Rod-Shaped Mesoporous Zinc-Containing Bioactive Glass Nanoparticles: Structural, Physico-Chemical, Antioxidant, and Immuno-Regulation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zn-Containing Rod-Shaped Mesoporous Bioactive Glass Nanoparticles (ZnRBGNs)
2.2. Characterization of the Physical and Chemical Properties of the Samples
2.3. Cytocompatibility Evaluation In Vitro
2.3.1. Cell Culture
2.3.2. Preparation of Leaching Solution of Samples
2.3.3. Cytotoxicity and Proliferation
2.3.4. EdU Assay
2.3.5. Live–Dead Assay
2.3.6. Flow Cytometry
2.4. Antioxidant Effect Evaluation
2.4.1. ROS (Reactive Oxygen Species) Generation Assay
2.4.2. DPPH Assay
2.5. Statistical Analysis
3. Results
3.1. Characterization of the Physical and Chemical Properties of the Samples
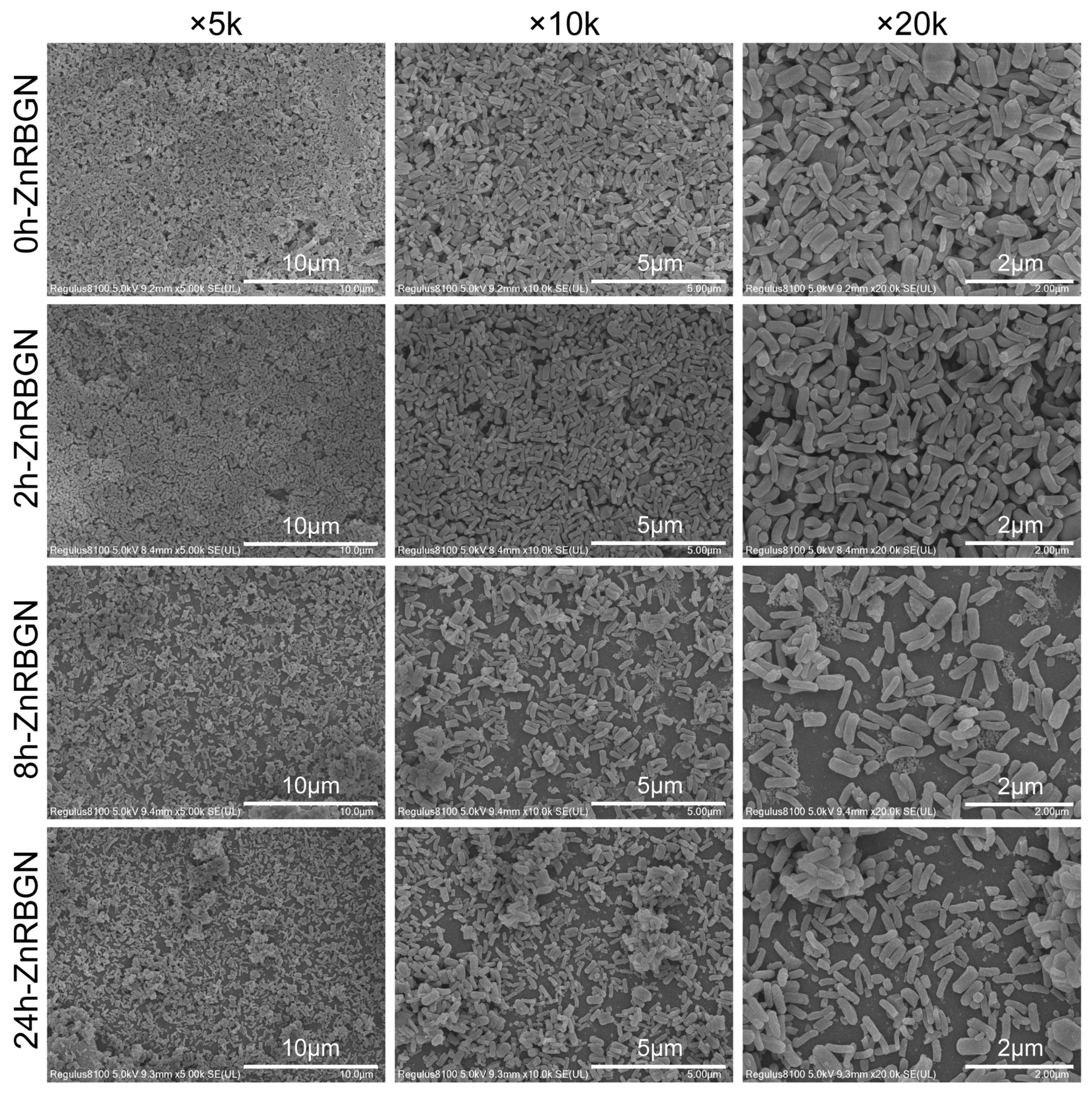
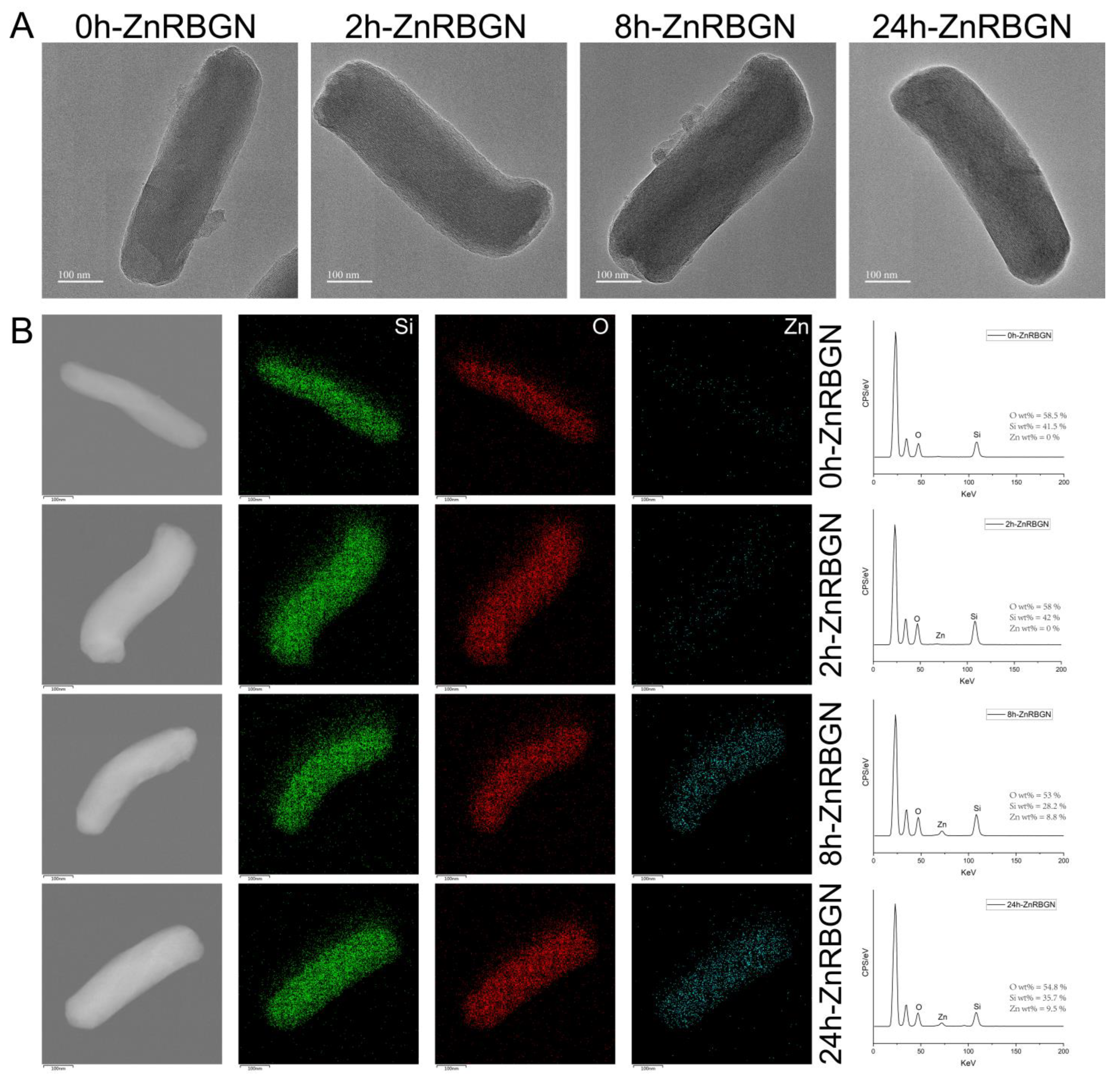
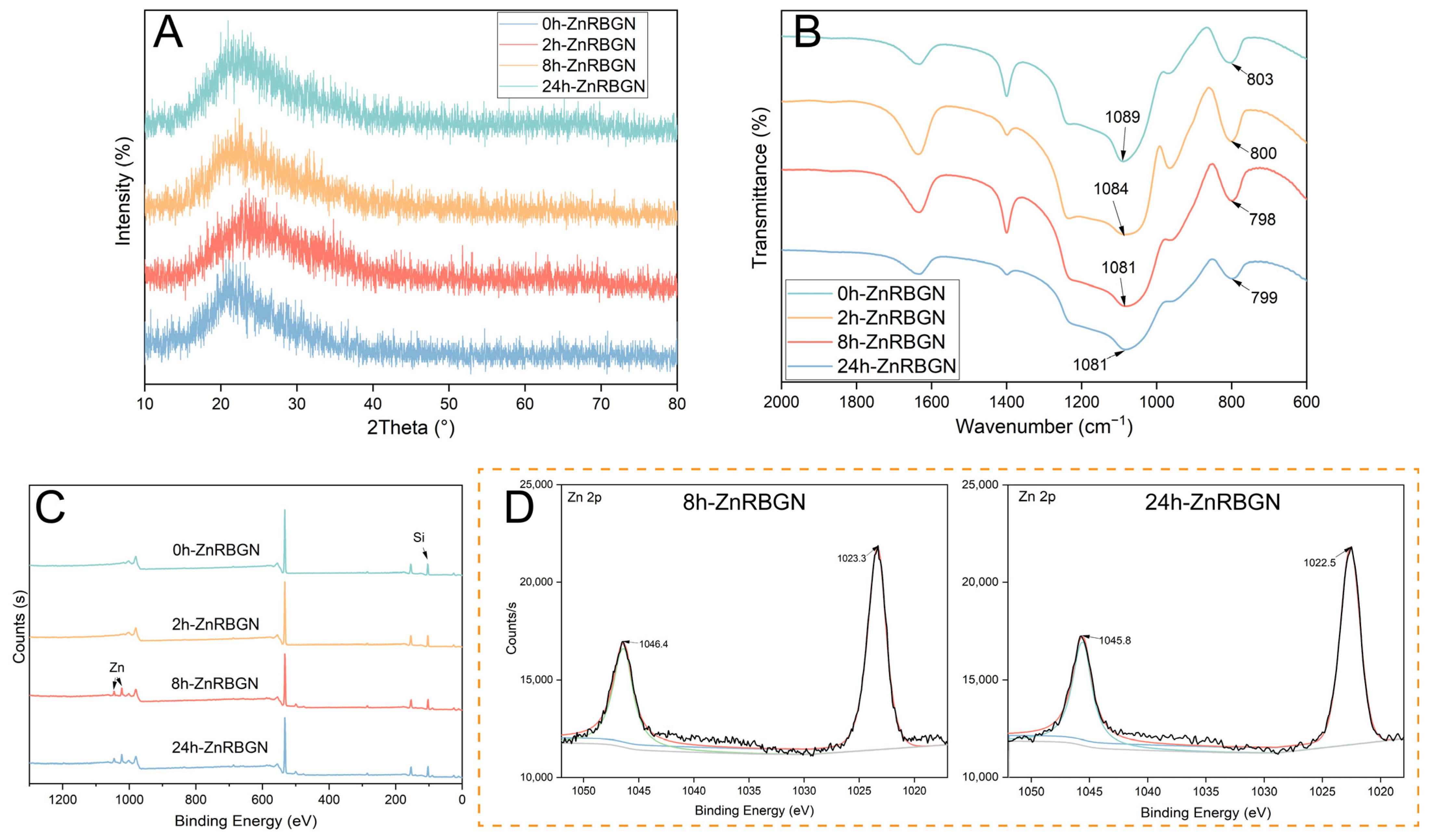
3.1.1. The Morphology and Composition of ZnRBGNs
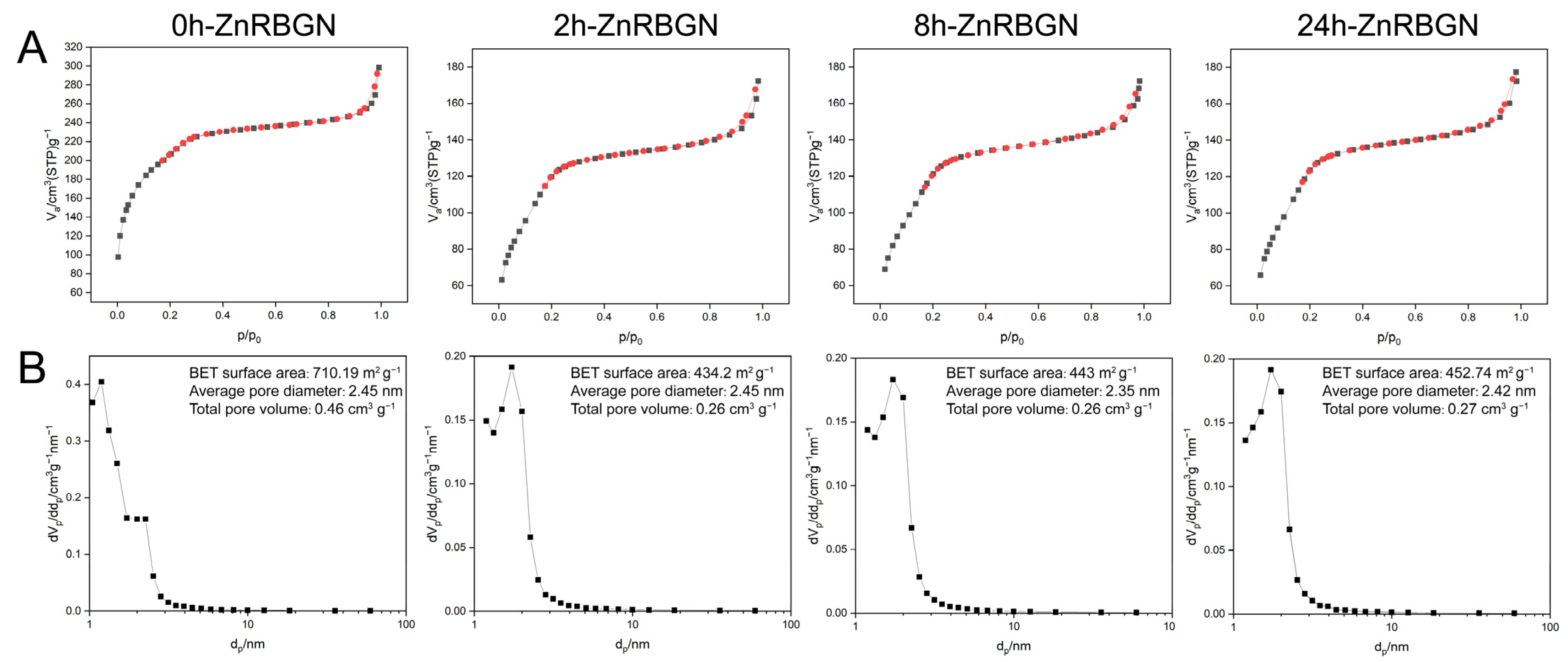
3.1.2. The Mesoporous Structural Analysis of ZnRBGN
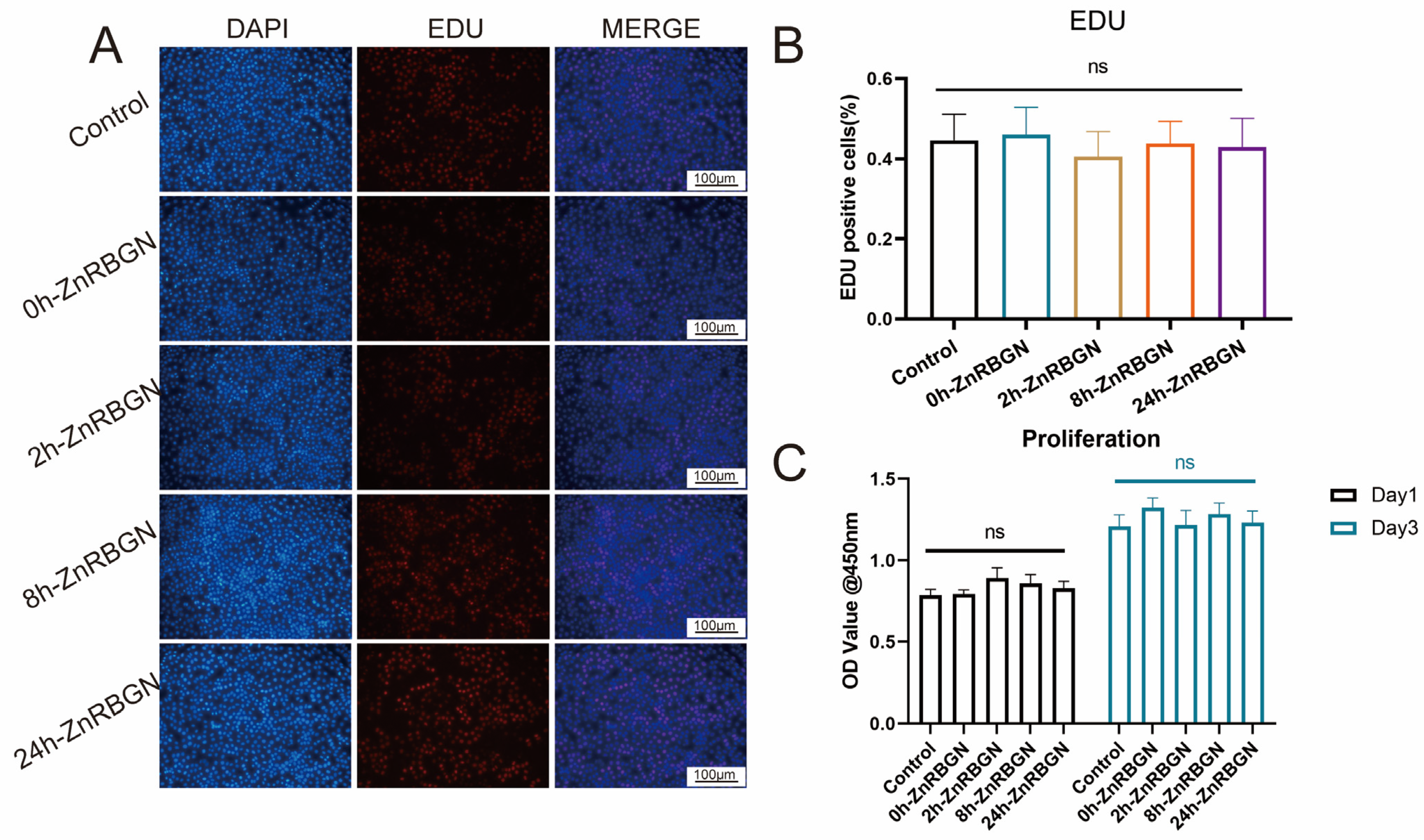
3.2. Cytocompatibility Evaluation In Vitro
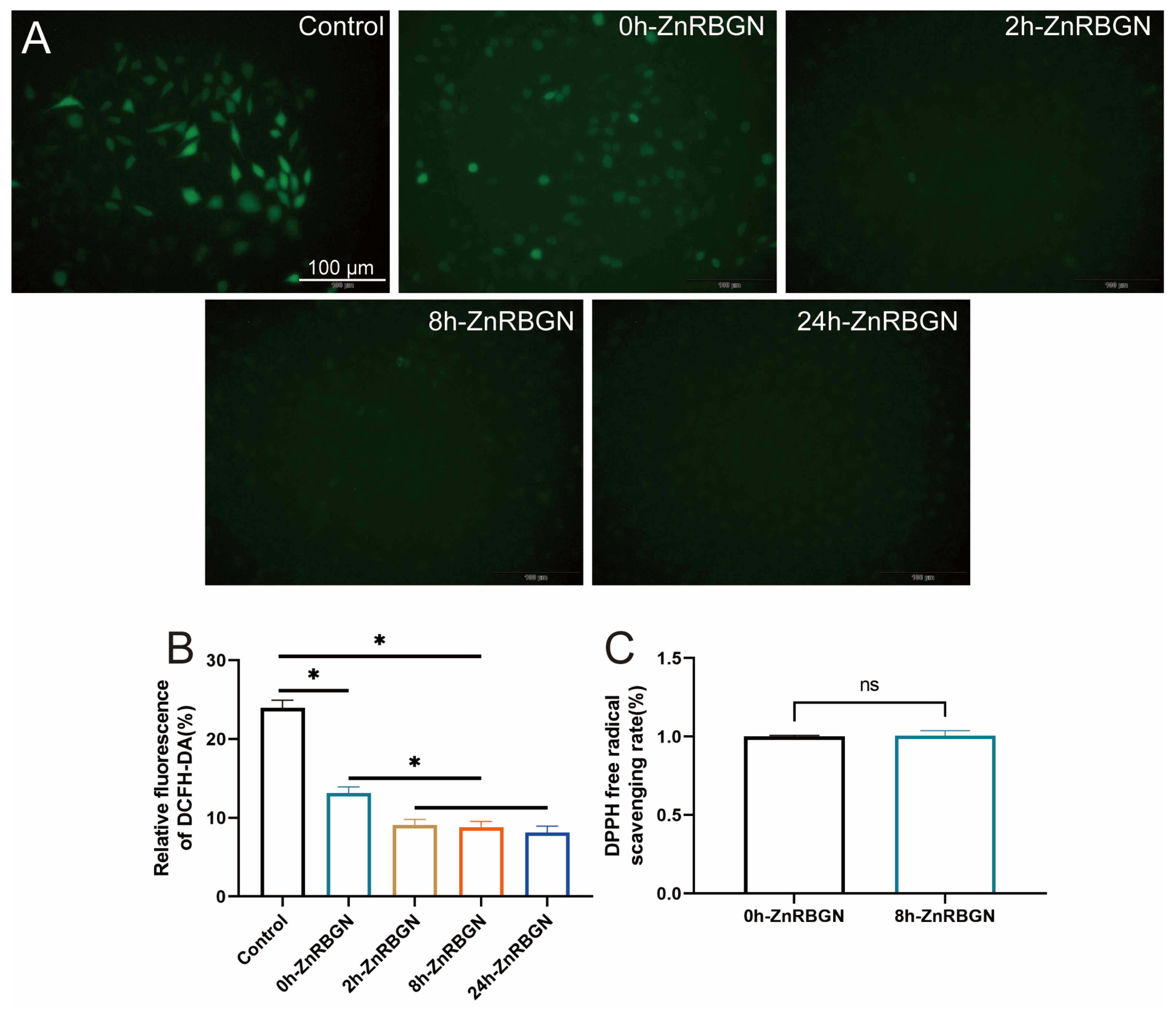
3.3. Antioxidant Effect Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francesco, B.; Sepideh, H.; Saeid, K. Bioactive glasses: Where Are We and Where Are We Going. J. Func. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Denissen, H.W.; Groot, D.K.; Makkes, P.C.; Hooff, V.D.; Klopper, P.J. Tissue Response to Dense Apatite Implants in Rats. J. Biomed. Mater. Res. 1980, 14, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Salinas, A.J. Mesoporous Bioactive Glasses for Regenerative Medicine. Mater. Today Bio. 2021, 11, 100121. [Google Scholar] [CrossRef] [PubMed]
- Charlotte, V.; Jean-Marie, N. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Kozon, D.; Zheng, K.; Boccardi, E.; Liu, Y.; Liverani, L.; Boccaccini, A.R. Synthesis of Monodispersed Ag-Doped Bioactive Glass Nanoparticles via Surface Modification. Materials 2016, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Cudal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wang, Z.; Liu, C.; Chen, X.; Cao, X. High Performance Injectable Mg Doped Bioactive Glass Bone Cement for the Regulation of Osteogenic Immune Microenvironment. Biomater. Adv. 2024, 160, 213864. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Mortera, R.; Onida, B.; Vitale-Brovarone, C. Mesoporous Bioactive Glass as a Multifunctional System for Bone Regeneration and Controlled Drug Release. J. Appl. Biomater. Func. Mater. 2012, 10, 12. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, F.; Lin, Z.; Cao, X.; Chen, D.; Chen, X. Local Delivery of Naringin in Beta-cyclodextrin Modified Mesoporous Bioactive Glass Promotes Bone Regeneration: From Anti-inflammatory to Synergistic Osteogenesis and Osteoclastogenesis. Biomater. Sci. 2022, 10, 1697–1712. [Google Scholar] [CrossRef]
- Schlundt, C.; Fischer, H.; Bucher, C.H.; Rendenbach, C.; Duda, G.N.; SchmidtBleek, K. The Multifaceted Roles of Macrophages in Bone Regeneration: A Story of Polarization, Activation and Time. Acta Biomater. 2021, 133, 46–57. [Google Scholar] [CrossRef]
- Wynn, T.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Natural 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Dziak, R. Osteoimmunology: Cross-talk between Bone and Immune Cells. Immunol. Investig. 2013, 42, 657–660. [Google Scholar] [CrossRef]
- Walsh, M.C.; Takegahara, N.; Kim, H.; Choi, Y. Updating Osteoimmunology: Regulation of Bone Cells by Innate and Adaptive Immunity. Nat. Rev. Rheumatol. 2018, 14, 146–156. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, B.; Bao, J.; Zhang, Y.H.; Lei, L.; Yan, F.H. Macrophage Polarization in Periodontal Ligament Stem Cells Enhanced Periodontal Regeneration. Stem Cell Res. Ther. 2019, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory Bioactive Glasses for Tissue Regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Cerqueni, G.; Scalzone, A.; Licini, C.; Gentile, P.; Mattioli-Belmonte, M. Insights into Oxidative Stress in Bone Tissue and Novel Challenges for Biomaterials. Mat. Sci. Eng. C Mater. 2021, 130, 112433. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Tu, Z.D.; Zhu, Z.; Liu, D.C.; Meng, Q.C.; Yu, Q.F.; Wang, Y.; Chen, J.Q.; Liu, T.; Han, F.X.; et al. Targeting Endogenous Reactive Oxygen Species Removal and Regulating Regenerative Microenvironment at Annulus Fibrosus Defects Promote Tissue Repair. ACS Nano 2023, 17, 7645–7661. [Google Scholar] [CrossRef]
- Day, R.M.; Boccaccini, A.R. Effect of Particulate Bioactive Glasses on Human Macrophages and Monocytes in vitro. J. Biomad. Mater. Res. B 2005, 73, 73. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, C.T.; Zhang, X.; Chang, J.; Dai, K. Regulation of Immune Response by Bioactive Ions Released from Silicate Bioceramics for Bone Regeneration. Acta Biomater. 2018, 66, 81–92. [Google Scholar] [CrossRef]
- Huo, S.; Wang, F.; Lyu, Z.; Hong, Q.; Nie, B.; Wei, J.; Wang, Y.; Zhang, J.; Yue, B. Dual-functional Polyetheretherketone Surface Modification for Regulating Immunity and Bone Metabolism. Chem. Eng. J. 2021, 8, 130806. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S. Nutritional Modulation of Immune Function: Analysis of Evidence Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef]
- Qian, G.; Lu, T.; Zhang, J.; Liu, R.; Wang, Z.; Yu, B.; Li, H.; Shi, H.; Ye, J. Promoting Bone Regeneration of Calcium Phosphate Cement by Addition of PLGA Microspheres and Zinc Silicate via Synergistic Effect of In-situ Pore Generation, Bioactive Ion Stimulation and Macrophage Immunomodulation. Appl. Mater. Today 2020, 19, 100615. [Google Scholar] [CrossRef]
- Yu, D.; Li, B.; Yu, M.; Guo, S.; Guo, Z.; Han, Y. Cubic Multi-ions-doped Na2TiO3 Nanorod-like Coatings: Structure-stable, Highly Efficient Platform for Ions Exchanged Release to Immunomodulatory Promotion on Vascularized Bone Apposition. Bioact. Mater. 2022, 18, 72–90. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Ananda, S.P. Zinc: An Antioxidant and Anti-inflammatory Agent: Role of Zinc in Degenerative Diorders of Aging. J. Trace Elem. Med. Bio. 2014, 28, 364–371. [Google Scholar] [CrossRef]
- Kargozar, S.; Hooshmand, S.; Hosseini, S.A.; Gorgani, S.; Kermani, F.; Baino, F. Antioxidant Effects of Bioactive Glasses (BGs) and Their Significance in Tissue Engineering Strategies. Molecule 2022, 27, 6642. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Dong, Z.; Liu, K.; He, H.; Lu, Y.; Wu, W.; Qi, J. Rod-like Mesoporous Silica Nanoparticles Facilitate Oral Drug Delivery via Enhanced Permeation and Retention Effect in Mucus. Nano Res. 2022, 15, 9243–9252. [Google Scholar] [CrossRef]
- Yang, G.; Gong, H.; Qian, X.; Tan, P.; Li, Z.; Teng, L.; Liu, J.; Li, Y.; Liu, Z. Mesoporous Silica Nanorods Intrinsically doped with Photosensitizers as a Multifunctional Drug Carrier for Combination Therapy of Cancer. Nano Res. 2015, 8, 751–764. [Google Scholar] [CrossRef]
- Katunar, M.R.; Diaz, F.; Boccaccini, A.R.; Ballarre, J. SiO2-CaO Rod-like Particles in Chitosan Matrix as Bioactive Coatings for Stainless Steel Implants. Ceram. Int. 2023, 49, 38535–38543. [Google Scholar] [CrossRef]
- Boccacini, A.R.; Erol, M.; Stark, W.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive Glass Nanocomposites for Biomedical Applications: A Review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Shen, J.; Cai, Z.; Zhao, C.; Chen, H.; Luo, X.; Hu, N.; Cui, W.; Huang, W. Injectable Hydrogel Microspheres with Self-renewable Hydration Layers Alleviate Osteoarthritis. Sci. Adv. 2022, 8, 6449. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, L.; Zhao, Y.; Yang, L.; Cheng, J.; Hua, R.; Zhang, Z.; Li, Q. Melatonin Protects Mouse Testes from Palmitic Acid-induced Lipotoxicity by Attenuating Oxidative Stress and DNA Damage in a SIRT1-dependent Manner. J. Pineal Res. 2020, 69, e12690. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, Y.T.; Li, B.; Qiu, X.; Hu, H.; Zhang, X.; Lu, Z.; Zheng, F. Transcriptional Characteristics and Functional Validation of Three Monocyte Subsets during Aging. Immun. Ageing 2023, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zou, Y.; Zhang, Y.; Wang, P.; Zhang, H. Electrospun Pullulan Nanofiber Loading Zanthoxylum Bungeanum Essential oil/β-cyclodextrin Inclusion Complexes for Active Packaging. Int. J. Biol. Macromol. 2022, 210, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Prince, A.A.M.; Raghavan, P.S.; Gopalan, R.; Panneersevam, G.; Narasimhan, S.V. Formation of Zinc Ferrite by Solid-state Reaction and Its Characterization by XRD and XPS. J. Mater. Sci. 2001, 36, 5379–5384. [Google Scholar] [CrossRef]
- Ege, D.; Lu, H.; Boccaccini, A.R. Bioactive Glass and Silica Particles for Skeletal and Cardiac Muscle Tissue Regeneration. Tissue Eng. Part B Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zambon, A.; Malavasi, G.; Pallini, A.; Fraulini, F.; Lusvardi, G. Cerium Containing Bioactive Glasses: A Review. ACS Biomater-Sci. Eng. 2021, 7, 4388–4401. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional Silica-based Mesoporous Materials for Simultaneous Delivery of Biologically Active Ions and Therapeutic Biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Cong, V.T.; Gaus, K.; Tilley, R.D.; Gooding, J.J. Rod-shaped mesoporous silica nanoparticles for nanomedicine: Recent progress and perspectives. Expert Opin. Drug Del. 2018, 15, 881–892. [Google Scholar] [CrossRef]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc silicate/nano-hydroxyapatite/collagen scaffolds promote angiogenesis and bone regeneration via the p38 MAPK pathway in activated monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, J.; Diao, Y.; Chen, Y.; Liang, X.; Li, H.; Miao, Y.; Gao, Q.; Gui, L.; Wang, X.; et al. ROS-responsive capsules engineered from EGCG-Zinc networks improve therapeutic angiogenesis in mouse limb ischemia. Bioact. Mater. 2021, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Wen, W.; Yan, J.; Wang, Y.; Wang, R.; Ma, X.; Ren, D.; Zheng, K.; Deng, C.; Zhang, J. Rod-Shaped Mesoporous Zinc-Containing Bioactive Glass Nanoparticles: Structural, Physico-Chemical, Antioxidant, and Immuno-Regulation Properties. Antioxidants 2024, 13, 875. https://doi.org/10.3390/antiox13070875
Zhu X, Wen W, Yan J, Wang Y, Wang R, Ma X, Ren D, Zheng K, Deng C, Zhang J. Rod-Shaped Mesoporous Zinc-Containing Bioactive Glass Nanoparticles: Structural, Physico-Chemical, Antioxidant, and Immuno-Regulation Properties. Antioxidants. 2024; 13(7):875. https://doi.org/10.3390/antiox13070875
Chicago/Turabian StyleZhu, Xiuan, Wenjie Wen, Jingjing Yan, Yuran Wang, Rumeng Wang, Xiang Ma, Dandan Ren, Kai Zheng, Chao Deng, and Jue Zhang. 2024. "Rod-Shaped Mesoporous Zinc-Containing Bioactive Glass Nanoparticles: Structural, Physico-Chemical, Antioxidant, and Immuno-Regulation Properties" Antioxidants 13, no. 7: 875. https://doi.org/10.3390/antiox13070875




