent interaction between rice protein hydrolysates and chlorogenic acid: Improving the stability of oil-in-water emulsions. J. Agric. Food Chem. 2019, 67, 4023–4030. [Google Scholar] [CrossRef]
- Elsebaie, E.; Ali, M. Antioxidants, antimicrobial and anticancer activities of whey protein isolate covalently modified with chlorogenic and rosmarinic acids. J. Agroaliment. Process. Technol. 2022, 28, 277–282. [Google Scholar]
- Lin, X.; Ye, L.; He, K.; Zhang, T.; Sun, F.; Mei, T.; Wu, X. A new method to reduce allergenicity by improving the functional properties of soybean 7S protein through covalent modification with polyphenols. Food Chem. 2022, 373, 131589. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Sethi, D.S.; van der Goot, A.J.; Keppler, J.K. Covalent and non-covalent modification of sunflower protein with chlorogenic acid: Identifying the critical ratios that affect techno-functionality. Food Hydrocoll. 2022, 131, 107800. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Y.; Yokoyama, W.; Yi, J. β-Lactoglobulin–chlorogenic acid conjugate-based nanoparticles for delivery of (−)-epigallocatechin-3-gallate. RSC Adv. 2017, 7, 21366–21374. [Google Scholar] [CrossRef]
- He, D.; Xing, Y.; Wang, Y.; Zeng, W.; Gao, W.; Su, N.; Zhang, C.; Chen, H.; Xing, X.H. Improved functional properties of wheat gluten hydrolysate by covalent conjugation with chlorogenic acid. Int. J. Food Sci. Technol. 2023, 58, 454–462. [Google Scholar] [CrossRef]
- Sun, J.; Jing, H.; Mu, Y.; McClements, D.J.; Dong, S.; Xu, B. Fabrication of antioxidant emulsifiers from natural ingredients: Conjugation of egg white proteins with catechin and chlorogenic acid. Food Hydrocoll. 2020, 108, 106019. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, Q.; Li, T.; Liu, Q.; Wang, Y.; Huang, J. Comparison of interaction mechanism between chlorogenic acid/luteolin and glutenin/gliadin by multi-spectroscopic and thermodynamic methods. J. Mol. Struct. 2021, 1246, 131219. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Yang, Y.; Li, S.; Wang, C.; Wang, C.; Zhang, T. Comparison of non-covalent binding interactions between three whey proteins and chlorogenic acid: Spectroscopic analysis and molecular docking. Food Biosci. 2021, 41, 101035. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, Y.; Qi, X.; Yan, H.; Zhao, Y.; Wu, Y.; Zhang, N.; Ding, Z.; Yuan, L.; Liu, M. Noncovalent interaction of chlorogenic acid and/or gallocatechin gallate with β-lactoglobulin: Effect on stability and bioaccessibility of complexes and nanoparticles. LWT 2023, 175, 114493. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, D.; Luo, D.; Zhou, Z.; Liu, W.; Zeng, W.; Dinnyés, A.; Xiong, Y.L.; Sun, Q. Non-covalent binding of chlorogenic acid to myofibrillar protein improved its bio-functionality properties and metabolic fate. Food Chem. 2024, 440, 138208. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Zhang, H.; Yu, D.; Jin, S.; Ren, F. Interaction of chlorogenic acid with milk proteins analyzed by spectroscopic and modeling methods. Spectrosc. Lett. 2016, 49, 44–50. [Google Scholar] [CrossRef]
- Zhou, S.; Meng, L.; Lin, Y.; Dong, X.; Dong, M. Exploring the Interactions of Soybean 7S Globulin with Gallic Acid, Chlorogenic Acid and (−)-Epigallocatechin Gallate. Foods 2023, 12, 4013. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xue, J.; Zhang, H.; Wang, F.; Liu, J. Mechanistic understanding of the effect of zein–chlorogenic acid interaction on the properties of electrospun nanofiber films. Food Chem. X 2022, 16, 100454. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The type and concentration of milk increase the in vitro bioaccessibility of coffee chlorogenic acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef]
- Meng, Y.; Li, C. Conformational changes and functional properties of whey protein isolate-polyphenol complexes formed by non-covalent interaction. Food Chem. 2021, 364, 129622. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, T.; Lu, Y.; Lin, X.; Hu, X.; Liu, L.; He, Z.; Wu, X. Effect of chlorogenic acid covalent conjugation on the allergenicity, digestibility and functional properties of whey protein. Food Chem. 2019, 298, 125024. [Google Scholar] [CrossRef]
- Qie, X.; Chen, W.; Zeng, M.; Wang, Z.; Chen, J.; Goff, H.D.; He, Z. Interaction between β-lactoglobulin and chlorogenic acid and its effect on antioxidant activity and thermal stability. Food Hydrocoll. 2021, 121, 107059. [Google Scholar] [CrossRef]
- Ma, G.; Tang, C.; Sun, X.; Zhang, J. The interaction mechanism of β-casein with oligomeric proanthocyanidins and its effect on proanthocyanidin bioaccessibility. Food Hydrocoll. 2021, 113, 106485. [Google Scholar] [CrossRef]
- McSweeney, P.L.; Fox, P.F. Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Yin, Z.; Qie, X.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Li, W.; He, Z. Effect of thermal treatment on the molecular-level interactions and antioxidant activities in β-casein and chlorogenic acid complexes. Food Hydrocoll. 2022, 123, 107177. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Zhao, J.; Liu, Y. The effect of non-covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of in vitro protein digests. Food Chem. 2018, 268, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, X. Modification of myofibrillar protein functional properties prepared by various strategies: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 458–500. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qiu, H.; Deng, X.; Mao, X.; Guo, X.; Xu, C.; Zhang, J. Effect of chlorogenic acid on the physicochemical and functional properties of Coregonus peled myofibrillar protein through hydroxyl radical oxidation. Molecules 2019, 24, 3205. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yang, X.; Li, J.; Fu, Q.; Zhou, J.; Zhao, J.; Zhang, N.; Liu, Q.; Wang, T.; Wang, H. Improvement of physicochemical and gel properties of chlorogenic acid-modified oxidized myofibrillar proteins by transglutaminase. LWT 2023, 178, 114582. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, D.; Xu, X.; Dai, J.; Lao, G.; Zhang, S.; Xu, X.; Dinnyés, A.; Xiong, Y.; Sun, Q. Myofibrillar protein-chlorogenic acid complexes ameliorate glucose metabolism via modulating gut microbiota in a type 2 diabetic rat model. Food Chem. 2023, 409, 135195. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, J.; Xue, H.; Hu, Y.; Liu, Y.-n.; Lin, G.; Liu, L.; Xu, R. Applications of human and bovine serum albumins in biomedical engineering: A review. Int. J. Biol. Macromol. 2023, 253, 126914. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, Y.; Xie, M.-X.; Li, S.; Jiang, M.; Wang, Y.-D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2004, 1674, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Xu, J.; Du, W. Comparison of the binding affinity of chlorogenic acid with two serum albumins. Int. J. Biol. Macromol. 2011, 48, 81–86. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Chen, C.-H.; Zhou, S.; Bai, A.-M.; Ou-Yang, Y. The specific binding of chlorogenic acid to human serum albumin. Mol. Biol. Rep. 2012, 39, 2781–2787. [Google Scholar] [CrossRef]
- Tang, B.; Huang, Y.; Ma, X.; Liao, X.; Wang, Q.; Xiong, X.; Li, H. Multispectroscopic and docking studies on the binding of chlorogenic acid isomers to human serum albumin: Effects of esteryl position on affinity. Food Chem. 2016, 212, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Rohn, S.; Kruse, H.-P.; Kroll, J. Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid. Food Chem. 2002, 78, 443–455. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Tang, L.; Jiang, J.; Sun, K.; Manirafasha, E.; Zhang, M. Effect of Cu2+ and Al3+ on the interaction of chlorogenic acid and caffeic acid with serum albumin. Food Chem. 2023, 410, 135406. [Google Scholar] [CrossRef]
- Abeyrathne, E.N.S.; Lee, H.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, F.; Liu, T.; Jing, H.; Huang, Y.; Obadi, M.; Xu, B. Ultrasound-enhanced egg white proteins conjugated with polyphenols: The structure of the polyphenols on their functional properties. LWT 2022, 164, 113600. [Google Scholar] [CrossRef]
- Perumal, M.; Marimuthu, P.; Chen, X. Investigation into the site-specific binding interactions between chlorogenic acid and ovalbumin using multi-spectroscopic and in silico simulation studies. J. Biomol. Struct. Dyn. 2022, 40, 6619–6633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, C.; Liu, H.; Liu, Q.; Kong, B. Enhanced physical and oxidative stability of porcine plasma protein hydrolysates based oil-in-water emulsions by adding oxidized chlorogenic acid. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 330–337. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Chen, Q.; Liu, Q.; Kong, B. Antioxidant activities and emulsifying properties of porcine plasma protein hydrolysates modified by oxidized tannic acid and oxidized chlorogenic acid. Process Biochem. 2019, 79, 105–113. [Google Scholar] [CrossRef]
- Fu, S.; Wu, C.; Wu, T.; Yu, H.; Yang, S.; Hu, Y. Preparation and characterisation of Chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef]
- Shi, J.; Cui, Y.-f.; Zhou, G.; Li, N.; Sun, X.; Wang, X.; Xu, N. Covalent interaction of soy protein isolate and chlorogenic acid: Effect on protein structure and functional properties. LWT 2022, 170, 114081. [Google Scholar] [CrossRef]
- Budryn, G.; Rachwal-Rosiak, D. Interactions of hydroxycinnamic acids with proteins and their technological and nutritional implications. Food Rev. Int. 2013, 29, 217–230. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Pałecz, B.; Rachwał-Rosiak, D.; Belica, S.; den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H. Interactions of free and encapsulated hydroxycinnamic acids from green coffee with egg ovalbumin, whey and soy protein hydrolysates. LWT-Food Sci. Technol. 2016, 65, 823–831. [Google Scholar] [CrossRef]
- Guo, K.; Zhou, G.; Lok, U.S.; Wang, X.; Jiang, L. Improving interface-related functions and antioxidant activities of soy protein isolate by covalent conjugation with chlorogenic acid. J. Food Meas. Charact. 2022, 16, 202–213. [Google Scholar] [CrossRef]
- Glusac, J.; Fishman, A. Enzymatic and chemical modification of zein for food application. Trends Food Sci. Technol. 2021, 112, 507–517. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, Q.; Li, T.; Zhang, Y.; Huang, J.; Huang, Q.; Gao, L. Effect of covalent conjugation with chlorogenic acid and luteolin on allergenicity and functional properties of wheat gliadin. J. Cereal Sci. 2022, 106, 103484. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Yao, J.; Luo, S.; Wang, X.; Zhang, N.; Wang, L.; Zhu, X. Effect of ultrasonic power on the emulsion stability of rice bran protein-chlorogenic acid emulsion. Ultrason. Sonochem. 2022, 84, 105959. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Tsuge, I.; Tokuda, R.; Teshima, R. Analysis of the distribution of rice allergens in brown rice grains and of the allergenicity of products containing rice bran. Food Chem. 2019, 276, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, X.; Wang, W.; Wang, L.; Jiang, L.; Yu, D.; Xie, F. Effect of ultrasound on the properties of rice bran protein and its chlorogenic acid complex. Ultrason. Sonochem. 2021, 79, 105758. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Ghoshal, G. Sunflower protein isolates-composition, extraction and functional properties. Adv. Colloid Interface Sci. 2022, 306, 102725. [Google Scholar] [CrossRef]
- Karefyllakis, D.; Salakou, S.; Bitter, J.H.; van der Goot, A.J.; Nikiforidis, C.V. Covalent bonding of chlorogenic acid induces structural modifications on sunflower proteins. ChemPhysChem 2018, 19, 459–468. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.; Huang, X.; Lian, Y.; Yang, F.; Yu, D. Hemp seed protein and chlorogenic acid complex: Effect of ultrasound modification on its structure and functional properties. Int. J. Biol. Macromol. 2023, 233, 123521. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, L.; Zhao, J.; Jiang, J. Possible role of polypeptide-chlorogenic acid interaction in the physicochemical and sensory characteristics of quinoa-modified coffee beverage. Food Chem. 2023, 425, 136359. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, J.; Ma, S.; Chen, X.; Dai, X.; Zhang, L.; Guo, M.; Li, L.; Liu, W.; Ren, G. Structure Characterization and Functional Properties of Flaxseed Protein–Chlorogenic Acid Complex. Foods 2023, 12, 4449. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Sun, J.; Pei, M.; Zhang, G.; Li, C.; Li, C.; Ma, X.; He, S.; Liu, L. Impact of non-covalent bound polyphenols on conformational, functional properties and in vitro digestibility of pea protein. Food Chem. 2022, 383, 132623. [Google Scholar] [CrossRef] [PubMed]
- Shchekoldina, T.; Aider, M. Production of low chlorogenic and caffeic acid containing sunflower meal protein isolate and its use in functional wheat bread making. J. Food Sci. Technol. 2014, 51, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Schuppe, S.; Rohn, S.; Rawel, H.M.; Kroll, J. Chlorogenic acid moderately decreases the quality of whey proteins in rats. J. Agric. Food Chem. 2005, 53, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Duarte, G.S.; Farah, A. Effect of simultaneous consumption of milk and coffee on chlorogenic acids’ bioavailability in humans. J. Agric. Food Chem. 2011, 59, 7925–7931. [Google Scholar] [CrossRef] [PubMed]
- Felberg, I.; Farah, A.; Monteiro, M.C.; Godoy, R.L.d.O.; Pacheco, S.; Calado, V.; Donangelo, C.M. Effect of simultaneous consumption of soymilk and coffee on the urinary excretion of isoflavones, chlorogenic acids and metabolites in healthy adults. J. Funct. Foods 2015, 19, 688–699. [Google Scholar] [CrossRef]
- Alongi, M.; Calligaris, S.; Anese, M. Fat concentration and high-pressure homogenization affect chlorogenic acid bioaccessibility and α-glucosidase inhibitory capacity of milk-based coffee beverages. J. Funct. Foods 2019, 58, 130–137. [Google Scholar] [CrossRef]
- Zhang, W.; Hedayati, S.; Tarahi, M.; Karaca, A.C.; Hadidi, M.; Assadpour, E.; Jafari, S.M. Advances in transglutaminase cross-linked protein-based food packaging films; a review. Int. J. Biol. Macromol. 2023, 253, 127399. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Deng, C.; Zhang, D.; Li, H.; Zhou, C.; Hong, P. Gelatin/wheat Gliadin Electrospun Film Containing Chlorogenic Acid: Fabrication, Characterization, and Application in the Preservation of Grass Carp (Ctenopharyngodon idella) Fillets. Food Biophys. 2023, 18, 580–595. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Wang, X.; Yang, H.; Cheng, J.; Lin, Y.; Tang, D. Influence of Gelatin-Chitosan-Glycerol Edible Coating Incorporated with Chlorogenic Acid, Gallic Acid, and Resveratrol on the Preservation of Fresh Beef. Foods 2022, 11, 3813. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, H.; Yu, Y.; Sun, J.; Zheng, H.; Zhang, Y.; Xu, J.; Zhu, X. The improvement of nanoemulsion stability and antioxidation via protein-chlorogenic acid-dextran conjugates as emulsifiers. Nanomaterials 2020, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, D.; Sun, C.; McClements, D.J.; Gao, Y. Utilization of interfacial engineering to improve physicochemical stability of β-carotene emulsions: Multilayer coatings formed using protein and protein–polyphenol conjugates. Food Chem. 2016, 205, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Dong, C.; Zhao, R.; Zhang, X.; Wang, C. Lycopene-loaded emulsions stabilized by whey protein covalently modified with pectin or/and chlorogenic acid: Enhanced physicochemical stability and reduced bio-accessibility. Food Chem. 2023, 417, 135879. [Google Scholar] [CrossRef]
- Iacomino, M.; Weber, F.; Gleichenhagen, M.; Pistorio, V.; Panzella, L.; Pizzo, E.; Schieber, A.; d’Ischia, M.; Napolitano, A. Stable benzacridine pigments by oxidative coupling of chlorogenic acid with amino acids and proteins: Toward natural product-based green food coloring. J. Agric. Food Chem. 2017, 65, 6519–6528. [Google Scholar] [CrossRef]
- Liang, S.; Tran, H.L.; Were, L. Lowering greening of cookies made from sunflower butter using acidic ingredients and effect on reducing capacity, tryptophan and protein oxidation. Food Chem. 2018, 252, 318–326. [Google Scholar] [CrossRef]
- Bongartz, V.; Brandt, L.; Gehrmann, M.L.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Evidence for the formation of benzacridine derivatives in alkaline-treated sunflower meal and model solutions. Molecules 2016, 21, 91. [Google Scholar] [CrossRef]
- Moccia, F.; Martín, M.Á.; Ramos, S.; Goya, L.; Marzorati, S.; DellaGreca, M.; Panzella, L.; Napolitano, A. A new cyanine from oxidative coupling of chlorogenic acid with tryptophan: Assessment of the potential as red dye for food coloring. Food Chem. 2021, 348, 129152. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarahi, M.; Gharagozlou, M.; Niakousari, M.; Hedayati, S. Protein–Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications. Antioxidants 2024, 13, 777. https://doi.org/10.3390/antiox13070777
Tarahi M, Gharagozlou M, Niakousari M, Hedayati S. Protein–Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications. Antioxidants. 2024; 13(7):777. https://doi.org/10.3390/antiox13070777
Chicago/Turabian StyleTarahi, Mohammad, Maryam Gharagozlou, Mehrdad Niakousari, and Sara Hedayati. 2024. "Protein–Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications" Antioxidants 13, no. 7: 777. https://doi.org/10.3390/antiox13070777
APA StyleTarahi, M., Gharagozlou, M., Niakousari, M., & Hedayati, S. (2024). Protein–Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications. Antioxidants, 13(7), 777. https://doi.org/10.3390/antiox13070777








