Exploitation of Natural By-Products for the Promotion of Healthy Outcomes in Humans: Special Focus on Antioxidant and Anti-Inflammatory Mechanisms and Modulation of the Gut Microbiota
Abstract
:1. Introduction
2. Major By-Products Extracted from Fruit, Vegetables, and Cereals
2.1. Grape By-Products
2.2. Olive By-Products
2.3. Apple By-Products
2.4. Pumpkin By-Products
2.5. Tomato By-Products
2.6. Rice By-Products
3. By-Products from Marine Food Sources
4. Effects of Food By-Products on the Intestinal Microbiota
5. Conclusions
Funding
Conflicts of Interest
References
- Radha, A.; Ahluwalia, V.; Rai, A.K.; Varjani, S.; Awasthi, M.K.; Sindhu, R.; Binod, P.; Saran, S.; Kumar, V. The way forward to produce nutraceuticals from agri-food processing residues: Obstacle, solution, and possibility. J. Food Sci. Technol. 2024, 61, 429–443. [Google Scholar] [CrossRef] [PubMed]
- de Brito Nogueira, T.B.; da Silva, T.P.M.; de Araújo Luiz, D.; de Andrade, C.J.; de Andrade, L.M.; Ferreira, M.S.L.; Fai, A.E.C. Fruits and vegetable-processing waste: A case study in two markets at Rio de Janeiro, RJ, Brazil. Environ. Sci. Pollut. Res. Int. 2020, 27, 18530–18540. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products-A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Camana, D.; Manzardo, A.; Toniolo, S.; Gallo, F.; Scipioni, A. Assessing Environmental Sustainability of Local Waste Management Policies in Italy from a Circular Economy Perspective. An Overview of Existing Tools. Sustain. Prod. Consum. 2021, 27, 613–629. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A Review on Circular Economy: The Expected Transition to a Balanced Interplay of Environmental and Economic Systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Anbarasan, R.; Tiwari, B.K.; Mahendran, R. Upcycling of seafood side streams for circularity. Adv. Food Nutr. Res. 2024, 108, 179–221. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef] [PubMed]
- Zen, C.K.; Tiepo, C.B.V.; da Silva, R.V.; Reinehr, C.O.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of functional pasta with microencapsulated Spirulina: Technological and sensorial effects. J. Sci. Food Agric. 2020, 30, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Lian, P.; Braber, S.; Garssen, J.; Wichers, H.J.; Folkerts, G.; Fink-Gremmels, J.; Varasteh, S. Beyond Heat Stress: Intestinal Integrity Disruption and Mechanism-Based Intervention Strategies. Nutrients 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. Editorial on the Occasion of the 20th Anniversary of Endocrine Metabolic Immune Disorders-Drug Targets Journal with a Kaleidoscopic Vision of Selected Publications. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health: (Revision 1). EFSA J. 2018, 16, e05136. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, Y.Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Sousa Silva, M. Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef] [PubMed]
- Lantzouraki, D.Z.; Tsiaka, T.; Soteriou, N.; Asimomiti, G.; Spanidi, E.; Natskoulis, P.; Gardikis, K.; Sinanoglou, V.J.; Zoumpoulakis, P. Antioxidant Profiles of Vitis vinifera L. and Salvia triloba L. Leaves Using High-Energy Extraction Methodologies. J. AOAC Int. 2020, 103, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rasane, P.; Kaur, R.; Kaur, H.; Garg, R.; Kaur, S.; Ercisli, S.; Choudhary, R.; Skrovankova, S.; Mlcek, J. Valorization of grape (Vitis vinifera) leaves for bioactive compounds: Novel green extraction technologies and food-pharma applications. Front. Chem. 2023, 11, 1290619. [Google Scholar] [CrossRef] [PubMed]
- Bajomo, E.M.; Aing, M.S.; Ford, L.S.; Niemeyer, E.D. Chemo-typing of commercially available basil (Ocimum basilicum L.) varieties: Cultivar and morphotype influence the phenolic acid composition and antioxidant properties. NFS J. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Babri, S.; Mohaddes, G.; Feizi, I.; Mohammadnia, A.; Niapour, A.; Alihemmati, A.; Amani, M. Effect of troxerutin on synaptic plasticity of hippocampal dentate gyrus neurons in a β-amyloid model of Alzheimer’s disease: An electrophysiological study. Eur. J. Pharmacol. 2014, 732, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Restani, P.; Fradera, U.; Ruf, J.C.; Stockley, C.; Teissedre, P.L.; Biella, S.; Colombo, F.; Lorenzo, C.D. Grapes and their derivatives in modulation of cognitive decline: A critical review of epidemiological and randomized-controlled trials in humans. Crit. Rev. Food Sci. Nutr. 2021, 61, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Amen, Y.; Sherif, A.E.; Shawky, N.M.; Abdelrahman, R.S.; Wink, M.; Sobeh, M. Grape-Leaf Extract Attenuates Alcohol-Induced Liver Injury via Interference with NF-κB Signaling Pathway. Biomolecules 2020, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, T.K.; Wohlenberg, M.F.; Medeiros, N.; Martins, J.B.; Agostini, F.; Funchal, C.; Dani, C. Evaluation of antioxidant activity of grapevine leaves extracts (Vitis labrusca) in liver of Wistar rats. An. Acad. Bras. Cienc. 2016, 88, 187–196. [Google Scholar] [CrossRef]
- Saadaoui, N.; Mathlouthi, A.; Zaiter, A.; El-Bok, S.; Mokni, M.; Harbi, M.; Ghanem-Boughanmi, N.; Dicko, A.; Ben-Attia, M. Phytochemical profiling, antioxidant potential and protective effect of leaves extract of tunisian Vitis vinifera autochthonous accessions against acute CCl4-injured hepatotoxicity in mice. Heliyon 2023, 9, e16377. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.L.; Carpa, R.; Fizeșan, I.; Vlase, L.; Bogdan, C.; Iurian, S.M.; Benedec, D.; Pop, A. Phytochemical Profile and Biological Activities of Tendrils and Leaves Extracts from a Variety of Vitis vinifera L. Antioxidants 2020, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; Rebollo-Hernanz, M.; Martin-Cabrejas, M.A.; Moreno-Arribas, M.V.; Bartolomé, B. Grape Pomace as a Cardiometabolic Health-Promoting Ingredient: Activity in the Intestinal Environment. Antioxidants 2023, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Montagnani, M.; Santacroce, L.; Charitos, I.A.; Bottalico, L. Ancient herbal therapy: A brief history of Panax ginseng. J. Ginseng. Res. 2023, 47, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.C.; Bulgaru, C.V.; Marin, D.E.; Oancea, A.G.; Taranu, I. Dietary Grape Seed Meal Bioactive Compounds Alleviate Epithelial Dysfunctions and Attenuates Inflammation in Colon of DSS-Treated Piglets. Foods 2021, 10, 530. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary Fiber Pectin Directly Blocks Toll-Like Receptor 2-1 and Prevents Doxorubicin-Induced Ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Minuzzi, N.M.; Nichelle, S.M.; Lamas, C.A.; Cagnon, V.H.A.; Morari, J.; Velloso, L.A.; Maróstica Júnior, M.R.; et al. Grape peel powder attenuates the inflammatory and oxidative response of experimental colitis in rats by modulating the NF-κB pathway and activity of antioxidant enzymes. Nutr. Res. 2020, 76, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Boussenna, A.; Goncalves-Mendes, N.; Joubert-Zakeyh, J.; Pereira, B.; Fraisse, D.; Vasson, M.P.; Texier, O.; Felgines, C. Impact of basal diet on dextran sodium sulphate (DSS)-induced colitis in rats. Eur. J. Nutr. 2015, 54, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Bahena-Garrido, S.M.; Ohama, T.; Suehiro, Y.; Hata, Y.; Isogai, A.; Iwashita, K.; Goto-Yamamoto, N.; Koyama, K. The potential aroma and flavor compounds in Vitis sp. cv. Koshu and V. vinifera L. cv. Chardonnay under different environmental conditions. J. Sci. Food Agric. 2019, 99, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, H.; Fang, H.; Jin, Y.; Zhao, Y.; Shen, J.; Zhou, C.; Li, R.; Wang, J.; Fu, Y.; et al. Effects of dietary grape pomace on the intestinal microbiota and growth performance of weaned piglets. Arch. Anim. Nutr. 2020, 74, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Martínez, I.; Arreaza-Gil, V.; Muguerza, B.; Arola-Arnal, A.; Bravo, F.I.; Torres-Fuentes, C.; Suárez, M. Administration Time Significantly Affects Plasma Bioavailability of Grape Seed Proanthocyanidins Extract in Healthy and Obese Fischer 344 Rats. Mol. Nutr. Food Res. 2022, 66, e2100552. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Kaspar, J.W.; Shen, J.; Jaiswal, A.K. Nrf2 signaling and cell survival. Toxicol. Appl. Pharmacol. 2010, 244, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Arola, L.; Blay, M.; Terra, X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal alterations in obese rats. Mol. Nutr. Food Res. 2017, 61, 1601039. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolomé, B.; Moreno-Arribas, M.V. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. (Landmark Ed.) 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Shah, I.M.; Surek, E.; Barile, D. Uncovering the promising role of grape pomace as a modulator of the gut microbiome: An in-depth review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos, K.; Stagos, D.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2017, 101, e108–e121. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Pugliese, V.; Fontana, S.; Jirillo, E. Human use of Leucoselect® Phytosome® with special reference to inflammatory-allergic pathologies in frail elderly patients. Curr. Pharm. Des. 2014, 20, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Topi, S.; Charitos, I.A.; Lovero, R.; Luperto, P.; Palmirotta, R.; Jirillo, E. Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products. Pathophysiology 2024, 31, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Marzulli, G.; Magrone, T.; Vonghia, L.; Kaneko, M.; Takimoto, H.; Kumazawa, Y.; Jirillo, E. Immunomodulating and anti-allergic effects of Negroamaro and Koshu Vitis vinifera fermented grape marc (FGM). Curr. Pharm. Des. 2014, 20, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Esteban-Fernández, A.; González de Llano, D.; Sanz-Buenhombre, M.; Guadarrana, A.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilánc, C.G.; Martín Gómez, L.; García Bermejo, M.L.; et al. Supplementation with grape pomace in healthy women: Changes in biochemical parameters, gut microbiota and related metabolic biomarkers. J. Funct. Foods 2018, 45, 34–46. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Martínez-Maqueda, D.; Hereu, M.; Amézqueta, S.; Torres, J.L.; Pérez-Jiménez, J. Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial. Foods 2020, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Bottalico, L.; Charitos, I.A.; Haxhirexha, K.; Topi, S.; Jirillo, E. Healthy Diets and Lifestyles in the World: Mediterranean and Blue Zone People Live Longer. Special Focus on Gut Microbiota and Some Food Components. Endocr. Metab. Immune Disord. Drug Targets, 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Yuan, Y.; Tian, Y.; Gao, S.; Zhang, X.M.; Gao, X.F.; He, J.P. Effects of environmental factors and fermentation on red raspberry anthocyanins stability. LWT 2023, 173, 114252. [Google Scholar] [CrossRef]
- Akazawa, T.; Itami, H.; Furumoto, T.; Nozaki, C.; Koike, H.; Iritani, S.; Amimoto, N.; Ogawa, M. Impact of an Olive Leaf Polyphenol 3,4-DHPEA-EDA on Physical Properties of Food Protein Gels. J. Agric. Food Chem. 2021, 69, 14250–14258. [Google Scholar] [CrossRef] [PubMed]
- González-Rámila, S.; Mateos, R.; García-Cordero, J.; Seguido, M.A.; Bravo-Clemente, L.; Sarriá, B. Olive Pomace Oil versus High Oleic Sunflower Oil and Sunflower Oil: A Comparative Study in Healthy and Cardiovascular Risk Humans. Foods 2022, 11, 2186. [Google Scholar] [CrossRef]
- Palmieri, D.; Aliakbarian, B.; Casazza, A.A.; Ferrari, N.; Spinella, G.; Pane, B.; Cafueri, G.; Perego, P.; Palombo, D. Effects of polyphenol extract from olive pomace on anoxia-induced endothelial dysfunction. Microvasc. Res. 2012, 83, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Katsinas, N.; Rodríguez-Rojo, S.; Enríquez-de-Salamanca, A. Olive Pomace Phenolic Compounds and Extracts Can Inhibit Inflammatory- and Oxidative-Related Diseases of Human Ocular Surface Epithelium. Antioxidants 2021, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Katsinas, N.; Gehlsen, U.; García-Posadas, L.; Rodríguez-Rojo, S.; Steven, P.; González-García, M.J.; Enríquez-de-Salamanca, A. Olive Pomace Phenolic Compounds: From an Agro-Industrial By-Product to a Promising Ocular Surface Protection for Dry Eye Disease. J. Clin. Med. 2022, 11, 4703. [Google Scholar] [CrossRef] [PubMed]
- Radić, K.; Vinković Vrček, I.; Pavičić, I.; Čepo, D.V. Cellular Antioxidant Activity of Olive Pomace Extracts: Impact of Gastrointestinal Digestion and Cyclodextrin Encapsulation. Molecules 2020, 25, 5027. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Vargas, B.K.; Monteiro, C.S.; Pappis, L.; Mello, R.O.; Machado, A.K.; Emanuelli, T.; Ayub, M.A.Z.; Moreira, J.C.F.; Augusti, P.R. Bioavailable Phenolic Compounds from Olive Pomace Present Anti-Neuroinflammatory Potential on Microglia Cells. Foods 2023, 12, 4048. [Google Scholar] [CrossRef]
- Detopoulou, M.; Ntzouvani, A.; Petsini, F.; Gavriil, L.; Fragopoulou, E.; Antonopoulou, S. Consumption of Enriched Yogurt with PAF Inhibitors from Olive Pomace Affects the Major Enzymes of PAF Metabolism: A Randomized, Double Blind, Three Arm Trial. Biomolecules 2021, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, O.; Moulas, I.; Loukas, T.; Magkoutis, A.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Trends in Food Innovation: An Interventional Study on the Benefits of Consuming Novel Functional Cookies Enriched with Olive Paste. Sustainability 2021, 13, 11472. [Google Scholar] [CrossRef]
- Ronca, C.L.; Marques, S.S.; Ritieni, A.; Giménez-Martínez, R.; Barreiros, L.; Segundo, M.A. Olive Oil Waste as a Source of Functional Food Ingredients: Assessing Polyphenolic Content and Antioxidant Activity in Olive Leaves. Foods. 2024, 13, 189. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101 (Suppl. S1), S75–S77. [Google Scholar] [CrossRef]
- Gill, C.I.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [CrossRef]
- Nashwa, F.S.M.; Abdel-Aziz, M.E. Efficiency of olive (Olea europaea L.) leaf extract as antioxidant and anticancer agents. J. Agroaliment Process. Technol. 2014, 20, 46–53. [Google Scholar]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the usage of olive leaves, bioactive compounds, biological activities, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Spagnoletta, A.; Salvatore, R.; Magrone, M.; Dentamaro, F.; Russo, M.A.; Difonzo, G.; Summo, C.; Caponio, F.; Jirillo, E. Olive Leaf Extracts Act as Modulators of the Human Immune Response. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.D.; Pérez-Guerrero, C.; Marhuenda, E.; Ruiz-Gutiérrez, V. Effects of dietary oleic-rich oils (virgin olive and high-oleic-acid sunflower) on vascular reactivity in Wistar-Kyoto and spontaneously hypertensive rats. Br. J. Nutr. 2001, 86, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, A.; Rauwald, H.W.; Kampa, B.; Mann, U.; Mohr, F.W.; Dhein, S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. J. Ethnopharmacol. 2008, 120, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M.; Reig, M. A green approach to phenolic compounds recovery from olive mill and winery wastes. Sci. Total Environ. 2022, 835, 155552. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From antiquity to contemporary times: How olive oil by-products and waste water can contribute to health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Romeo, R.; Cassano, A. Concentration of Bioactive Phenolic Compounds in Olive Mill Wastewater by Direct Contact Membrane Distillation. Molecules 2021, 26, 1808. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Bassani, B.; Gallo, C.; Maramotti, S.; Noonan, D.N.; Albini, A.; Bruno, A. Effect of a Purified Extract of Olive Mill Waste Waters on Endothelial Cell Proliferation, Apoptosis, Migration and Capillary-Like Structure in vitro and in vivo. Bioanal. Biomed. 2015, S12, 6. [Google Scholar] [CrossRef]
- Albini, A.; Benelli, R. The chemoinvasion assay: A method to assess tumor and endothelial cell invasion and its modulation. Nat. Protoc. 2007, 2, 504–511. [Google Scholar] [CrossRef]
- Roberts, J.D.; Lillis, J.B.; Pinto, J.M.; Chichger, H.; López-Samanes, Á.; Coso, J.D.; Zacca, R.; Willmott, A.G.B. The Effect of a Hydroxytyrosol-Rich, Olive-Derived Phytocomplex on Aerobic Exercise and Acute Recovery. Nutrients 2023, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Pierno, S.; Tricarico, D.; Liantonio, A.; Mele, A.; Digennaro, C.; Rolland, J.F.; Bianco, G.; Villanova, L.; Merendino, A.; Camerino, G.M.; et al. An olive oil-derived antioxidant mixture ameliorates the age-related decline of skeletal muscle function. Age 2014, 36, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Pantano, D.; Luccarini, I.; Nardiello, P.; Servili, M.; Stefani, M.; Casamenti, F. Oleuropein aglycone and polyphenols from olive mill waste water ameliorate cognitive deficits and neuropathology. Br. J. Clin. Pharmacol. 2017, 83, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Bassani, B.; Rossi, T.; De Stefano, D.; Pizzichini, D.; Corradino, P.; Macrì, N.; Noonan, D.M.; Albini, A.; Bruno, A. Potential chemopreventive activities of a polyphenol rich purified extract from olive mill wastewater on colon cancer cells. J. Funct. Foods 2016, 27, 236–248. [Google Scholar] [CrossRef]
- Benedetto, N.; Calabrone, N.; Gutmanska, K.; Macrì, N.; Cerrito, M.G.; Ricotta, R.; Pelosi, G.; Bruno, A.; Nooman, D.M.; Albini, A. An olive oil mill wastewater extract improves chemotherapeutic activity against breast cancer cells while protecting from cardiotoxicity. Front. Cardiovasc. Med. 2022, 9, 867867. [Google Scholar] [CrossRef] [PubMed]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.; Erkan, N.; Yasmin, A. Antioxidant protection of eicosapentaenoic acid and fish oil oxidation by polyphenolic-enriched apple skin extract. J. Agric. Food Chem. 2010, 58, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sychrová, A.; Škovranová, G.; Čulenová, M.; Bittner Fialová, S. Prenylated Flavonoids in Topical Infections and Wound Healing. Molecules 2022, 27, 4491. [Google Scholar] [CrossRef] [PubMed]
- Abu-Niaaj, L.F.; Al-Daghistani, H.I.; Katampe, I.; Abu-Irmaileh, B.; Bustanji, Y.K. Pomegranate peel: Bioactivities as antimicrobial and cytotoxic agents. Food Sci. Nutr. 2024, 12, 2818–2832. [Google Scholar] [CrossRef] [PubMed]
- Pistol, G.C.; Pertea, A.M.; Taranu, I. The Use of Fruit and Vegetable by-Products as Enhancers of Health Status of Piglets after Weaning: The Role of Bioactive Compounds from Apple and Carrot Industrial Wastes. Vet. Sci. 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef] [PubMed]
- Asma, U.; Bertotti, M.L.; Zamai, S.; Arnold, M.; Amorati, R.; Scampicchio, M. A Kinetic Approach to Oxygen Radical Absorbance Capacity (ORAC): Restoring Order to the Antioxidant Activity of Hydroxycinnamic Acids and Fruit Juices. Antioxidants 2024, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Asif, H.M.; Kamal, S.; Rehman, A.U.; Rasool, S.; Hamid Akash, M.S. Synthesis, Characterization, and Enzyme Inhibition Properties of 1,2,4-Triazole Bearing Azinane Analogues. ACS Omega 2022, 7, 32360–32368. [Google Scholar] [CrossRef]
- Aziz, A.; Noreen, S.; Khalid, W.; Ejaz, A.; Faiz Ul Rasool, I.; Maham Munir, A.; Farwa Javed, M.; Ercisli, S.; Okcu, Z.; Marc, R.A.; et al. Pumpkin and Pumpkin Byproducts: Phytochemical Constitutes, Food Application and Health Benefits. ACS Omega 2023, 8, 23346–23357. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, G.; Kanbur, M.; Aslan, Ö.; Karabacak, M. The antioxidant effects of pumpkin seed oil on subacute aflatoxin poisoning in mice. Environ. Toxicol. 2013, 28, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, J.; Carani Venkatraman, A. Cissus quadrangularis stem alleviates insulin resistance, oxidative injury and fatty liver disease in rats fed high fat plus fructose diet. Food Chem. Toxicol. 2010, 48, 2021–2029. [Google Scholar] [CrossRef]
- Koraqi, H.; Petkoska, A.T.; Khalid, W.; Sehrish, A.; Ambreen, S.; Lorenzo, J.M. Optimization of the Extraction Conditions of Antioxidant Phenolic Compounds from Strawberry Fruits (Fragaria x ananassa Duch.) Using Response Surface Methodology. Food Anal. Methods 2023, 16, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Z.; Arshad, M.S.; Ali, A.; Aziz, A.; Khalid, W.; Afzal, M.F.; Bangar, S.P.; Addi, M.; Hano, C.; Lorenzo, J.M. Potential Role of Phytochemical Extract from Saffron in Development of Functional Foods and Protection of Brain-Related Disorders. Oxid. Med. Cell. Longev. 2022, 2022, 6480590. [Google Scholar] [CrossRef] [PubMed]
- Quanhong, L.; Caili, F.; Yukui, R.; Guanghui, H.; Tongyi, C. Effects of protein-bound polysaccharide isolated from pumpkin on insulin in diabetic rats. Plant Foods Hum. Nutr. 2005, 60, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Potukuchi, A.; Addepally, U.; Sindhu, K.; Manchala, R. Increased total DNA damage and oxidative stress in brain are associated with decreased longevity in high sucrose diet fed WNIN/Gr-Ob obese rats. Nutr. Neurosci. 2018, 21, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Fahim, A.T.; Abd-el Fattah, A.A.; Agha, A.M.; Gad, M.Z. Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacol. Res. 1995, 31, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Gual-Grau, A.; Guirro, M.; Crescenti, A.; Boqué, N.; Arola, L. In vitro fermentability of a broad range of natural ingredients by fecal microbiota from lean and obese individuals: Potential health benefits. Int. J. Food Sci. Nutr. 2022, 73, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Long, S.R.; Kutikova, L.; Bowman, L.; Finley, D.; Crown, W.H.; Bennett, C.L. Estimating the cost of cancer: Results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J. Clin. Oncol. 2004, 22, 3524–3530. [Google Scholar] [CrossRef] [PubMed]
- Silva RCd Ferdaus, M.J.; Foguel, A.; da Silva, T.L.T. Oleogels as a Fat Substitute in Food: A Current Review. Gels 2023, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.H.; Matielo, C.E.L.; Curvelo, L.A.; Rocco, R.A.; Felga, G.; Della Guardia, B.; Boteon, Y.L. Update on the management and treatment of viral hepatitis. World J. Gastroenterol. 2021, 27, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Stajčić, S.; Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Mandić, A.; Četojević-Simin, D. Tomato waste: Carotenoids content, antioxidant and cell growth activities. Food Chem. 2015, 172, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Sun, J.; Bailoni, E.; Chen, P.; Li, Y.; Gao, B.; Wang, T.T.Y.; Rao, J.; Yu, L.L. Chemical Composition of Tomato Seed Flours, and Their Radical Scavenging, Anti-Inflammatory and Gut Microbiota Modulating Properties. Molecules 2021, 26, 1478. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; AREDS2 Research Group. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022, 140, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Chopra, K. Lycopene abrogates Aβ(1-42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Thomas-Ahner, J.M.; Wan, L.; Zuniga, K.E.; Erdman, J.W.; Clinton, S.K. Tomatoes, Lycopene, and Prostate Cancer: What Have We Learned from Experimental Models? J. Nutr. 2022, 152, 1381–1403. [Google Scholar] [CrossRef] [PubMed]
- Ba, W.; Xu, W.; Deng, Z.; Zhang, B.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of the Main Carotenoids from Tomatoes via Nrf2 and NF-κB Signaling Pathways. Nutrients 2023, 15, 4652. [Google Scholar] [CrossRef] [PubMed]
- Ascenso, A.; Pinho, S.; Eleutério, C.; Praça, F.G.; Bentley, M.V.; Oliveira, H.; Santos, C.; Silva, O.; Simões, S. Lycopene from tomatoes: Vesicular nanocarrier formulations for dermal delivery. J. Agric. Food Chem. 2013, 61, 7284–7293. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Moretto, G.; Barberis, M.; Frosi, I.; Papetti, A. Rice Byproduct Compounds: From Green Extraction to Antioxidant Properties. Antioxidants 2023, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Amankwah-Amoah, J.; Ahen, F. Editorial: Sustainable Waste Management Innovations: Developing New Ventures for Improved Health and Environmental Wellbeing. Sustainability 2021, 13, 7132. [Google Scholar] [CrossRef]
- Kyu, M.T.; Dar, B.; Aye, S.S.; Matsuda, T. Prebiotic Oligosaccharides Prepared by Enzymatic Degradation of Dietary Fibers in Rice Grains. J. Nutr. Sci. Vitaminol. 2019, 65, S143–S147. [Google Scholar] [CrossRef] [PubMed]
- Sahini, M.G.; Mutegoa, E. Extraction, phytochemistry, nutritional, and therapeutical potentials of rice bran oil: A review. Phytomed. Plus 2023, 3, 100453. [Google Scholar] [CrossRef]
- Rebello, C.J.; Burton, J.; Heiman, M.; Greenway, F.L. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial. J. Diabetes Complicat. 2015, 29, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Jaramillo-Madrid, A.C.; Ashworth, J.; Fabris, M.; Ralph, P.J. Phytosterol biosynthesis and production by diatoms (Bacillariophyceae). Phytochemistry 2019, 163, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Nieri, P.; Carpi, S.; Esposito, R.; Costantini, M.; Zupo, V. Bioactive Molecules from Marine Diatoms and Their Value for the Nutraceutical Industry. Nutrients 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Lakshmegowda, S.B.; Rajesh, S.K.; Kandikattu, H.K.; Nallamuthu, I.; Khanum, F. In vitro and in vivo Studies on Hexane Fraction of Nitzschia palea, a Freshwater Diatom for Oxidative Damage Protective and Anti-Inflammatory Response. Rev. Bras. Farmacogn. 2020, 30, 189–201. [Google Scholar] [CrossRef]
- Świderska-Kołacz, G.; Jefimow, M.; Klusek, J.; Rączka, N.; Zmorzyński, S.; Wojciechowska, A.; Stanisławska, I.; Łyp, M.; Czerwik-Marcinkowska, J. Influence of Algae Supplementation on the Concentration of Glutathione and the Activity of Glutathione Enzymes in the Mice Liver and Kidney. Nutrients 2021, 13, 1996. [Google Scholar] [CrossRef]
- Pekkoh, J.; Phinyo, K.; Thurakit, T.; Lomakool, S.; Duangjan, K.; Ruangrit, K.; Pumas, C.; Jiranusornkul, S.; Yooin, W.; Cheirsilp, B.; et al. Lipid Profile, Antioxidant and Antihypertensive Activity, and Computational Molecular Docking of Diatom Fatty Acids as ACE Inhibitors. Antioxidants 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, M.; Šantek, B.; Zorić, Z.; Čošić, Z.; Vrana, I.; Gašparović, B.; Čož-Rakovac, R.; Ivančić Šantek, M. Bioprospecting of Microalgae Isolated from the Adriatic Sea: Characterization of Biomass, Pigment, Lipid and Fatty Acid Composition, and Antioxidant and Antimicrobial Activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef] [PubMed]
- Gügi, B.; Le Costaouec, T.; Burel, C.; Lerouge, P.; Helbert, W.; Bardor, M. Diatom-Specific Oligosaccharide and Polysaccharide Structures Help to Unravel Biosynthetic Capabilities in Diatoms. Mar. Drugs 2015, 13, 5993–6018. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.; Chronopoulou, E.G.; Letsiou, S.; Maya, C.; Labrou, N.E.; Infante, C.; Power, D.M.; Manchado, M. Antioxidant capacity and immunomodulatory effects of a chrysolaminarin-enriched extract in Senegalese sole. Fish Shellfish Immunol. 2018, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zheng, J.; Xia, C.; Chen, X.; Xu, Q.; Duan, B. The potential, challenges, and prospects of the genus Spirulina polysaccharides as future multipurpose biomacromolecules. Int. J. Biol. Macromol. 2023, 253 Pt 8, 127482. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Chang, H.C. Improvements in the quality and shelf life of kimchi by fermentation with the induced bacteriocin-producing strain, Leuconostoc citreum GJ7 as a starter. J. Food Sci. 2010, 75, M103–M110. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Dananjaya, S.H.S.; Nikapitiya, C.; Liyanage, T.D.; Lee, K.A.; Oh, C.; Kang, D.H.; De Zoysa, M. Novel pectin isolated from Spirulina maxima enhances the disease resistance and immune responses in zebrafish against Edwardsiella piscicida and Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 94, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liang, C.H.; Wu, H.T.; Pang, H.Y.; Chen, C.; Wang, G.H.; Chan, L.P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018, 55, 2310–2317. [Google Scholar] [CrossRef]
- Auti, A.; Alessio, N.; Ballini, A.; Dioguardi, M.; Cantore, S.; Scacco, S.; Vitiello, A.; Quagliuolo, L.; Rinaldi, B.; Santacroce, L.; et al. Protective Effect of Resveratrol against Hypoxia-Induced Neural Oxidative Stress. J. Pers. Med. 2022, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Johns, M.R. Screening of diatoms for heterotrophic eicosapentaenoic acid production. J. Appl. Phycol. 1996, 8, 59–64. [Google Scholar] [CrossRef]
- Wang, M.J.; Wang, M.; Zhan, X.Q.; Liu, L.; Wu, Q.; An, F.L.; Lu, Y.B.; Guo, L.L.; Zhang, Z.X.; Fei, D.Q. Antimicrobial diterpenoids from the leaves and twigs of Croton kongensis Gagnepain. Fitoterapia 2023, 164, 105350. [Google Scholar] [CrossRef] [PubMed]
- Chandrarathna, H.; Liyanage, T.D.; Edirisinghe, S.L.; Dananjaya, S.H.S.; Thulshan, E.H.T.; Nikapitiya, C.; Oh, C.; Kang, D.H.; De Zoysa, M. Marine Microalgae, Spirulina maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina platensis ameliorates diphenoxylate-induced constipation symptoms in mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Long, H.; Yu, L.; Xia, X.; Zhu, Z.; Liu, X. Selenium-containing polysaccharide from Spirulina platensis alleviates Cd-induced toxicity in mice by inhibiting liver inflammation mediated by gut microbiota. Front. Nutr. 2022, 9, 950062. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Mo, X.; Zhou, N.; Wang, Y.; Wei, G.; Chen, J.; Chen, K.; Ouyang, P. A novel bacterial β-N-acetyl glucosaminidase from Chitinolyticbacter meiyuanensis possessing transglycosylation and reverse hydrolysis activities. Biotechnol. Biofuels 2020, 13, 115. [Google Scholar] [CrossRef]
- Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.I.; Sotelo, C.G. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Mar. Drugs 2018, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; He, J.L.; Li, W.M.; Yang, Z.; Wang, Y.X.; Bai, X.F.; Yu, C.; Du, Y.G. Chitosan oligosaccharides protect human umbilical vein endothelial cells from hydrogen peroxide induced apoptosis. Carbohydr. Polym. 2010, 80, 1062–1071. [Google Scholar] [CrossRef]
- Pangestuti, R.; Bak, S.S.; Kim, S.K. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Fukamizo, T.; Suginta, W. Green-Chemical Strategies for Production of Tailor-Made Chitooligosaccharides with Enhanced Biological Activities. Molecules 2023, 28, 6591. [Google Scholar] [CrossRef] [PubMed]
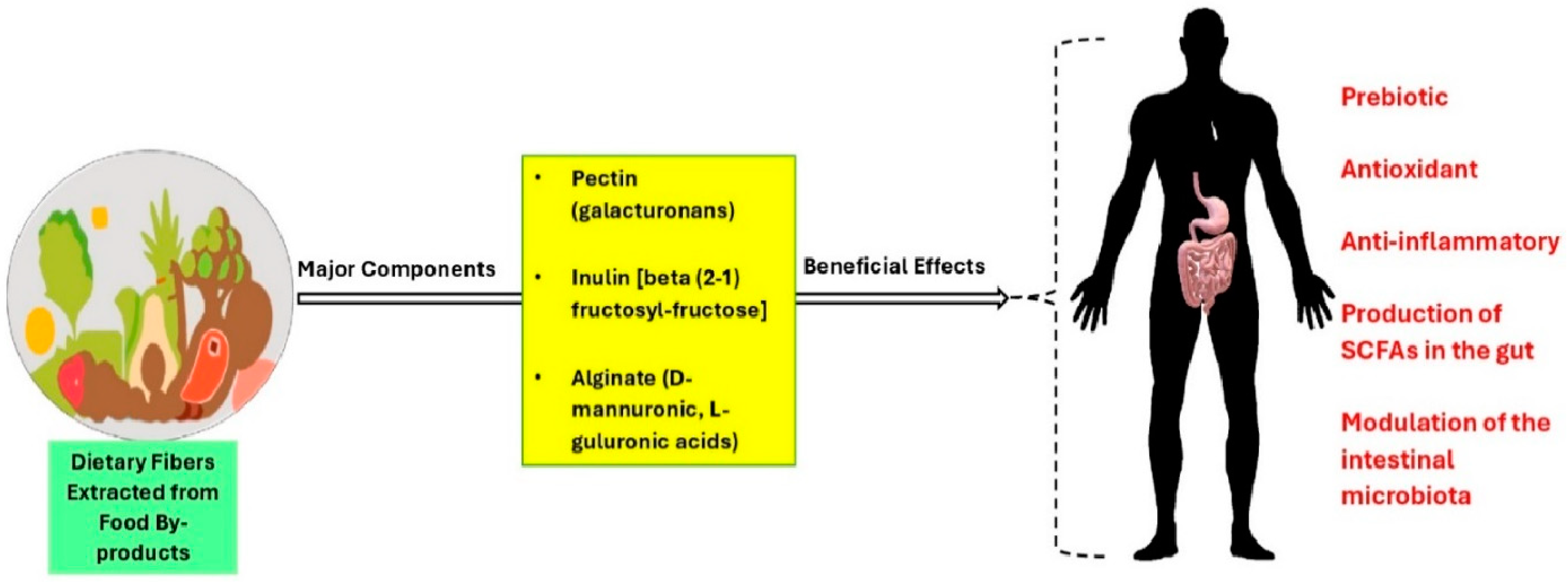
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [PubMed]
- Expósito-Almellón, X.; Duque-Soto, C.; López-Salas, L.; Quirantes-Piné, R.; de Menezes, C.R.; Borrás-Linares, I.; Lozano-Sánchez, J. Non-Digestible Carbohydrates: Green Extraction from Food By-Products and Assessment of Their Effect on Microbiota Modulation. Nutrients 2023, 15, 3880. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. Artificial neural network modelling of inflammatory markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Sasaki, H.; Miyakawa, H.; Nakayama, Y.; Lyu, Y.; Shibata, S. Effect of Dose and Timing of Burdock (Arctium lappa) Root Intake on Intestinal Microbiota of Mice. Microorganisms 2020, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Montagnani, M.; Potenza, M.A.; Corsalini, M.; Barile, G.; Charitos, I.A.; de Giacomo, A.; Jirillo, E.; Colella, M.; Santacroce, L. Current View on How Human Gut Microbiota Mediate Metabolic and Pharmacological Activity of Panax ginseng. A Scoping Review. Endocr. Metab. Immune Disord. Drug Targets, 2024; epub ahead of print. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Kosaka, M.; Hirano, S.; Kawahara, M.; Sato, M.; Kaneshima, T.; Nishizawa, M.; Takahashi, H.; Kimura, B. Effect of sodium-alginate and laminaran on Salmonella Typhimurium infection in human enterocyte-like HT-29-Luc cells and BALB/c mice. Carbohydr. Polym. 2015, 125, 113–119. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Thissen, J.P.; Delzenne, N.M. Gut microbial metabolites of polyunsaturated fatty acids correlate with specific fecal bacteria and serum markers of metabolic syndrome in obese women. Lipids 2014, 49, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Meyer, D.; Gibson, G.R. A double-blind placebo-controlled study to establish the bifidogenic dose of inulin in healthy humans. Eur. J. Clin. Nutr. 2007, 61, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef]
- Bondy, S.C. Relationships between Diabetes and the Intestinal Microbial Population. Int J Mol Sci. 2022, 24, 566. [Google Scholar] [CrossRef] [PubMed]

| GRAPE BY-PRODUCTS |
| Major components: polyphenols, fibers [17]. |
| Activities |
| In vitro anti-inflammatory activities with activation of T regulatory cells and release of the anti- inflammatory cytokine, IL-10 [15,32,33]. |
| In obese rats, modulation of gut microbiota with production of SCFAs and expansion of Enterococcus, Prevotella, Bifidobacterium, and Faecalibacterium [45]. |
| Potentiation of T helper 1 activity in elderly subjects [50], and mitigation of symptoms in contact dermatitis to nickel in patients, with production of IL-10 [51,52]. |
| OLIVE BY-PRODUCTS (olive pomace, olive leaf extract, and oil mill wastewater) |
| Major components: polyphenols, fatty acids, stearic acids, carotenoids, vitamin C and E [55,56,57,96]. |
| Activities |
| In animal models, anti-inflammatory activities (inhibition of NF-kβ, TNF-α, PAF), increase in antioxidant activities, and decrease in serum triglycerides [64,65,68,69,79,81]. |
| In animal models, cardioprotective effects and prevention of arterial plaque formation [70,74]. |
| In vitro, inhibition of proliferation and angiogenesis in cancer cells [85]. |
| In animal models, prevention of Alzheimer’ disease outcome with attenuation of Ab peptide aggregation [75]. |
| In obese rats, effects on the gut microbiota with increase in SCFAs and enhancement in intestinal barrier integrity [87]. |
| APPLE BY-PRODUCTS |
| Major components: polyphenols, carotenoids, vitamins C and E. |
| Activities |
| Antioxidant and anti-inflammatory activities [88]. |
| In piglets, effects on the gut microbiota with expansion of lactobacilli and Faecalibacterium [94]. |
| PUMPKIN BY-PRODUCTS |
| Major components: polyphenols, carotenoids, vitamins C and E, linoleic acid, selenium [96]. |
| Activities |
| In animal models: |
| Decreased risk of Alzheimer’s disease [100,101]. |
| Prevention of energy malnutrition [103]. |
| Attenuation of arthritis [104]. |
| Increase in SCFA production in the gut and expansion of Lactobacillaceae and Faecalibacterium spp. [106]. |
| TOMATO BY-PRODUCTS |
| Major components: polyphenols, dietary fibers, proteins, lycopene [107]. |
| Activities |
| In animal models: |
| Lycopene-mediated anti-inflammatory and cardioprotective effects [111,112]. |
| Lycopene-mediated production of IL-17 with protection against bacterial infections [115]. |
| RICE BY-PRODUCTS |
| Major components: carbohydrates, proteins, fats, minerals, fibers, polyphenols, vitamin E [117]. |
| Activities |
| In animal models: |
| Enzymatic degradation of dietary fibers followed by generation of oligosaccharides in the gut with probiotic effects [122]. |
| In humans: |
| Limited use of rice bran oil for its high levels of free fatty acids [120]. |
| MARINE-BY PRODUCTS |
|---|
| MICROALGAE Major components: carotenoids, polysaccharides, polyphenols, sterols, proteins/peptides, and fatty acids [124]. Activities Fucoxantin (carotenoid): in vitro antioxidant and anti-inflammatory activities [125]. Polyphenols: in vitro inhibition of pro-inflamamtory cytokine and nitric oxide [127]. Polyunsaturared fatty acids: in animal models, antioxidant, anti-inflammatory, and anti-cholesteroligenic activities [130]. Polysaccharides (Spirulina): in animal models, increase in innate immunity functions and B cell activities [135,136,137,138], inhibition of lipid peroxidation [139,140], modulation of gut microbiota [141], and improvement in constipation symptoms and liver inflammation [142,143]. Proteins: in animal models, antihypertensive and antioxidant roles [144]. |
| ARTHROPODS Major component: chitooligosaccharides (COS) [145]. Activities COS: in vitro, antioxidant, anti-inflammatory, immune-enhancing, and antitumor activities [145,146,147,148,149]. |
| FISH BY-PRODUCTS (fish scales, skin, bones) Activities Collagen peptides from fish scales: in vitro antioxidant, anti-inflammatory, and DNA-protective functions [151]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santacroce, L.; Bottalico, L.; Charitos, I.A.; Castellaneta, F.; Gaxhja, E.; Topi, S.; Palmirotta, R.; Jirillo, E. Exploitation of Natural By-Products for the Promotion of Healthy Outcomes in Humans: Special Focus on Antioxidant and Anti-Inflammatory Mechanisms and Modulation of the Gut Microbiota. Antioxidants 2024, 13, 796. https://doi.org/10.3390/antiox13070796
Santacroce L, Bottalico L, Charitos IA, Castellaneta F, Gaxhja E, Topi S, Palmirotta R, Jirillo E. Exploitation of Natural By-Products for the Promotion of Healthy Outcomes in Humans: Special Focus on Antioxidant and Anti-Inflammatory Mechanisms and Modulation of the Gut Microbiota. Antioxidants. 2024; 13(7):796. https://doi.org/10.3390/antiox13070796
Chicago/Turabian StyleSantacroce, Luigi, Lucrezia Bottalico, Ioannis Alexandros Charitos, Francesca Castellaneta, Elona Gaxhja, Skender Topi, Raffaele Palmirotta, and Emilio Jirillo. 2024. "Exploitation of Natural By-Products for the Promotion of Healthy Outcomes in Humans: Special Focus on Antioxidant and Anti-Inflammatory Mechanisms and Modulation of the Gut Microbiota" Antioxidants 13, no. 7: 796. https://doi.org/10.3390/antiox13070796
APA StyleSantacroce, L., Bottalico, L., Charitos, I. A., Castellaneta, F., Gaxhja, E., Topi, S., Palmirotta, R., & Jirillo, E. (2024). Exploitation of Natural By-Products for the Promotion of Healthy Outcomes in Humans: Special Focus on Antioxidant and Anti-Inflammatory Mechanisms and Modulation of the Gut Microbiota. Antioxidants, 13(7), 796. https://doi.org/10.3390/antiox13070796











