Resolvin D5 Protects Female Hairless Mouse Skin from Pathological Alterations Caused by UVB Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Protocol
2.4. Irradiation Protocol
2.5. Skin Edema
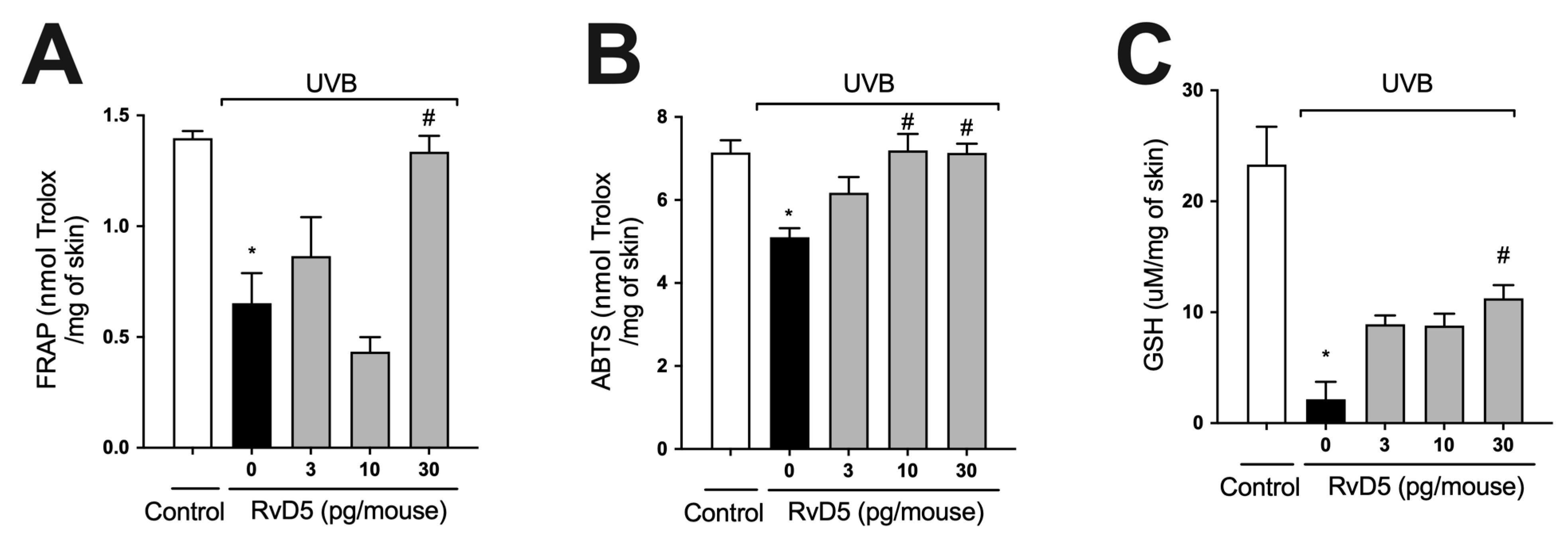
2.6. Total Antioxidant Capacity: FRAP and ABTS Assays
2.7. Quantification of Endogenous Antioxidant: Reduced Glutathione (GSH)
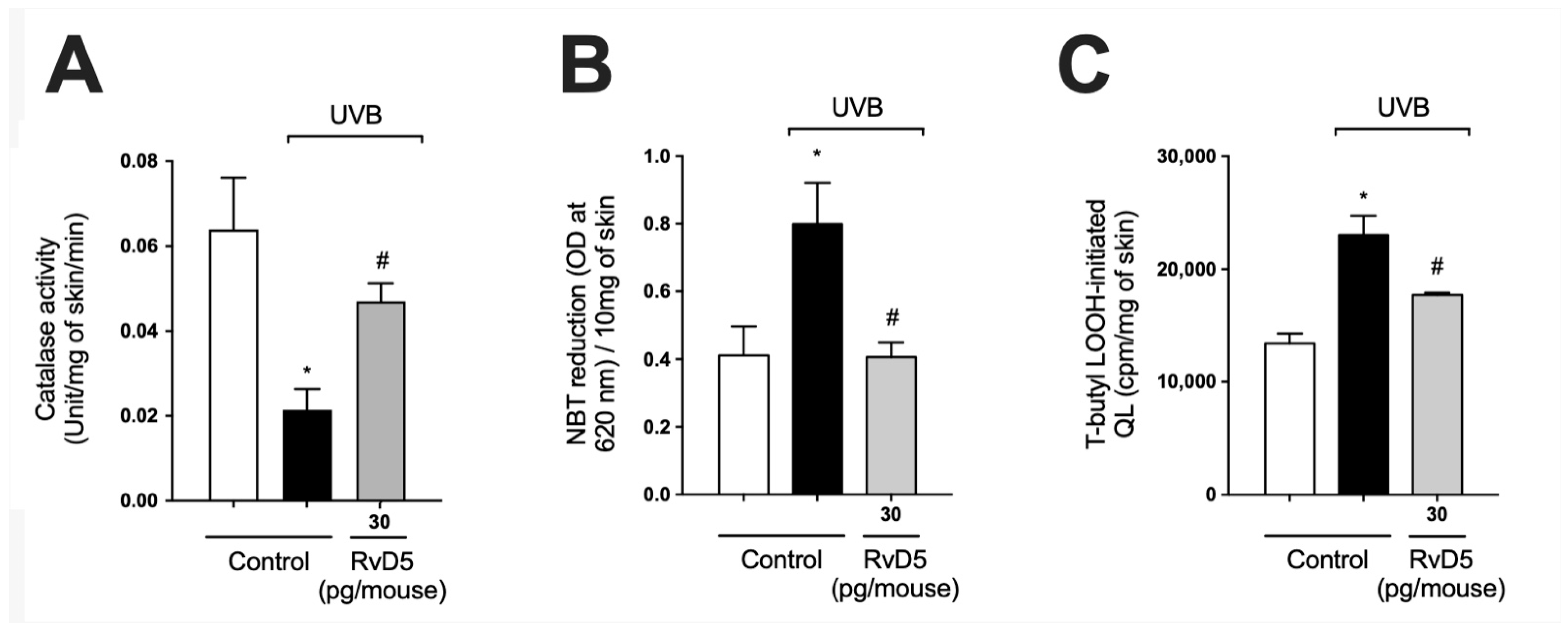
2.8. Catalase Assay
2.9. Superoxide Anion Production
2.10. Lipid Peroxidation Assay (LPO)
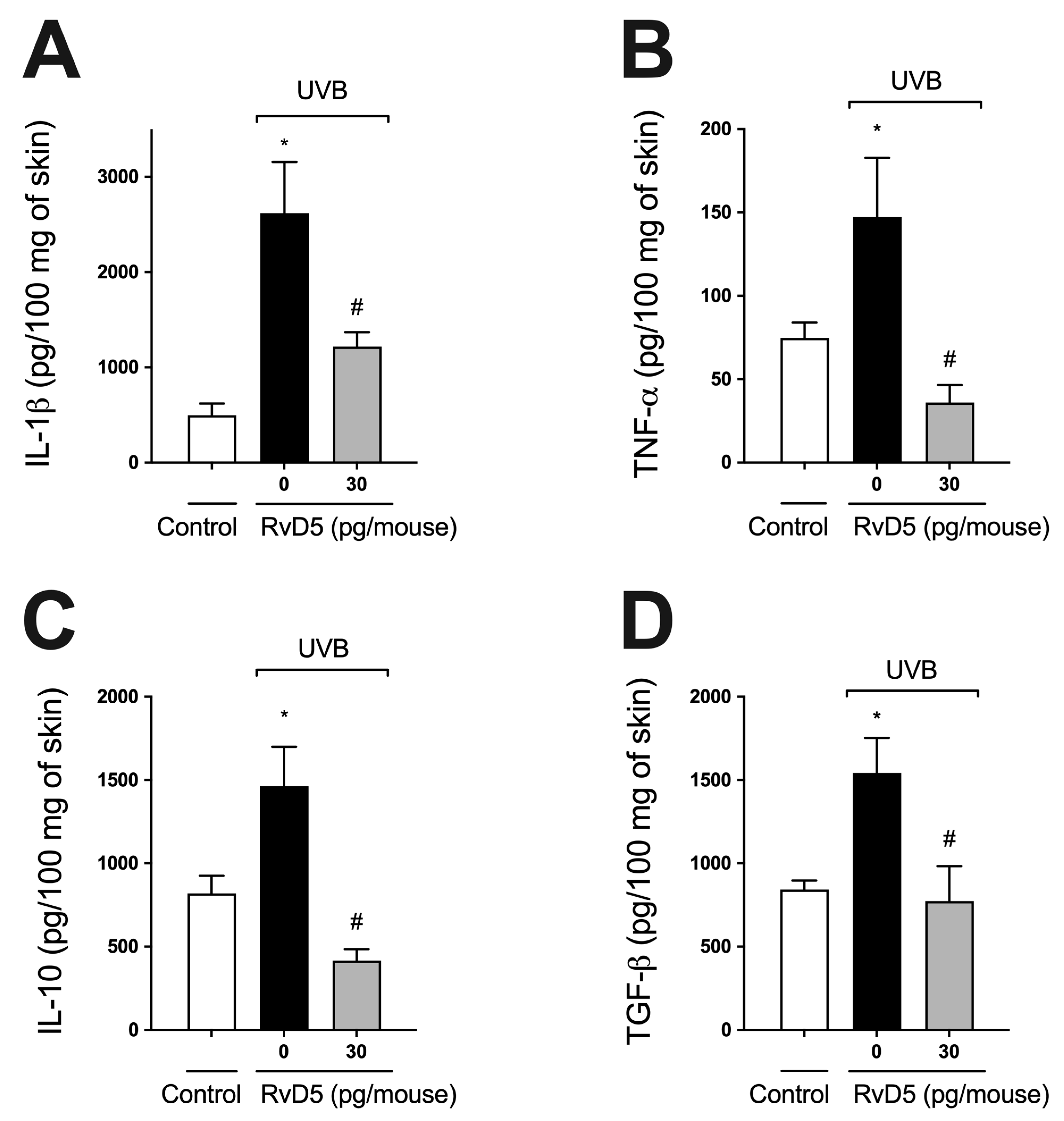
2.11. Cytokine Measurement by ELISA
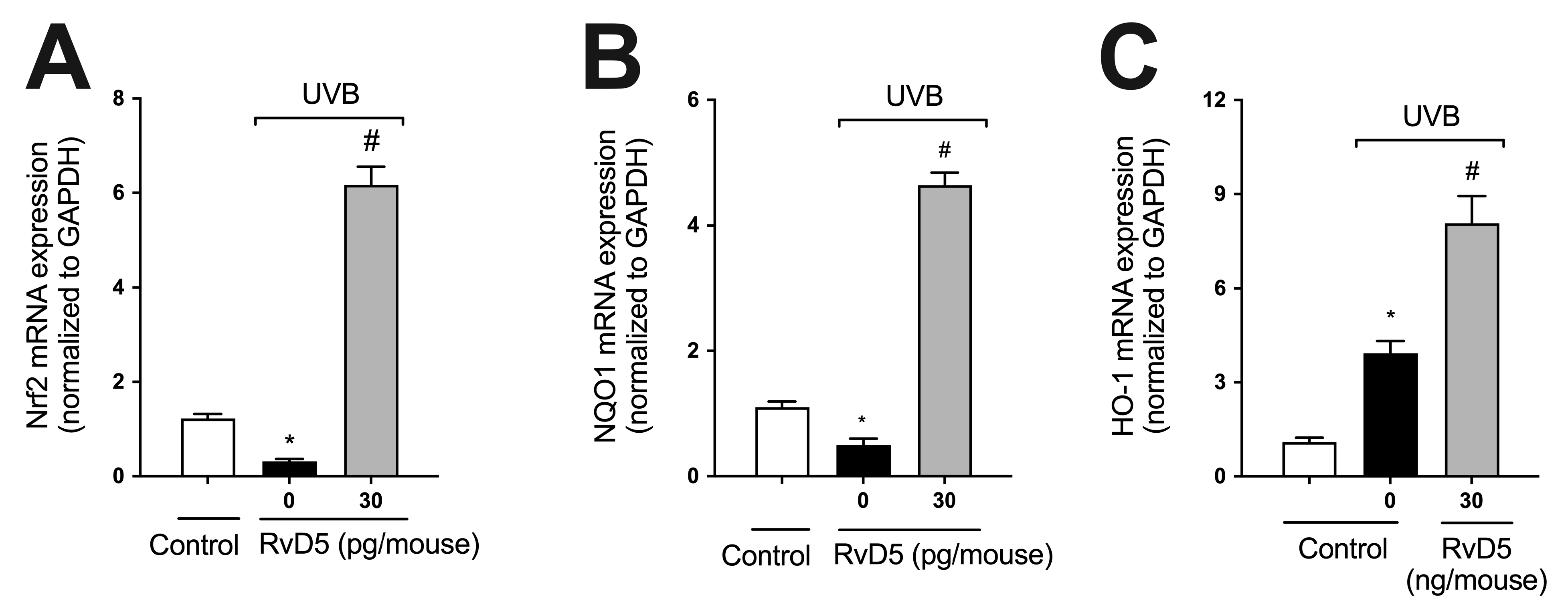
2.12. Real-Time and Quantitative Polymerase Chain Reaction (RT-qPCR)
2.13. Skin Histologic Evaluation
2.14. Fluorescence Assay
2.15. Matrix Metalloproteinase (MMP)-9 Activity Measurement
2.16. Statistical Analysis
3. Results
3.1. Effect of Resolvin D5 (RvD5) against Skin Edema Caused by UVB Irradiation
3.2. RvD5 Inhibits UVB Irradiation-Induced Depletion of Skin Antioxidants
3.3. RvD5 Interferes with ROS Metabolization, Production and Generation of Lipid Peroxidation End-Products in UVB Irradiation
3.4. RvD5 Up-Regulates the mRNA Expression of Genes Involved in Skin Endogenous Antioxidant Response during UVB Irradiation Inflammation
3.5. RvD5 Inhibits UVB Irradiation-Induced Cytokine Production
3.6. RvD5 Reduces Mast Cell Counts in UVB Irradiation
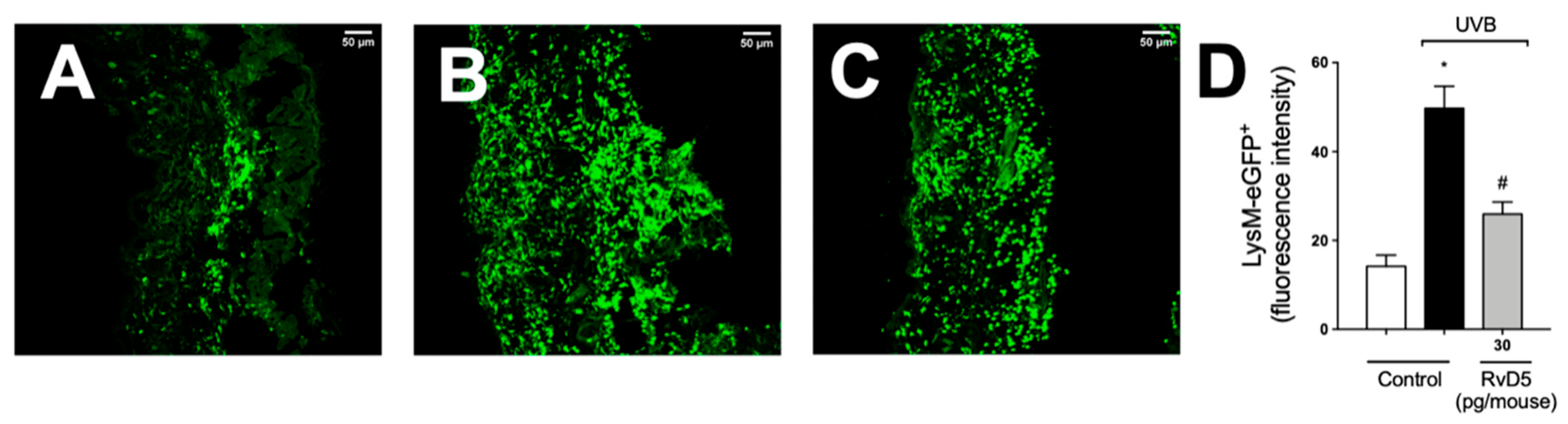
3.7. RvD5 Treatment Inhibits Skin Recruitment of LysM+ Neutrophils/Macrophages in UVB Irradiation
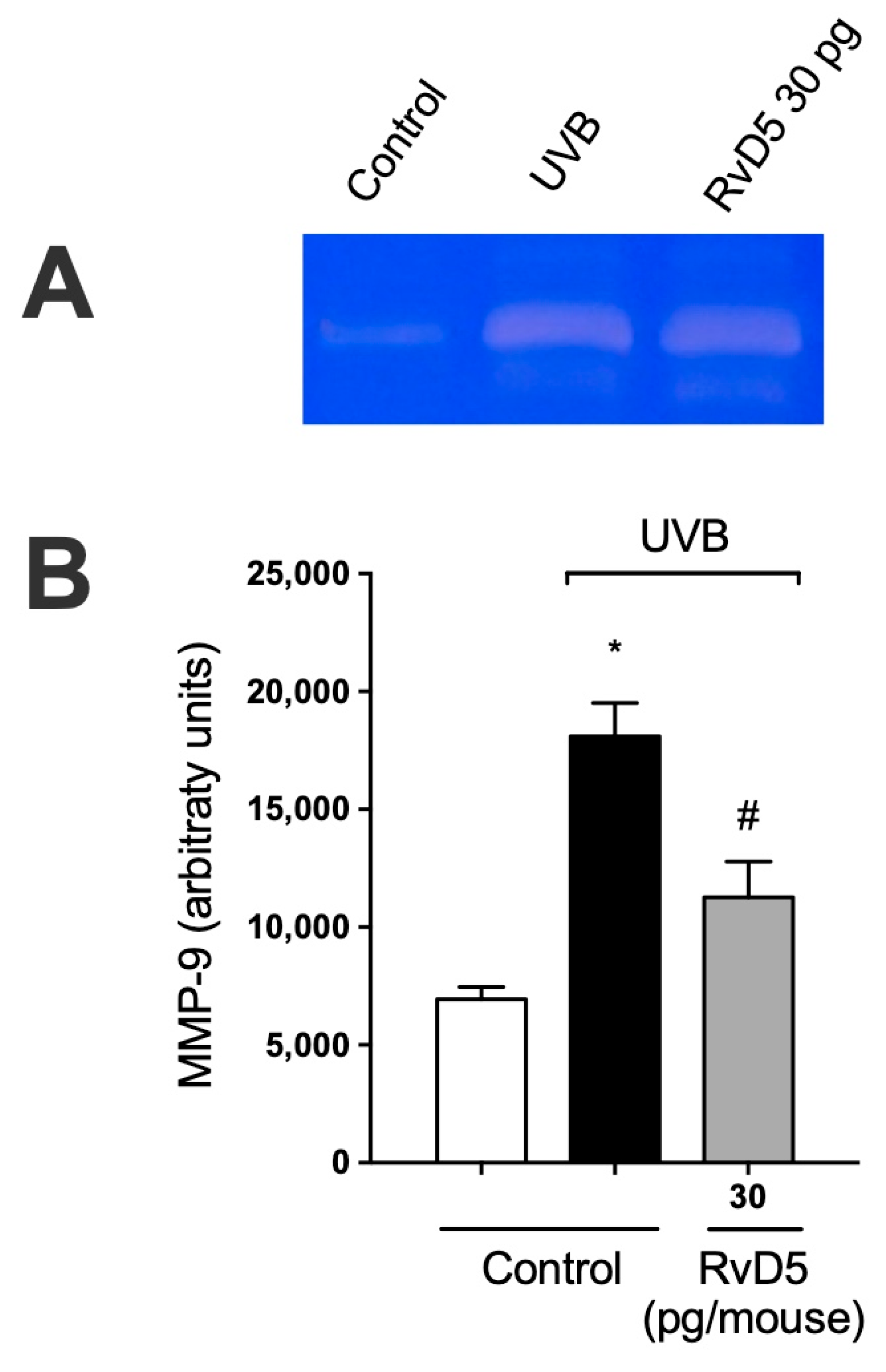
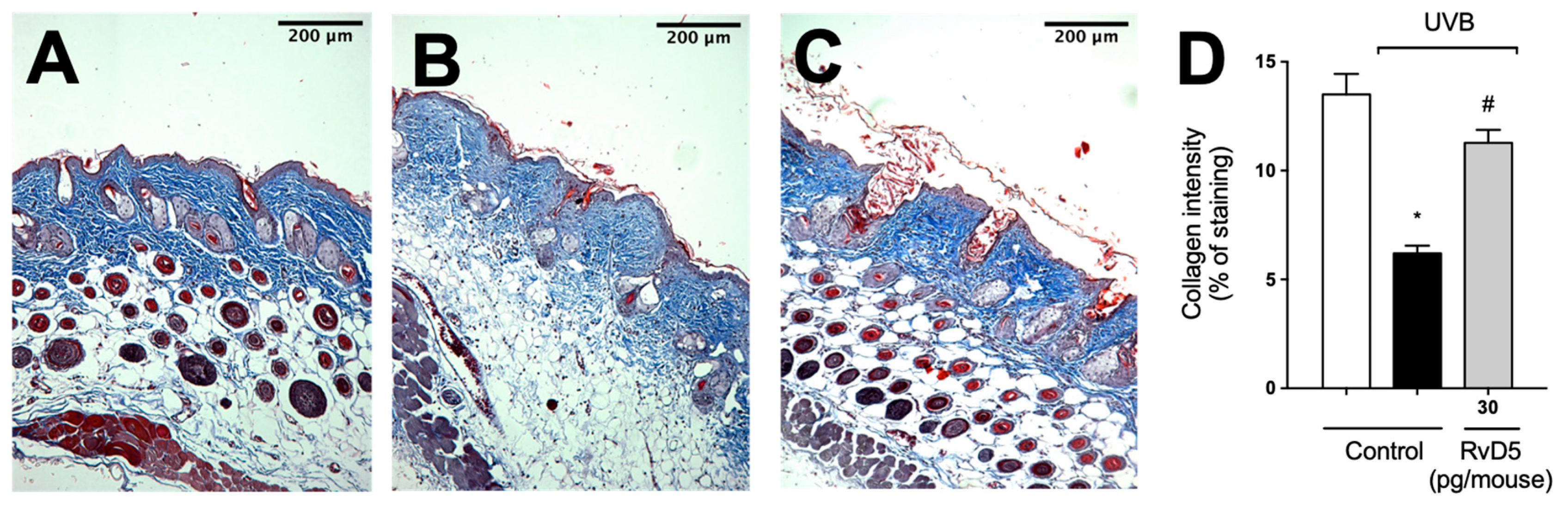
3.8. RvD5 Inhibits MMP-9 Activity and Collagen Fibers Degradation in UVB Irradiation
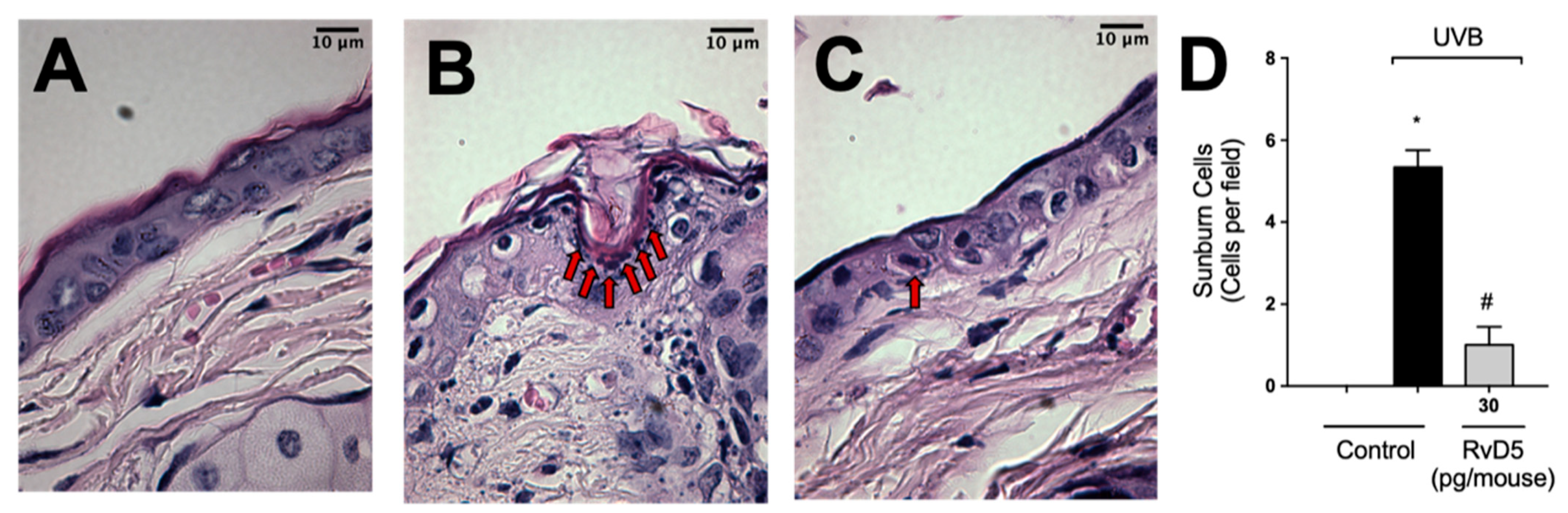
3.9. RvD5 Inhibits UVB Irradiation-Induced Apoptosis of Keratinocytes
3.10. RvD5 Inhibits UVB Irradiation-Induced Epidermal Thickening
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of Ultraviolet B Signaling and Photocarcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Cardoso, J.C.; Cavalcanti De Albuquerque Junior, R.L.; Vieira Fonseca, M.J. Efficacy of Marigold Extract-Loaded Formulations against UV-Induced Oxidative Stress. J. Pharm. Sci. 2011, 100, 2182–2193. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A.F. Aging Skin: Histology, Physiology, and Pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef]
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Packer, L. Dose-Response Effects of Acute Ultraviolet Irradiation on Antioxidants and Molecular Markers of Oxidation in Murine Epidermis and Dermis. J. Investig. Dermatol. 1994, 102, 470–475. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Maioli, N.A.; Zarpelon, A.C.; Mizokami, S.S.; Calixto-Campos, C.; Guazelli, C.F.S.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Carvalho, T.T.; Manchope, M.F.; Ferraz, C.R.; et al. The Superoxide Anion Donor, Potassium Superoxide, Induces Pain and Inflammation in Mice through Production of Reactive Oxygen Species and Cyclooxygenase-2. Braz. J. Med. Biol. Res. 2015, 48, 321–331. [Google Scholar] [CrossRef]
- Balupillai, A.; Prasad, R.N.; Ramasamy, K.; Muthusamy, G.; Shanmugham, M.; Govindasamy, K.; Gunaseelan, S. Caffeic Acid Inhibits UVB-Induced Inflammation and Photocarcinogenesis through Activation of Peroxisome Proliferator-Activated Receptor-γ in Mouse Skin. Photochem. Photobiol. 2015, 91, 1458–1468. [Google Scholar] [CrossRef]
- Yamacita-Borin, F.Y.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Fattori, V.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M.; Casagrande, R.; Verri, W.A. Superoxide Anion-Induced Pain and Inflammation Depends on TNFα/TNFR1 Signaling in Mice. Neurosci. Lett. 2015, 605, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Bhardwaj, R.; Aragane, Y.; Mahnke, K.; Riemann, H.; Metze, D.; Luger, T.A.; Schwarz, T. Ultraviolet-B-Induced Apoptosis of Keratinocytes: Evidence for Partial Involvement of Tumor Necrosis Factor-α in the Formation of Sunburn Cells. J. Investig. Dermatol. 1995, 104, 922–927. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-Inflammatory Cytokines Increase Reactive Oxygen Species through Mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp. Eye Res. 2007, 85, 462. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Verri, W.A.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive Role of Cytokines and Chemokines: Targets for Analgesic Drug Development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti- and Pro-Inflammatory Roles of TGF-β, IL-10, and IL-22 In Immunity and Autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447. [Google Scholar] [CrossRef]
- Ansar, W.; Ghosh, S. Inflammation and Inflammatory Diseases, Markers, and Mediators: Role of CRP in Some Inflammatory Diseases; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9788132226802. [Google Scholar]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-Derived Resolvins, Protectins, and Maresins in Airway Inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Sasaki, T.; Ueda, T.; Arita, M. Resolvins as Regulators of the Immune System. Sci. World J. 2010, 10, 818–831. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Martinez, R.M.; Fattori, V.; Saito, P.; Melo, C.B.P.; Borghi, S.M.; Pinto, I.C.; Bussmann, A.J.C.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; et al. Lipoxin A4 Inhibits UV Radiation-Induced Skin Inflammation and Oxidative Stress in Mice. J. Dermatol. Sci. 2018, 91, 164–174. [Google Scholar] [CrossRef]
- Saito, P.; Melo, C.P.B.; Martinez, R.M.; Fattori, V.; Cezar, T.L.C.; Pinto, I.C.; Bussmann, A.J.C.; Vignoli, J.A.; Georgetti, S.R.; Baracat, M.M.; et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Cezar, T.L.C.; Martinez, R.M.; da Rocha, C.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with Maresin 1, a Docosahexaenoic Acid-Derived pro-Resolution Lipid, Protects Skin from Inflammation and Oxidative Stress Caused by UVB Irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef]
- Melo, C.P.B.; Saito, P.; Martinez, R.M.; Staurengo-Ferrari, L.; Pinto, I.C.; Rodrigues, C.C.A.; Badaro-Garcia, S.; Vignoli, J.A.; Baracat, M.M.; Bussmann, A.J.C.; et al. Aspirin-Triggered Resolvin D1 (AT-RvD1) Protects Mouse Skin against UVB-Induced Inflammation and Oxidative Stress. Molecules 2023, 28, 2417. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, C.M.; Martinez, R.M.; Colombo, B.B.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Vale, D.L.; Matos, R.L.N.; Bracarense, A.P.F.R.L.; Baracat, M.M.; et al. Topical Administration of 15-Deoxy-Δ12,14-Prostaglandin J2 Using a Nonionic Cream: Effect on UVB-Induced Skin Oxidative, Inflammatory, and Histopathological Modifications in Mice. Mediat. Inflamm. 2021, 2021, 9330596. [Google Scholar] [CrossRef]
- Chun, H.W.; Lee, J.; Pham, T.H.; Lee, J.; Yoon, J.H.; Lee, J.; Oh, D.K.; Oh, J.; Yoon, D.Y. Resolvin D5, a Lipid Mediator, Inhibits Production of Interleukin-6 and CCL5 Via the ERK-NF-ΚB Signaling Pathway in Lipopolysaccharide-Stimulated THP-1 Cells. J. Microbiol. Biotechnol. 2020, 30, 85–92. [Google Scholar] [CrossRef]
- Rasquel-Oliveira, F.S.; da Silva, M.D.V.; Martelossi-Cebinelli, G.; Fattori, V.; Casagrande, R.; Verri, W.A. Specialized Pro-Resolving Lipid Mediators: Endogenous Roles and Pharmacological Activities in Infections. Molecules 2023, 28, 5032. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection Regulates Pro-Resolving Mediators That Lower Antibiotic Requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Flak, M.B.; Koenis, D.S.; Sobrino, A.; Smith, J.; Pistorius, K.; Palmas, F.; Dalli, J. GPR101 Mediates the Pro-Resolving Actions of RvD5n-3 DPA in Arthritis and Infections. J. Clin. Investig. 2020, 130, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Flak, M.B.; Koenis, D.S.; Gonzalez-Nunez, M.; Chopo-Pizarro, A.; Dalli, J. Deletion of Macrophage Gpr101 Disrupts Their Phenotype and Function Dysregulating Host Immune Responses in Sterile and Infectious Inflammation. Biochem. Pharmacol. 2023, 207, 115348. [Google Scholar] [CrossRef] [PubMed]
- Leão, F.F.; Waltrick, A.P.F.; Verri, W.A.; da Cunha, J.M.; Zanoveli, J.M. Resolvin D5 Disrupts Anxious- and Depressive-like Behaviors in a Type 1 Diabetes Mellitus Animal Model. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Henkels, K.M.; Shah, K.; De La Rosa, X.; Libreros, S.; Cheemarla, N.R.; Serhan, C.N.; Gomez-Cambronero, J. D-Series Resolvins Activate Phospholipase D in Phagocytes during Inflammation and Resolution. FASEB J. 2020, 34, 15888–15906. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Altman, N.H.; Khan, W.N.; Serhan, C.N. Specialized Proresolving Mediators Citrobacter Rodentium Infection and Promote Immunity against Reinfection. Am. Soc. Microbiol. 2018, 85, 1–10. [Google Scholar]
- Colas, R.A.; Ashton, A.W.; Mukherjee, S.; Dalli, J.; Akide-Ndunge, O.B.; Huang, H.; Desruisseaux, M.S.; Guan, F.; Jelicks, L.A.; dos Santos, F.M.; et al. Trypanosoma Cruzi Produces the Specialized Proresolving Mediators Resolvin D1, Resolvin D5, and Resolvin E2. Infect. Immun. 2018, 86, e00688-17. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Saegusa, J.; Sendo, S.; Ueda, Y.; Okano, T.; Shinohara, M.; Morinobu, A. Effect of Resolvin D5 on T Cell Differentiation and Osteoclastogenesis Analyzed by Lipid Mediator Profiling in the Experimental Arthritis. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Su, Y.; Han, Y.; Choi, H.S.; Lee, G.Y.; Cho, H.W.; Choi, H.; Jang, Y.S.; Choi, J.H.; Seo, J.W. Lipid Mediators Derived from DHA Alleviate DNCB-Induced Atopic Dermatitis and Improve the Gut Microbiome in BALB/c Mice. Int. Immunopharmacol. 2023, 124, 110900. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Gu, Y.; Tao, X.; Serhan, C.N.; Ji, R.R. Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Front. Pharmacol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Baggio, D.F.; da Luz, F.M.R.; Lopes, R.V.; Ferreira, L.E.N.; Araya, E.I.; Chichorro, J.G. Sex Dimorphism in Resolvin D5-Induced Analgesia in Rat Models of Trigeminal Pain. J. Pain. 2023, 24, 717–729. [Google Scholar] [CrossRef]
- Cardoso, R.D.R.; Chambo, S.D.; Zaninelli, T.H.; Bianchini, B.H.S.; da Silva, M.D.V.; Bertozzi, M.M.; Saraiva-Santos, T.; Franciosi, A.; Martelossi-Cebinelli, G.; Garcia-Miguel, P.E.; et al. Resolvin D5 (RvD5) Reduces Renal Damage Caused by LPS Endotoxemia in Female Mice. Molecules 2022, 28, 121. [Google Scholar] [CrossRef]
- Prasad, R.; Katiyar, S.K. Crosstalk Among UV-Induced Inflammatory Mediators, DNA Damage and Epigenetic Regulators Facilitates Suppression of the Immune System. Photochem. Photobiol. 2017, 93, 930. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 996, pp. 15–23. [Google Scholar]
- Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Staurengo-Ferrari, L.; Fattori, V.; Amaral, F.A.; Teixeira, M.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; et al. Hesperidin Methylchalcone Suppresses Experimental Gout Arthritis in Mice by Inhibiting NF-ΚB Activation. J. Agric. Food Chem. 2018, 66, 6269–6280. [Google Scholar] [CrossRef]
- Ivan, A.L.M.; Campanini, M.Z.; Martinez, R.M.; Ferreira, V.S.; Steffen, V.S.; Vicentini, F.T.M.C.; Vilela, F.M.P.; Martins, F.S.; Zarpelon, A.C.; Cunha, T.M.; et al. Pyrrolidine Dithiocarbamate Inhibits UVB-Induced Skin Inflammation and Oxidative Stress in Hairless Mice and Exhibits Antioxidant Activity in Vitro. J. Photochem. Photobiol. B 2014, 138, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Vale, D.L.; Steffen, V.S.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Trans-Chalcone Added in Topical Formulation Inhibits Skin Inflammation and Oxidative Stress in a Model of Ultraviolet B Radiation Skin Damage in Hairless Mice. J. Photochem. Photobiol. B 2017, 171, 139–146. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Hesperidin Methyl Chalcone Inhibits Oxidative Stress and Inflammation in a Mouse Model of Ultraviolet B Irradiation-Induced Skin Damage. J. Photochem. Photobiol. B 2015, 148, 145–153. [Google Scholar] [CrossRef]
- Campanini, M.Z.; Pinho-Ribeiro, F.A.; Ivan, A.L.M.; Ferreira, V.S.; Vilela, F.M.P.; Vicentini, F.T.M.C.; Martineza, R.M.; Zarpelon, A.C.; Fonseca, M.J.V.; Faria, T.J.; et al. Efficacy of Topical Formulations Containing Pimenta Pseudocaryophyllus Extract against UVB-Induced Oxidative Stress and Inflammation in Hairless Mice. J. Photochem. Photobiol. B 2013, 127, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Modun, D.; Music, I.; Boban, M. Gender Differences in Antioxidant Capacity of Rat Tissues Determined by 2,2???-Azinobis (3-Ethylbenzothiazoline 6-Sulfonate; ABTS) and Ferric Reducing Antioxidant Power (FRAP) Assays. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 47–52. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sabitha, K.E.; Shyamaladevi, C.S. Attenuation of 4-Nitroquinoline 1-Oxide Induced in Vitro Lipid Peroxidation by Green Tea Polyphenols. Life Sci. 2007, 80, 1080–1086. [Google Scholar] [CrossRef]
- Yasui, H.; Hakozaki, T.; Date, A.; Yoshii, T.; Sakurai, H. Real-Time Chemiluminescent Imaging and Detection of Reactive Oxygen Species Generated in the UVB-Exposed Human Skin Equivalent Model. Biochem. Biophys. Res. Commun. 2006, 347, 83–88. [Google Scholar] [CrossRef]
- Gonzalez Flecha, B.; Llesuy, S.; Boveris, A. Hydroperoxide-Initiated Chemiluminescence: An Assay for Oxidative Stress in Biopsies of Heart, Liver, and Muscle. Free Radic. Biol. Med. 1991, 10, 93–100. [Google Scholar] [CrossRef]
- Verri, W.A.; Guerrero, A.T.G.; Fukada, S.Y.; Valerio, D.A.; Cunha, T.M.; Xu, D.; Ferreira, S.H.; Liew, F.Y.; Cunha, F.Q. IL-33 Mediates Antigen-Induced Cutaneous and Articular Hypernociception in Mice. Proc. Natl. Acad. Sci. USA 2008, 105, 2723–2728. [Google Scholar] [CrossRef]
- Deng, Y.; Ediriwickrema, A.; Yang, F.; Lewis, J.; Girardi, M.; Saltzman, W.M. A Sunblock Based on Bioadhesive Nanoparticles. Nat. Mater. 2015, 14, 1278–1285. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxid. Med. Cell Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The Wanderings of a Free Radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M. Inflammation, Gene Mutation and Photoimmunosuppression in Response to UVR-Induced Oxidative Damage Contributes to Photocarcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 571, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Lingnert, H.; Vallentin, K.; Eriksson, C.E. Measurement of Antioxidative Effect in Model System. J. Food Process Preserv. 1979, 3, 87–103. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, M.S.; Zaninelli, T.H.; Staurengo-Ferrari, L.; Manchope, M.F.; Badaro-Garcia, S.; de Freitas, A.; Casagrande, R.; Verri, W.A. Nrf2 in Immune Responses During Inflammation. Progress. Inflamm. Res. 2020, 85, 23–49. [Google Scholar] [CrossRef]
- Robinson, J.M.; Ohira, T.; Badwey, J.A. Regulation of the NADPH-Oxidase Complex of Phagocytic Leukocytes. Recent Insights from Structural Biology, Molecular Genetics, and Microscopy. Histochem. Cell Biol. 2004, 122, 293–304. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, Functions and Pathophysiological Aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The Role of the TGF-β Family in Wound Healing, Burns and Scarring: A Review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar] [PubMed]
- Hart, P.H.; Grimbaldeston, M.A.; Swift, G.J.; Jaksic, A.; Noonan, F.P.; Finlay-Jones, J.J. Dermal Mast Cells Determine Susceptibility to Ultraviolet B-Induced Systemic Suppression of Contact Hypersensitivity Responses in Mice. J. Exp. Med. 1998, 187, 2045–2053. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Fortino, V.; Maioli, E.; Torricelli, C.; Davis, P.; Valacchi, G. Cutaneous MMPs Are Differently Modulated by Environmental Stressors in Old and Young Mice. Toxicol. Lett. 2007, 173, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.; Tolvanen, K.; Virolainen, S.; Kuivanen, T.; Kyllönen, L.; Saarialho-Kere, U. Differential Expression of Stromal MMP-1, MMP-9 and TIMP-1 in Basal Cell Carcinomas of Immunosuppressed Patients and Controls. Virchows Arch. 2008, 452, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Song, J.H.; Youn, U.J.; Hyun, J.W.; Jeong, W.S.; Lee, M.Y.; Choi, H.J.; Lee, H.K.; Chae, S. Inhibition of UVB-Induced Wrinkle Formation and MMP-9 Expression by Mangiferin Isolated from Anemarrhena Asphodeloides. Eur. J. Pharmacol. 2012, 689, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Kobayashi, T.; Takemoto, Y.; Sasaki, I.; Shinkai, H. Induction of Matrix Metalloproteinase-9 Secretion from Human Keratinocytes in Culture by Ultraviolet B Irradiation. J. Dermatol. Sci. 2003, 33, 105–111. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix Metalloproteinase-1 Is the Major Collagenolytic Enzyme Responsible for Collagen Damage in UV-Irradiated Human Skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Grady, A.O.; Dunne, C.; Kelly, P.O.; Murphy, G.M.; Leader, M.; Kay, E. Differential Expression of Matrix Metalloproteinase (MMP)-2, MMP-9 and Tissue Inhibitor of Metalloproteinase (TIMP)-1 and TIMP-2 in Non-Melanoma Skin Cancer: Implications for Tumour Progression. Histopathology 2007, 51, 793–804. [Google Scholar] [CrossRef]
- Song, J.H.; Piao, M.J.; Han, X.; Ah Kang, K.; Kang, H.K.; Yoon, W.J.; Ko, M.H.; Lee, N.H.; Lee, M.Y.; Chae, S.; et al. T BA: A Review of Skin Ageing and Its Medical Therapy. Mol. Med. Rep. 2016, 14, 2937–2944. [Google Scholar] [CrossRef]
- Scott, T.L.; Christian, P.A.; Kesler, M.V.; Donohue, K.M.; Shelton, B.; Wakamatsu, K.; Shosuke, I.; D’Orazio, J. Pigment-Independent CAMP-Mediated Epidermal Thickening Protects against Cutaneous UV Injury by Keratinocyte Proliferation. Exp. Dermatol. 2012, 21, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Flori, E.; Briganti, S.; Camera, E.; Cario-André, M.; Taïeb, A.; Picardo, M. UVA-Induced Modification of Catalase Charge Properties in the Epidermis Is Correlated with the Skin Phototype. J. Investig. Dermatol. 2006, 126, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid Oxidation in the Skin. Free. Radic. Res. 2014, 49, 827–834. [Google Scholar] [CrossRef]
- Ambrogini, P.; Minelli, A.; Galati, C.; Betti, M.; Lattanzi, D.; Ciffolilli, S.; Piroddi, M.; Galli, F.; Cuppini, R. Post-Seizure α-Tocopherol Treatment Decreases Neuroinflammation and Neuronal Degeneration Induced by Status Epilepticus in Rat Hippocampus. Mol. Neurobiol. 2014, 50, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Luu, Q.; Tra, Q.; Thi, C.; Hae-, H.K.; Park, S.; Seob, Y. Administration of Vitamin E Attenuates Airway Inflammation through Restoration of Nrf2 in a Mouse Model of Asthma. J. Cell Mol. Med. 2021, 25, 6721–6732. [Google Scholar] [CrossRef]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-ΚB Regulates Phagocytic NADPH Oxidase by Inducing the Expression of Gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive Oxygen Species, Cellular Redox Systems, and Apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Meziani, L.; Deutsch, E.; Mondini, M. Macrophages in Radiation Injury: A New Therapeutic Target. Oncoimmunology 2018, 7, e1494488. [Google Scholar] [CrossRef]
- Werz, O.; Gerstmeier, J.; Libreros, S.; De La Rosa, X.; Werner, M.; Norris, P.C.; Chiang, N.; Serhan, C.N. Human Macrophages Differentially Produce Specific Resolvin or Leukotriene Signals That Depend on Bacterial Pathogenicity. Nat. Commun. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Fattori, V.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Melo, C.P.B.; Bussmann, A.J.C.; Staurengo-Ferrari, L.; Bezerra, J.R.; Vignoli, J.A.; et al. The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin against Ultraviolet B Radiation. Molecules 2020, 25, 2953. [Google Scholar] [CrossRef] [PubMed]
- Baram, D.; Vaday, G.G.; Salamon, P.; Drucker, I.; Hershkoviz, R.; Mekori, Y.A. Human Mast Cells Release Metalloproteinase-9 on Contact with Activated T Cells: Juxtacrine Regulation by TNF-Alpha. J. Immunol. 2001, 167, 4008–4016. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Zaninelli, T.H.; Rasquel-Oliveira, F.S.; Casagrande, R.; Verri, W.A. Specialized Pro-Resolving Lipid Mediators: A New Class of Non-Immunosuppressive and Non-Opioid Analgesic Drugs. Pharmacol. Res. 2020, 151, 104549. [Google Scholar] [CrossRef]
- Ji, R.R. Specialized Pro-Resolving Mediators as Resolution Pharmacology for the Control of Pain and Itch. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 273–293. [Google Scholar] [CrossRef]
- Boff, D.; Oliveira, V.L.S.; Queiroz Junior, C.M.; Galvão, I.; Batista, N.V.; Gouwy, M.; Menezes, G.B.; Cunha, T.M.; Verri Junior, W.A.; Proost, P.; et al. Lipoxin A4 Impairs Effective Bacterial Control and Potentiates Joint Inflammation and Damage Caused by Staphylococcus Aureus Infection. FASEB J. 2020, 34, 11498–11510. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; de Matos, R.L.N.; Vale, D.L.; Melo, C.P.B.; Fattori, V.; Saraiva-Santos, T.; Mendes-Pierotti, S.; Bertozzi, M.M.; et al. Resolvin D5 Protects Female Hairless Mouse Skin from Pathological Alterations Caused by UVB Irradiation. Antioxidants 2024, 13, 1008. https://doi.org/10.3390/antiox13081008
Saito P, Pinto IC, Rodrigues CCA, de Matos RLN, Vale DL, Melo CPB, Fattori V, Saraiva-Santos T, Mendes-Pierotti S, Bertozzi MM, et al. Resolvin D5 Protects Female Hairless Mouse Skin from Pathological Alterations Caused by UVB Irradiation. Antioxidants. 2024; 13(8):1008. https://doi.org/10.3390/antiox13081008
Chicago/Turabian StyleSaito, Priscila, Ingrid C. Pinto, Camilla C. A. Rodrigues, Ricardo L. N. de Matos, David L. Vale, Cristina P. B. Melo, Victor Fattori, Telma Saraiva-Santos, Soraia Mendes-Pierotti, Mariana M. Bertozzi, and et al. 2024. "Resolvin D5 Protects Female Hairless Mouse Skin from Pathological Alterations Caused by UVB Irradiation" Antioxidants 13, no. 8: 1008. https://doi.org/10.3390/antiox13081008





