(Chemical) Roles of HOCl in Rheumatic Diseases
Abstract
:1. Introduction
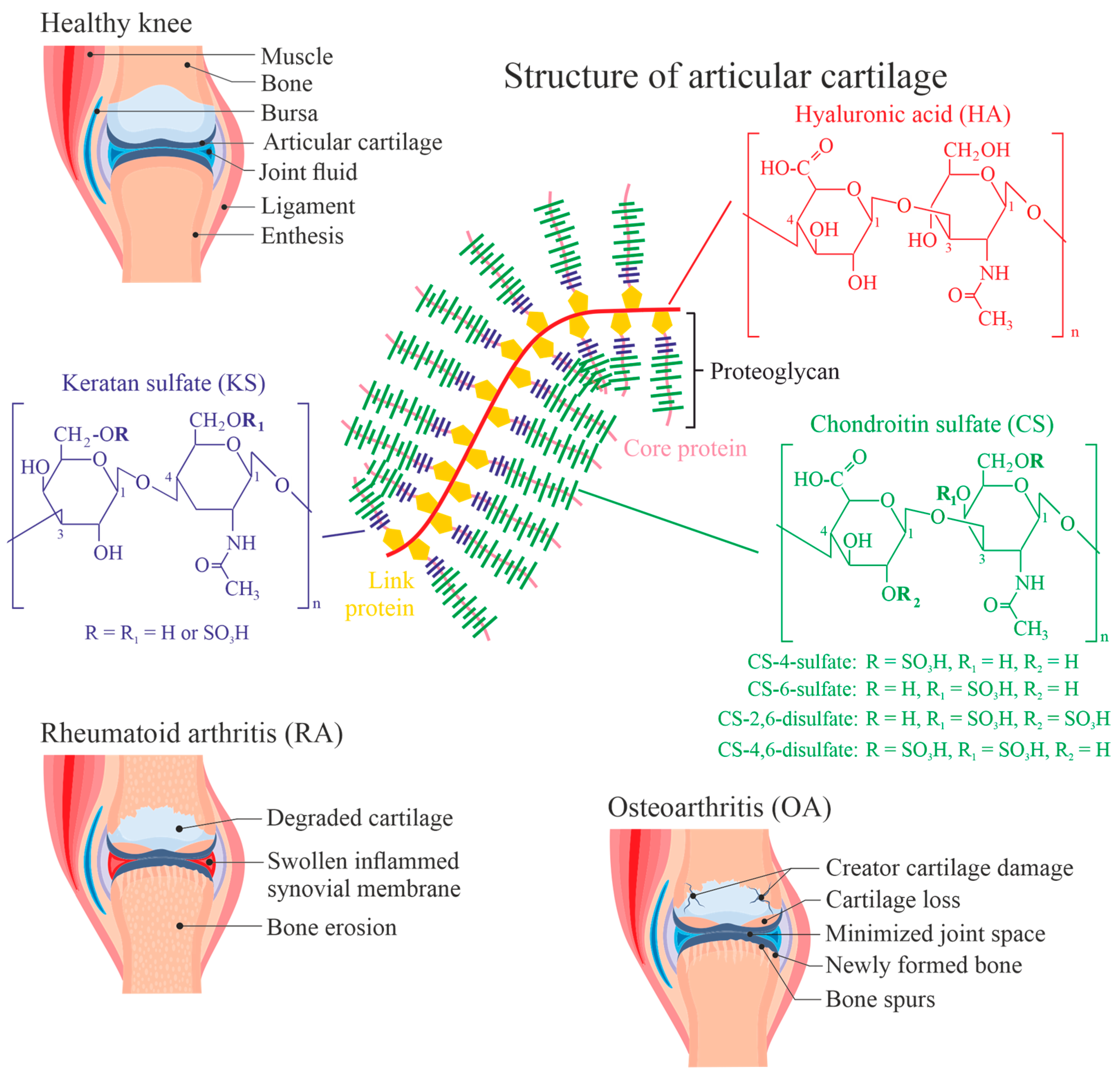
2. Composition of Articular Cartilage and Synovial Fluid
2.1. Collagen
2.2. Proteoglycans
3. Mechanisms of Cartilage Degradation
- Chondrocytes (in the cartilage) or synoviocytes (at the interior of the articular capsule) are negatively affected by products of typical inflammatory cells such as neutrophilic granulocytes (vide infra). This leads to a reduced overall ECM synthesis and the increased degradation of the ECM [34]. Additionally, the “quality” of the de novo generated ECM may be poor (fibrocartilage instead of hyaline cartilage).
- Inflammatory cells such as neutrophilic granulocytes, macrophages or T cells release harmful enzymes that can degrade the ECM. Matrixmetalloproteinases (collagenase, for instance) are often assumed to be particularly responsible for that degradation processes [35], especially in combination with ROS. This opinion is emphasized by the observation that the number of granulocytes is augmented in the blood from RA patients [36] and that polymorphonuclear granulocytes (abbreviated as both PMNs or neutrophils) can be found in large numbers in the pannus tissue, a replacement tissue as a consequence of ECM degradation, of cartilage [37,38].
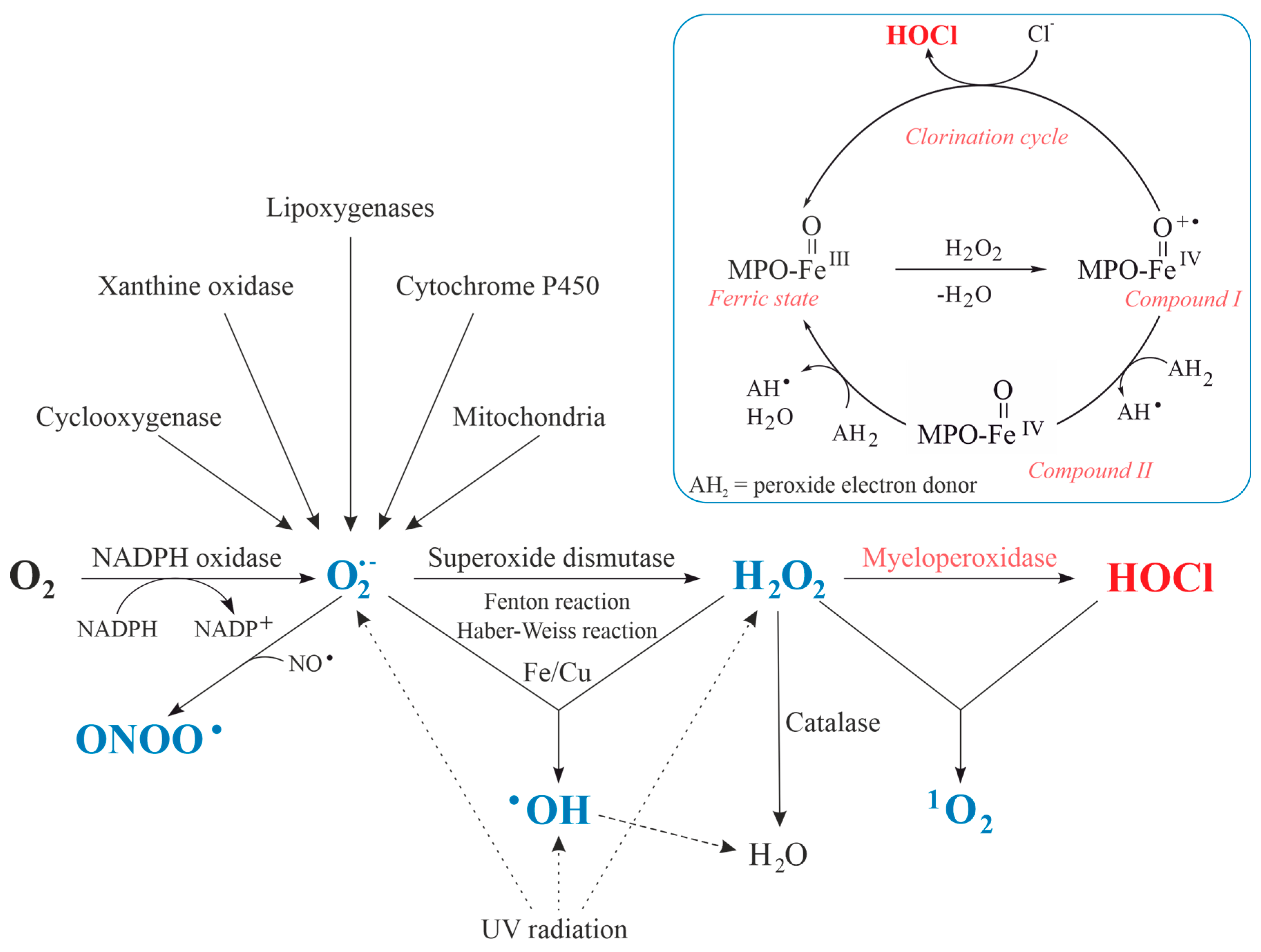
4. Reactive Oxygen/Nitrogen/Chlorine Species
5. Release of Enzymes into the Synovial Fluid—A Short Survey
6. Function and Modifications Induced by MPO
7. Reactions between HOCl and Cartilage Components
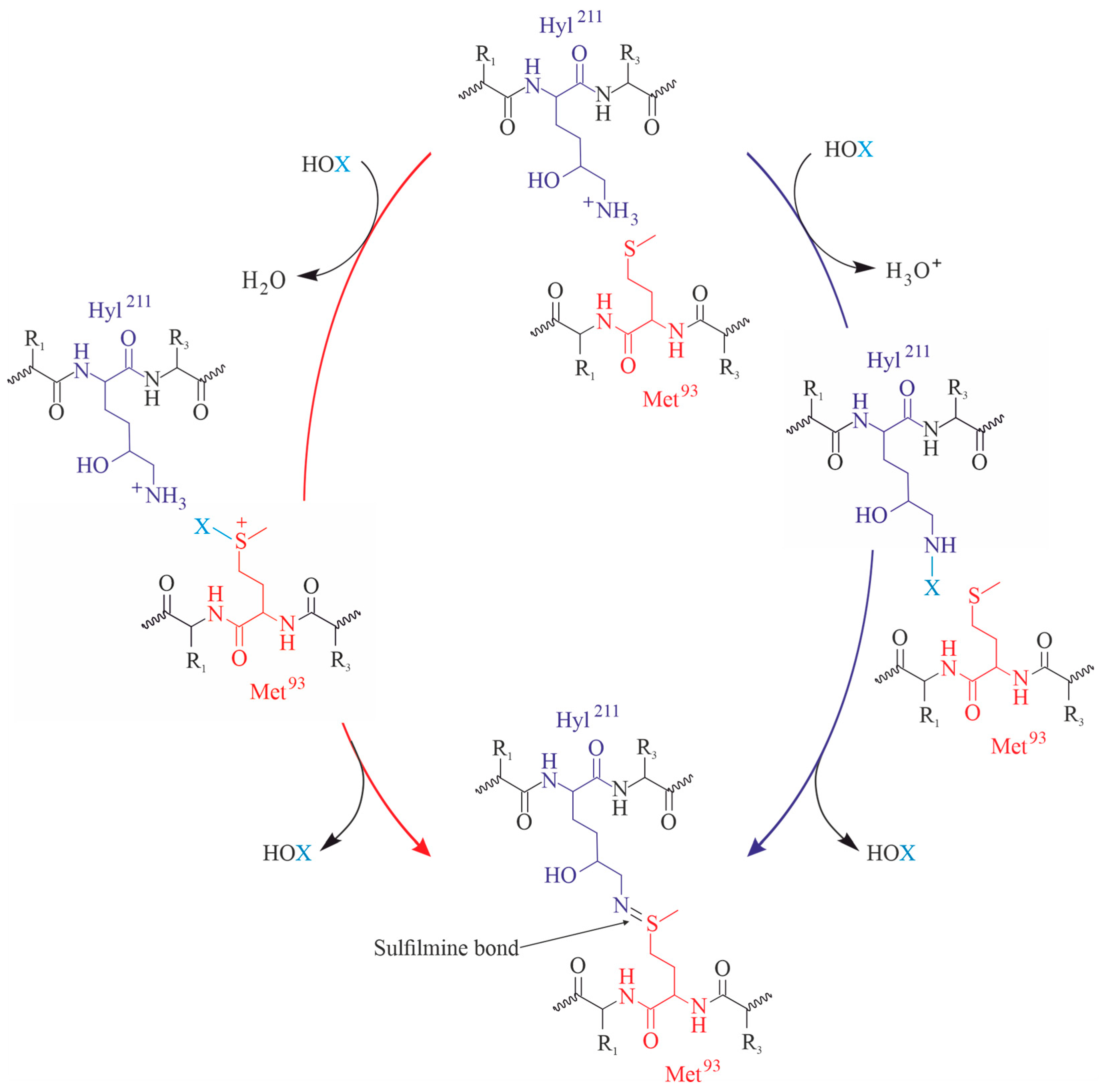
7.1. Collagen
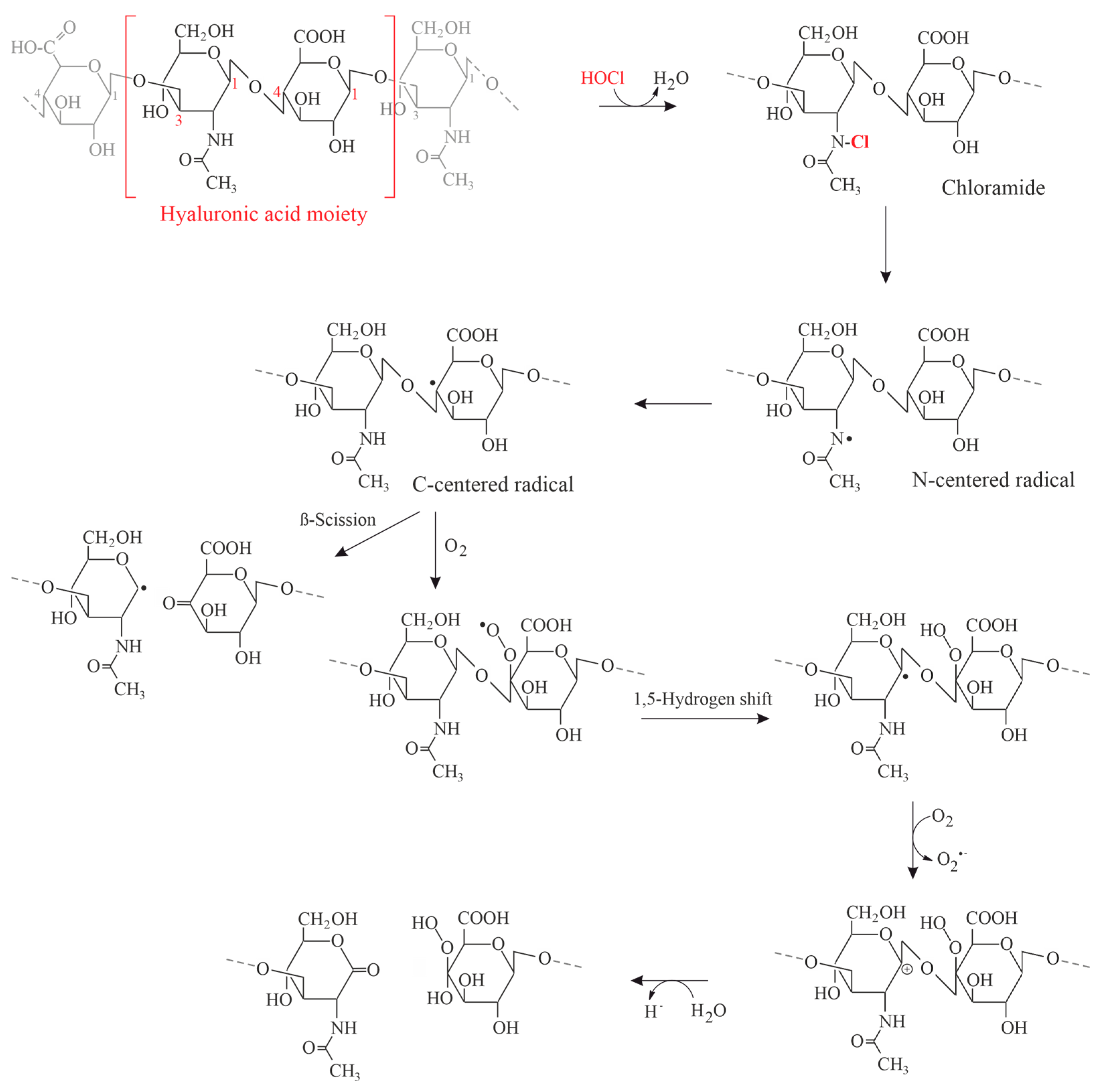
7.2. GAGs
7.3. DNA
7.4. Cellular Lipids
8. Antioxidants to Suppress HOCl-Induced Effects
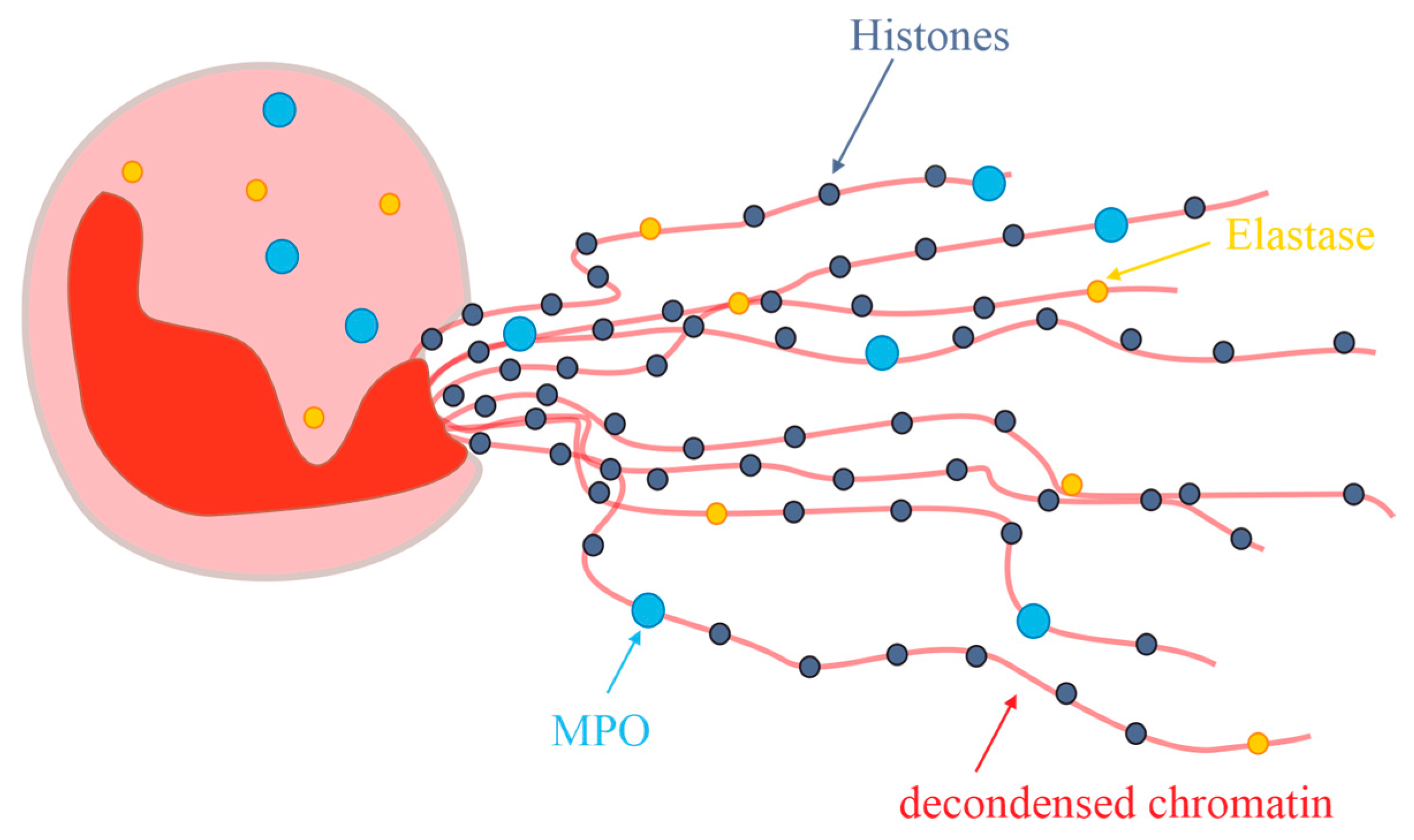
9. Neutrophil Extracellular Traps (NETs)
10. Conclusions
- The number of neutrophils is massively enhanced in the SF from patients suffering from RA [187].
- Since MPO is a very abundant protein within the neutrophils (it constitutes about 5% of all proteins), the contribution of this enzyme and its product, HOCl, to cartilage degradation during RA is obvious.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mela, G.S.; Cimmino, M.A. An overview of rheumatological research in the European Union. Ann. Rheum. Dis. 1998, 57, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Pincus, T.; Castrejon, I.; Yazici, Y.; Gibson, K.A.; Bergman, M.J.; Block, J.A. Osteoarthritis is as severe as rheumatoid arthritis: Evidence over 40 years according to the same measure in each disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 7–17. [Google Scholar] [PubMed]
- Lawrence, R.C.; Helmick, C.G.; Arnett, F.C.; Deyo, R.A.; Felson, D.T.; Giannini, E.H.; Heyse, S.P.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998, 41, 778–799. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Elkouzi, A.; Bit-Ivan, E.N.; Elble, R.J. Pure akinesia with gait freezing: A clinicopathologic study. J. Clin. Mov. Disord. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Flugge, L.A.; Miller-Deist, L.A.; Petillo, P.A. Towards a molecular understanding of arthritis. Chem. Biol. 1999, 6, R157–R166. [Google Scholar] [CrossRef]
- Thoms, B.L.; Bonnell, L.N.; Tompkins, B.; Nevares, A.; Lau, C. Predictors of inflammatory arthritis among new rheumatology referrals: A cross-sectional study. Rheumatol. Adv. Pract. 2023, 7, rkad067. [Google Scholar] [CrossRef]
- Schiller, J.; Fuchs, B.; Arnhold, J.; Arnold, K. Contribution of reactive oxygen species to cartilage degradation in rheumatic diseases: Molecular pathways, diagnosis and potential therapeutic strategies. Curr. Med. Chem. 2003, 10, 2123–2145. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Fuchs, B.; Arnold, K. The Molecular Organization of Polymers of Cartilage in Health and Disease. Curr. Org. Chem. 2006, 10, 1771–1789. [Google Scholar] [CrossRef]
- Rich, M.J.; Burnash, S.; Krishnan, R.R.; Chubinskaya, S.; Loeser, R.F.; Polacheck, W.J.; Diekman, B.O. Use of a novel magnetically actuated compression system to study the temporal dynamics of axial and lateral strain in human osteochondral plugs. J. Biomech. 2024, 162, 111887. [Google Scholar] [CrossRef] [PubMed]
- Bursac, P.; Arnoczky, S.; York, A. Dynamic compressive behavior of human meniscus correlates with its extra-cellular matrix composition. Biorheology 2009, 46, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, R.A. The cell density of human articular and costal cartilage. J. Anat. 1967, 101, 753–763. [Google Scholar]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Krane, S.M. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 2008, 35, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Li, Y.; Pan, J.; Liu, F.; Dai, H.; Fu, Y.; Huang, T.; Farooq, S.; Zhang, H. Collagen and gelatin: Structure, properties, and applications in food industry. Int. J. Biol. Macromol. 2024, 254, 128037. [Google Scholar] [CrossRef]
- Nimni, M.E. Collagen: Structure, function, and metabolism in normal and fibrotic tissues. Semin. Arthritis Rheum. 1983, 13, 1–86. [Google Scholar] [CrossRef] [PubMed]
- Knauss, R.; Schiller, J.; Fleischer, G.; Kärger, J.; Arnold, K. Self-diffusion of water in cartilage and cartilage components as studied by pulsed field gradient NMR. Magn. Reson. Med. 1999, 41, 285–292. [Google Scholar] [CrossRef]
- Rousseau, J.-C.; Chapurlat, R.; Garnero, P. Soluble biological markers in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211040300. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J. The structure and function of cartilage proteoglycans. Eur. Cells Mater. 2006, 12, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Hyc, A.; Osiecka-Iwan, A.; Jóźwiak, J.; Moskalewski, S. The morphology and selected biological properties of articular cartilage. Ortop. Traumatol. Rehabil. 2001, 3, 151–162. [Google Scholar] [PubMed]
- Kim, Y.S.; Guilak, F. Engineering Hyaluronic Acid for the Development of New Treatment Strategies for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 8662. [Google Scholar] [CrossRef] [PubMed]
- Torchia, D.A.; Hasson, M.A.; Hascall, V.C. Investigation of molecular motion of proteoglycans in cartilage by 13C magnetic resonance. J. Biol. Chem. 1977, 252, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Belcher, C.; Yaqub, R.; Fawthrop, F.; Bayliss, M.; Doherty, M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann. Rheum. Dis. 1997, 56, 299–307. [Google Scholar] [CrossRef]
- Gama, C.I.; Hsieh-Wilson, L.C. Chemical approaches to deciphering the glycosaminoglycan code. Curr. Opin. Chem. Biol. 2005, 9, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Guidolin, D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: Are the effects molecular weight dependent? Semin. Arthritis Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Seidman, A.J.; Limaiem, F. Synovial Fluid Analysis; StatPearls [Internet]: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ben-Trad, L.; Matei, C.I.; Sava, M.M.; Filali, S.; Duclos, M.-E.; Berthier, Y.; Guichardant, M.; Bernoud-Hubac, N.; Maniti, O.; Landoulsi, A.; et al. Synovial Extracellular Vesicles: Structure and Role in Synovial Fluid Tribological Performances. Int. J. Mol. Sci. 2022, 23, 11998. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Konttinen, Y.T.; Friman, C.; Sorsa, T. Differential effects of reactive oxygen species on native synovial fluid and purified human umbilical cord hyaluronate. Inflammation 1993, 17, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Arnhold, J.; Sonntag, K.; Arnold, K. NMR studies on human, pathologically changed synovial fluids: Role of hypochlorous acid. Magn. Reson. Med. 1996, 35, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Tieng, A.; Franchin, G. Knee Arthrocentesis in Adults. J. Vis. Exp. 2022, 180, e63135. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Hong, F.; Yang, S. Mitochondrial Dysfunction in Rheumatoid Arthritis. Biomolecules 2022, 12, 1216. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef] [PubMed]
- Pulik, Ł.; Łęgosz, P.; Motyl, G. Matrix metalloproteinases in rheumatoid arthritis and osteoarthritis: A state of the art review. Rheumatology 2023, 61, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 649693. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Ou, Q.; Zhou, X.; Nie, K.; Liu, J.; Zhang, S. Global research trends and focus on the link between rheumatoid arthritis and neutrophil extracellular traps: A bibliometric analysis from 1985 to 2023. Front. Immunol. 2023, 14, 1205445. [Google Scholar] [CrossRef] [PubMed]
- Mohr, W.; Westerhellweg, H.; Wessinghage, D. Polymorphonuclear granulocytes in rheumatic tissue destruction. III. an electron microscopic study of PMNs at the pannus-cartilage junction in rheumatoid arthritis. Ann. Rheum. Dis. 1981, 40, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Huang, W.; Zhang, W.; Mei, J.; Zhu, C. A Tale of Two Immune Cells in Rheumatoid Arthritis: The Crosstalk between Macrophages and T Cells in the Synovium. Front. Immunol. 2021, 12, 655477. [Google Scholar] [CrossRef]
- Chaney, S.; Vergara, R.; Qiryaqoz, Z.; Suggs, K.; Akkouch, A. The Involvement of Neutrophils in the Pathophysiology and Treatment of Osteoarthritis. Biomedicines 2022, 10, 1604. [Google Scholar] [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Rincón, E.; Rocha-Gregg, B.L.; Collins, S.R. A map of gene expression in neutrophil-like cell lines. BMC Genomics 2018, 19, 573. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.; Robinson, J.J.; Edwards, S.W. Neutrophil function in whole blood and after purification: Changes in receptor expression, oxidase activity and responsiveness to cytokines. Biosci. Rep. 1992, 12, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, M.P.; Laxer, R.M. NETosis in Rheumatic Diseases. Curr. Rheumatol. Rep. 2021, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Liu, C.; Su, C.; Liu, L.; Chen, P.; Li, X.; Zhang, X.; Yuan, B.; Wang, H.; Du, X. Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs. Front. Immunol. 2023, 14, 1107670. [Google Scholar] [CrossRef]
- Loh, J.T.; Lam, K.-P. Neutrophils in the Pathogenesis of Rheumatic Diseases. Rheumatol. Immunol. Res. 2022, 3, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Arve-Butler, S.; Schmidt, T.; Mossberg, A.; Berthold, E.; Gullstrand, B.; Bengtsson, A.A.; Kahn, F.; Kahn, R. Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res. Ther. 2021, 23, 109. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Lyon, M.; Chapman, E.A.; Moots, R.J.; Edwards, S.W. Rheumatoid Arthritis Synovial Fluid Neutrophils Drive Inflammation Through Production of Chemokines, Reactive Oxygen Species, and Neutrophil Extracellular Traps. Front. Immunol. 2020, 11, 584116. [Google Scholar] [CrossRef] [PubMed]
- MacWilliams, P.S.; Friedrichs, K.R. Laboratory evaluation and interpretation of synovial fluid. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 153–178. [Google Scholar] [CrossRef] [PubMed]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.-C.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Lanza, V.; Greco, V.; Bocchieri, E.; Sciuto, S.; Inturri, R.; Messina, L.; Vaccaro, S.; Bellia, F.; Rizzarelli, E. Synergistic Effect of L-Carnosine and Hyaluronic Acid in Their Covalent Conjugates on the Antioxidant Abilities and the Mutual Defense against Enzymatic Degradation. Antioxidants 2022, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef]
- Mohan, I.K.; Baba, K.S.S.S.; Iyyapu, R.; Thirumalasetty, S.; Satish, O.S. Advances in congestive heart failure biomarkers. Adv. Clin. Chem. 2023, 112, 205–248. [Google Scholar] [CrossRef] [PubMed]
- Rayner, B.S.; Zhang, Y.; Brown, B.E.; Reyes, L.; Cogger, V.C.; Hawkins, C.L. Role of hypochlorous acid (HOCl) and other inflammatory mediators in the induction of macrophage extracellular trap formation. Free Radic. Biol. Med. 2018, 129, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997, 3, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Flemmig, J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 2010, 500, 92–106. [Google Scholar] [CrossRef]
- Van Dalen, C.J.; Whitehouse, M.W.; Winterbourn, C.C.; Kettle, A.J. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997, 327, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bedouhène, S.; Dang, P.M.-C.; Hurtado-Nedelec, M.; El-Benna, J. Neutrophil Degranulation of Azurophil and Specific Granules. Methods Mol. Biol. 2020, 2087, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Heeschen, C.; Meinertz, T.; Zeiher, A.M.; Eiserich, J.P.; Münzel, T.; Simoons, M.L.; Hamm, C.W. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 2003, 108, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Malle, E. Halogenation Activity of Mammalian Heme Peroxidases. Antioxidants 2022, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Korotaeva, A.; Samoilova, E.; Pavlunina, T.; Panasenko, O.M. Halogenated phospholipids regulate secretory phospholipase A2 group IIA activity. Chem. Phys. Lipids 2013, 167–168, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Katrantzis, M.; Baker, M.S.; Handley, C.J.; Lowther, D.A. The oxidant hypochlorite (OCl−), a product of the myeloperoxidase system, degrades articular cartilage proteoglycan aggregate. Free Radic. Biol. Med. 1991, 10, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yin, C.; Huo, F. Small-Molecule Fluorescent Probes for Detecting Several Abnormally Expressed Substances in Tumors. Micromachines 2022, 13, 1328. [Google Scholar] [CrossRef] [PubMed]
- Haegens, A.; Vernooy, J.H.J.; Heeringa, P.; Mossman, B.T.; Wouters, E.F.M. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur. Respir. J. 2008, 31, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.I.; Pryatkin, S.A.; Kalinski, M.I.; Rogozkin, V.A. Effect of exercise to exhaustion on myeloperoxidase and lysozyme release from blood neutrophils. Eur. J. Appl. Physiol. 2003, 89, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hadler, N.M.; Spitznagel, J.K.; Quinet, R.J. Lysosomal Enzymes in Inflammatory Synovial Effusions. J. Immunol. 1979, 123, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [PubMed]
- Gotis-Graham, I.; Hogg, P.J.; McNeil, H.P. Significant correlation between thrombospondin 1 and serine proteinase expression in rheumatoid synovium. Arthritis Rheum. 1997, 40, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Momohara, S.; Kashiwazaki, S.; Inoue, K.; Saito, S.; Nakagawa, T. Elastase from polymorphonuclear leukocyte in articular cartilage and synovial fluids of patients with rheumatoid arthritis. Clin. Rheumatol. 1997, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Albrett, A.M.; Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leukoc. Biol. 2012, 91, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The Acid Ionization Constant of HOCl from 5 to 35°. J. Phys. Chem. 1966, 70, 3798–3805. [Google Scholar] [CrossRef]
- Wan, Z.; Zhu, C.; Francisco, J.S. Molecular Insights into the Spontaneous Generation of Cl2O in the Reaction of ClONO2 and HOCl at the Air-Water Interface. J. Am. Chem. Soc. 2023, 145, 17478–17484. [Google Scholar] [CrossRef] [PubMed]
- Sivey, J.D.; McCullough, C.E.; Roberts, A.L. Chlorine monoxide (Cl2O) and molecular chlorine (Cl2) as active chlorinating agents in reaction of dimethenamid with aqueous free chlorine. Environ. Sci. Technol. 2010, 44, 3357–3362. [Google Scholar] [CrossRef] [PubMed]
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; van der Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chuang, C.Y.; Hawkins, C.L.; Hägglund, P.; Davies, M.J. Identification and quantification of protein nitration sites in human coronary artery smooth muscle cells in the absence and presence of peroxynitrous acid/peroxynitrite. Redox Biol. 2023, 64, 102799. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim. Biophys. Acta 1985, 840, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Davies, M.J. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 2001, 14, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Hammerschmidt, S.; Arnold, K. Role of functional groups of human plasma and luminol in scavenging of NaOCl and neutrophil-derived hypochlorous acid. Biochim. Biophys. Acta 1991, 1097, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Prütz, W.A. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch. Biochem. Biophys. 1996, 332, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Armesto, X.L.; Canle, M.L.; Santaballa, J.A. α-amino acids chlorination in aqueous media. Tetrahedron 1993, 49, 275–284. [Google Scholar] [CrossRef]
- Pattison, D.I.; Hawkins, C.L.; Davies, M.J. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: Absolute rate constants, product analysis, and computational modeling. Chem. Res. Toxicol. 2003, 16, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, O.M.; Torkhovskaya, T.I.; Gorudko, I.V.; Sokolov, A.V. The Role of Halogenative Stress in Atherogenic Modification of Low-Density Lipoproteins. Biochemistry 2020, 85, S34–S55. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.; Shiue, G.G. Reaction Rates of Superoxide Radicals with the Essential Amino Acids; Excerpta Medica: Amsterdam, The Netherlands, 1978; pp. 43–56. [Google Scholar] [CrossRef]
- Burkhardt, H.; Schwingel, M.; Menninger, H.; Macartney, H.W.; Tschesche, H. Oxygen radicals as effectors of cartilage destruction. Direct degradative effect on matrix components and indirect action via activation of latent collagenase from polymorphonuclear leukocytes. Arthritis Rheum. 1986, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.; Flannelly, J.; Bowler, R.; Tran, K.; Nicks, M.; Carbone, B.D.; Glueck, D.; Heijnen, H.; Mason, R.; Crapo, J. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005, 52, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Arnhold, J.; Arnold, K. NMR studies of the action of hypochlorous acid on native pig articular cartilage. Eur. J. Biochem. 1995, 233, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Arnhold, J.; Zachäus, A.; Arnold, K. Reaction of hypochlorous acid with bovine nasal cartilage comparison to pig articular cartilage. Z. Naturforsch. C J. Biosci. 1997, 52, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Hadjigogos, K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003, 45, 7–13. [Google Scholar]
- Davies, J.M.; Horwitz, D.A.; Davies, K.J. Inhibition of collagenase activity by N-chlorotaurine, a product of activated neutrophils. Arthritis Rheum. 1994, 37, 424–427. [Google Scholar] [CrossRef]
- Stamp, L.K.; Khalilova, I.; Tarr, J.M.; Senthilmohan, R.; Turner, R.; Haigh, R.C.; Winyard, P.G.; Kettle, A.J. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology 2012, 51, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Odobasic, D.; Yang, Y.; Muljadi, R.C.M.; O’Sullivan, K.M.; Kao, W.; Smith, M.; Morand, E.F.; Holdsworth, S.R. Endogenous myeloperoxidase is a mediator of joint inflammation and damage in experimental arthritis. Arthritis Rheumatol. 2014, 66, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Garvican, E.R.; Vaughan-Thomas, A.; Innes, J.F.; Clegg, P.D. Biomarkers of cartilage turnover. Part 1: Markers of collagen degradation and synthesis. Vet. J. 2010, 185, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.; Lundberg, K.; Erlandsson Harris, H. Arthritogenicity of collagen type II is increased by chlorination. Clin. Exp. Immunol. 2006, 145, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Horwitz, D.A.; Davies, K.J. Potential roles of hypochlorous acid and N-chloroamines in collagen breakdown by phagocytic cells in synovitis. Free Radic. Biol. Med. 1993, 15, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Daumer, K.M.; Khan, A.U.; Steinbeck, M.J. Chlorination of pyridinium compounds. Possible role of hypochlorite, N-chloramines, and chlorine in the oxidation of pyridinoline cross-links of articular cartilage collagen type II during acute inflammation. J. Biol. Chem. 2000, 275, 34681–34692. [Google Scholar] [CrossRef] [PubMed]
- Olszowski, S.; Mak, P.; Olszowska, E.; Marcinkiewicz, J. Collagen type II modification by hypochlorite. Acta Biochim. Pol. 2003, 50, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Bochi, G.V.; Torbitz, V.D.; de Campos, L.P.; Sangoi, M.B.; Fernandes, N.F.; Gomes, P.; Moretto, M.B.; Barbisan, F.; Da Cruz, I.B.M.; Moresco, R.N. In Vitro Oxidation of Collagen Promotes the Formation of Advanced Oxidation Protein Products and the Activation of Human Neutrophils. Inflammation 2016, 39, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Gauld, J.W. Sulfilimine bond formation in collagen IV. Chem. Commun. 2024, 60, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Ronsein, G.E.; Winterbourn, C.C.; Di Mascio, P.; Kettle, A.J. Cross-linking methionine and amine residues with reactive halogen species. Free Radic. Biol. Med. 2014, 70, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Winyard, P.G.; Ryan, B.; Eggleton, P.; Nissim, A.; Taylor, E.; Lo Faro, M.L.; Burkholz, T.; Szabó-Taylor, K.E.; Fox, B.; Viner, N.; et al. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem. Soc. Trans. 2011, 39, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Schiller, J. Glycosaminoglycan degradation by selected reactive oxygen species. Antioxid. Redox Signal. 2014, 21, 1044–1062. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.S.; Green, S.P.; Lowther, D.A. Changes in the viscosity of hyaluronic acid after exposure to a myeloperoxidase-derived oxidant. Arthritis Rheum. 1989, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Martin-Alarcon, L.; Schmidt, T.A. Rheological effects of macromolecular interactions in synovial fluid. Biorheology 2016, 53, 49–67. [Google Scholar] [CrossRef]
- Cowman, M.K.; Matsuoka, S. Experimental approaches to hyaluronan structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Sprott, H.; Fleck, C. Hyaluronic Acid in Rheumatology. Pharmaceutics 2023, 15, 2247. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Xue, J.F.; Zheng, Z.Y.; Shuhaidi, M.; Thu, H.E.; Hussain, Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: A review of recent developments and critical appraisal of preclinical and clinical investigations. Int. J. Biol. Macromol. 2018, 116, 572–584. [Google Scholar] [CrossRef]
- Conrozier, T.; Mathieu, P.; Vignon, E.; Piperno, M.; Rinaudo, M. Differences in the osteoarthritic synovial fluid composition and rheology between patients with or without flare: A pilot study. Clin. Exp. Rheumatol. 2012, 30, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Arnhold, J.; Gründer, W.; Arnold, K. The action of hypochlorous acid on polymeric components of cartilage. Biol. Chem. Hoppe Seyler 1994, 375, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Degradation of hyaluronic acid, poly- and monosaccharides, and model compounds by hypochlorite: Evidence for radical intermediates and fragmentation. Free Radic. Biol. Med. 1998, 24, 1396–1410. [Google Scholar] [CrossRef] [PubMed]
- Grootveld, M.; Henderson, E.B.; Farrell, A.; Blake, D.R.; Parkes, H.G.; Haycock, P. Oxidative damage to hyaluronate and glucose in synovial fluid during exercise of the inflamed rheumatoid joint. Detection of abnormal low-molecular-mass metabolites by proton-n.m.r. spectroscopy. Biochem. J. 1991, 273 Pt 2, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Arnhold, J.; Schwinn, J.; Sprinz, H.; Brede, O.; Arnold, K. Reactivity of cartilage and selected carbohydrates with hydroxyl radicals: An NMR study to detect degradation products. Free Radic. Res. 1998, 28, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.D.; Hawkins, C.L.; Davies, M.J. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem. J. 2004, 381, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.; Prabutzki, P.; Nimptsch, A.; Schiller, J. Mass spectrometric investigations of the action of hypochlorous acid on monomeric and oligomeric components of glycosaminoglycans. Biochem. Biophys. Rep. 2023, 34, 101448. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kitamura, T.; Kato, M.; Otsu, H. Reusability of iron sludge as an iron source for the electrochemical Fenton-type process using Fe2+/HOCl system. Water Res. 2013, 47, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Akeel, A.; Sibanda, S.; Martin, S.W.; Paterson, A.W.J.; Parsons, B.J. Chlorination and oxidation of heparin and hyaluronan by hypochlorous acid and hypochlorite anions: Effect of sulfate groups on reaction pathways and kinetics. Free Radic. Biol. Med. 2013, 56, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Ginsburg, I. A Novel Hypothetical Approach to Explain the Mechanisms of Pathogenicity of Rheumatic Arthritis. Mediterr. J. Rheumatol. 2021, 32, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, M.M.; Pietraforte, D.; Di Lorenzo, A.S.; Minetti, M.; Marino, G. Reaction of peroxynitrite with hyaluronan and related saccharides. Free Radic. Res. 2004, 38, 343–353. [Google Scholar] [CrossRef]
- Lemmnitzer, K.; Köhling, S.; Freyse, J.; Rademann, J.; Schiller, J. Characterization of defined sulfated heparin-like oligosaccharides by electrospray ionization ion trap mass spectrometry. J. Mass Spectrom. 2021, 56, e4692. [Google Scholar] [CrossRef] [PubMed]
- Grabarics, M.; Lettow, M.; Kirschbaum, C.; Greis, K.; Manz, C.; Pagel, K. Mass Spectrometry-Based Techniques to Elucidate the Sugar Code. Chem. Rev. 2022, 122, 7840–7908. [Google Scholar] [CrossRef]
- Buffa, R.; Hermannová, M.; Sojka, M.; Svozil, V.; Šulc, P.; Halamková, P.; Pospíšilová, M.; Krejčí, H.; Velebný, V. Hyaluronic acid chloramide-Synthesis, chemical structure, stability and analysis of antimicrobials. Carbohydr. Polym. 2020, 250, 116928. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Szabó, M.; Fábián, I. The chlorination of glycine and α-alanine at excess HOCl: Kinetics and mechanism. J. Hazard. Mater. 2023, 447, 130794. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, M.J.; Nesti, L.J.; Sharkey, P.F.; Parvizi, J. Myeloperoxidase and chlorinated peptides in osteoarthritis: Potential biomarkers of the disease. J. Orthop. Res. 2007, 25, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Chokesuwattanaskul, S.; Phelan, M.M.; Welting, T.J.M.; Lian, L.-Y.; Peffers, M.J.; Wright, H.L. 1H NMR Metabolomics Identifies Underlying Inflammatory Pathology in Osteoarthritis and Rheumatoid Arthritis Synovial Joints. J. Proteome Res. 2018, 17, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Role of myeloperoxidase and oxidant formation in the extracellular environment in inflammation-induced tissue damage. Free Radic. Biol. Med. 2021, 172, 633–651. [Google Scholar] [CrossRef]
- Flemmig, J.; Spalteholz, H.; Schubert, K.; Meier, S.; Arnhold, J. Modification of phosphatidylserine by hypochlorous acid. Chem. Phys. Lipids 2009, 161, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; La Pérez de Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Karaman, A.; Binici, D.N.; Melikoğlu, M.A. Comet assay and analysis of micronucleus formation in patients with rheumatoid arthritis. Mutat. Res. 2011, 721, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Galita, G.; Sarnik, J.; Brzezinska, O.; Budlewski, T.; Dragan, G.; Poplawska, M.; Majsterek, I.; Poplawski, T.; Makowska, J.S. Polymorphisms in DNA Repair Genes and Association with Rheumatoid Arthritis in a Pilot Study on a Central European Population. Int. J. Mol. Sci. 2023, 24, 3804. [Google Scholar] [CrossRef] [PubMed]
- Galita, G.; Sarnik, J.; Brzezinska, O.; Budlewski, T.; Poplawska, M.; Sakowski, S.; Dudek, G.; Majsterek, I.; Makowska, J.; Poplawski, T. The Association between Inefficient Repair of DNA Double-Strand Breaks and Common Polymorphisms of the HRR and NHEJ Repair Genes in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2024, 25, 2619. [Google Scholar] [CrossRef] [PubMed]
- De Sandes Kimura, O.; Mozella, A.; Cobra, H.; Maciel Saraiva, A.C.; Carvalho de Almendra Freitas, E.H.; Cury Fernandes, M.B.; Matheus Guimarães, J.A.; Defino, H.; Leal, A.C. Neutrophil Extracellular Trap-related Biomarkers Are Increased in the Synovial Fluid of Patients with Periprosthetic Joint Infections. Clin. Orthop. Relat. Res. 2024, 482, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, R.A. Lipid content of human costal and articular cartilage. Ann. Rheum. Dis. 1967, 26, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Schröter, J.; Schiller, J. Chlorinated Phospholipids and Fatty Acids: (Patho)physiological Relevance, Potential Toxicity, and Analysis of Lipid Chlorohydrins. Oxid. Med. Cell. Longev. 2016, 2016, 8386362. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Antonny, B. Beyond Fluidity: The Role of Lipid Unsaturation in Membrane Function. Cold Spring Harb. Perspect. Biol. 2023, 15, a041409. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.; Schober, C.; Süss, R.; Fuchs, B.; Birkemeyer, C.; Schiller, J. Comparison of the positive and negative ion electrospray ionization and matrix-assisted laser desorption ionization-time-of-flight mass spectra of the reaction products of phosphatidylethanolamines and hypochlorous acid. Anal. Biochem. 2008, 376, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Osipov, A.N.; Spalteholz, H.; Panasenko, O.M.; Schiller, J. Effects of hypochlorous acid on unsaturated phosphatidylcholines. Free Radic. Biol. Med. 2001, 31, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; van den Berg, J.J.; Roitman, E.; Kuypers, F.A. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch. Biochem. Biophys. 1992, 296, 547–555. [Google Scholar] [CrossRef] [PubMed]
- De-Madaria, E.; Molero, X.; Bonjoch, L.; Casas, J.; Cárdenas-Jaén, K.; Montenegro, A.; Closa, D. Oleic acid chlorohydrin, a new early biomarker for the prediction of acute pancreatitis severity in humans. Ann. Intensive Care 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Schröter, J.; Griesinger, H.; Reuß, E.; Schulz, M.; Riemer, T.; Süß, R.; Schiller, J.; Fuchs, B. Unexpected products of the hypochlorous acid-induced oxidation of oleic acid: A study using high performance thin-layer chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2016, 1439, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.; Prabutzki, P.; Engel, K.M.; Schiller, J. From Oxidized Fatty Acids to Dimeric Species: In Vivo Relevance, Generation and Methods of Analysis. Molecules 2023, 28, 7850. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Vissers, M.C.; Domigan, N.M.; Winterbourn, C.C. Modification of red cell membrane lipids by hypochlorous acid and haemolysis by preformed lipid chlorohydrins. Redox Rep. 1997, 3, 263–271. [Google Scholar] [CrossRef] [PubMed]
- L’Huillier, N.; Pratten, M.K.; Clothier, R.H. The relative embryotoxicity of 1,3-dichloro-2-propanol on primary chick embryonic cells. Toxicol. Vitr. 2002, 16, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.J.; Blake, D.R.; Palmer, R.M.; Moncada, S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 1992, 51, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Fitzl, M.; Süss, R.; Arnold, K.; Schiller, J. Nitrite reduces the effects of HOCl on unsaturated phosphatidylcholines—A MALDI-TOF MS study. Chem. Phys. Lipids 2006, 140, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Rose, P.; Siau, J.L.; Halliwell, B. Nitrite-mediated protection against hypochlorous acid-induced chondrocyte toxicity: A novel cytoprotective role of nitric oxide in the inflamed joint? Arthritis Rheum. 2003, 48, 3140–3150. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Biological Functions of Plasmalogens. Adv. Exp. Med. Biol. 2020, 1299, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013, 65, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Herr, R.A.; Carlson, H.L.; Zoeller, R.A.; Albert, C.J.; Ford, D.A. Endothelial Cell Protein Targeting by Myeloperoxidase-Derived 2-Chlorofatty Aldehyde. Antioxidants 2022, 11, 940. [Google Scholar] [CrossRef] [PubMed]
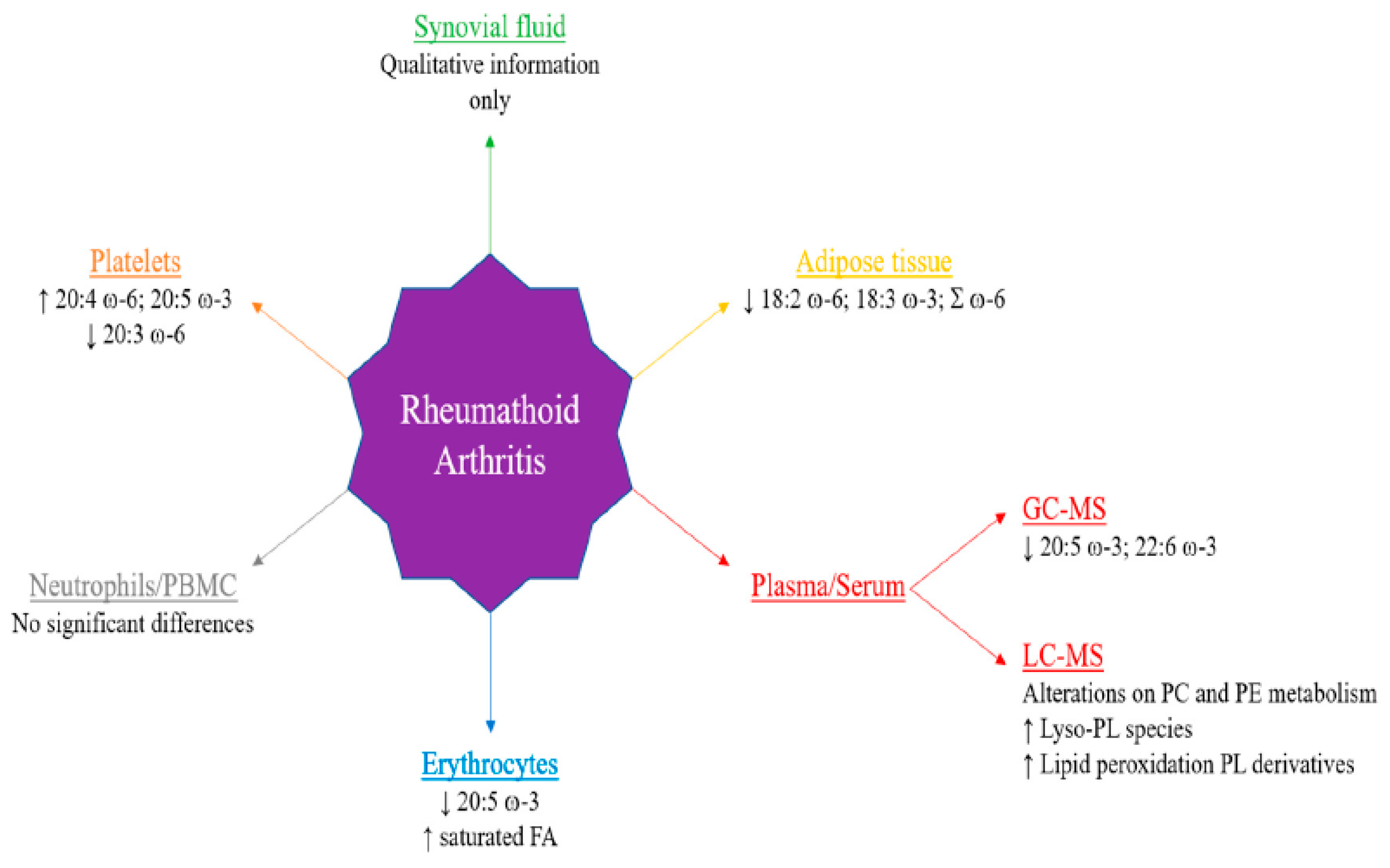
- Ferreira, H.B.; Melo, T.; Paiva, A.; Domingues, M.d.R. Insights in the Role of Lipids, Oxidative Stress and Inflammation in Rheumatoid Arthritis Unveiled by New Trends in Lipidomic Investigations. Antioxidants 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.H.; Hozayen, W.G.; Khaliefa, A.K.; El-Kenawy, A.E.; Ali, T.M.; Ahmed, O.M. Diosmin and Trolox Have Anti-Arthritic, Anti-Inflammatory and Antioxidant Potencies in Complete Freund’s Adjuvant-Induced Arthritic Male Wistar Rats: Roles of NF-κB, iNOS, Nrf2 and MMPs. Antioxidants 2022, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Ximenes, T.P.; Morgon, N.H.; de Souza, A.R. Taurine Chloramine and Hydrogen Peroxide as a Potential Source of Singlet Oxygen for Topical Application. Photochem. Photobiol. 2021, 97, 963–970. [Google Scholar] [CrossRef]
- Verdrengh, M.; Tarkowski, A. Inhibition of septic arthritis by local administration of taurine chloramine, a product of activated neutrophils. J. Rheumatol. 2005, 32, 1513–1517. [Google Scholar] [PubMed]
- Gottardi, W.; Debabov, D.; Nagl, M. N-chloramines, a promising class of well-tolerated topical anti-infectives. Antimicrob. Agents Chemother. 2013, 57, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takeuchi, R. Reactions of Methotrexate with Hypobromous Acid and Hypochlorous Acid. Chem. Pharm. Bull. 2019, 67, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, M.; Mallak, R.; Graham, G.G.; Kajer, T.; Milligan, M.K.; Nguyen, L.Q.; Newsham, D.W.; Keh, J.S.; Kettle, A.J.; Scott, K.F.; et al. Acetaminophen (paracetamol) inhibits myeloperoxidase-catalyzed oxidant production and biological damage at therapeutically achievable concentrations. Biochem. Pharmacol. 2010, 79, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Chaikijurajai, T.; Tang, W.H.W. Myeloperoxidase: A potential therapeutic target for coronary artery disease. Expert Opin. Ther. Targets 2020, 24, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.A.; Jing, X.; Teng, M.; Wells, C.; Jia, S.; Afolayan, A.J.; Jarzembowski, J.; Day, B.W.; Naylor, S.; Hessner, M.J.; et al. Role of endoplasmic reticulum stress in impaired neonatal lung growth and bronchopulmonary dysplasia. PLoS ONE 2022, 17, e0269564. [Google Scholar] [CrossRef] [PubMed]
- Tidén, A.-K.; Sjögren, T.; Svensson, M.; Bernlind, A.; Senthilmohan, R.; Auchère, F.; Norman, H.; Markgren, P.-O.; Gustavsson, S.; Schmidt, S.; et al. 2-thioxanthines are mechanism-based inactivators of myeloperoxidase that block oxidative stress during inflammation. J. Biol. Chem. 2011, 286, 37578–37589. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chen, H.; Chen, X.; Guo, C. The Roles of Neutrophil-Derived Myeloperoxidase (MPO) in Diseases: The New Progress. Antioxidants 2024, 13, 132. [Google Scholar] [CrossRef]
- Pahwa, R.; Modi, P.; Jialal, I. Myeloperoxidase Deficiency; StatPearls [Internet]: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zlatar, L.; Mahajan, A.; Muñoz-Becerra, M.; Weidner, D.; Bila, G.; Bilyy, R.; Titze, J.; Hoffmann, M.H.; Schett, G.; Herrmann, M.; et al. Suppression of neutrophils by sodium exacerbates oxidative stress and arthritis. Front. Immunol. 2023, 14, 1174537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, C.; Wang, M.; Zhao, H.; Zhu, Y. Ferroptosis as an emerging target in rheumatoid arthritis. Front. Immunol. 2023, 14, 1260839. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, B.; Xi, C.; Qin, Y.; Lin, X.; Wang, B.; Kong, P.; Yan, J. Ferroptosis Plays a Role in Human Chondrocyte of Osteoarthritis Induced by IL-1β In Vitro. Cartilage 2023, 14, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Frade-Sosa, B.; Sanmartí, R. Neutrophils, neutrophil extracellular traps, and rheumatoid arthritis: An updated review for clinicians. Reumatol. Clin. (Engl. Ed.) 2023, 19, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.; Bueb, J.-L.; Tolle, F.; Bréchard, S. Regulation of neutrophil pro-inflammatory functions sheds new light on the pathogenesis of rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 170–180. [Google Scholar] [CrossRef]
- Apel, F.; Zychlinsky, A.; Kenny, E.F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, C.; Zhang, Y.; Zhu, L. Composition and Function of Neutrophil Extracellular Traps. Biomolecules 2024, 14, 416. [Google Scholar] [CrossRef] [PubMed]
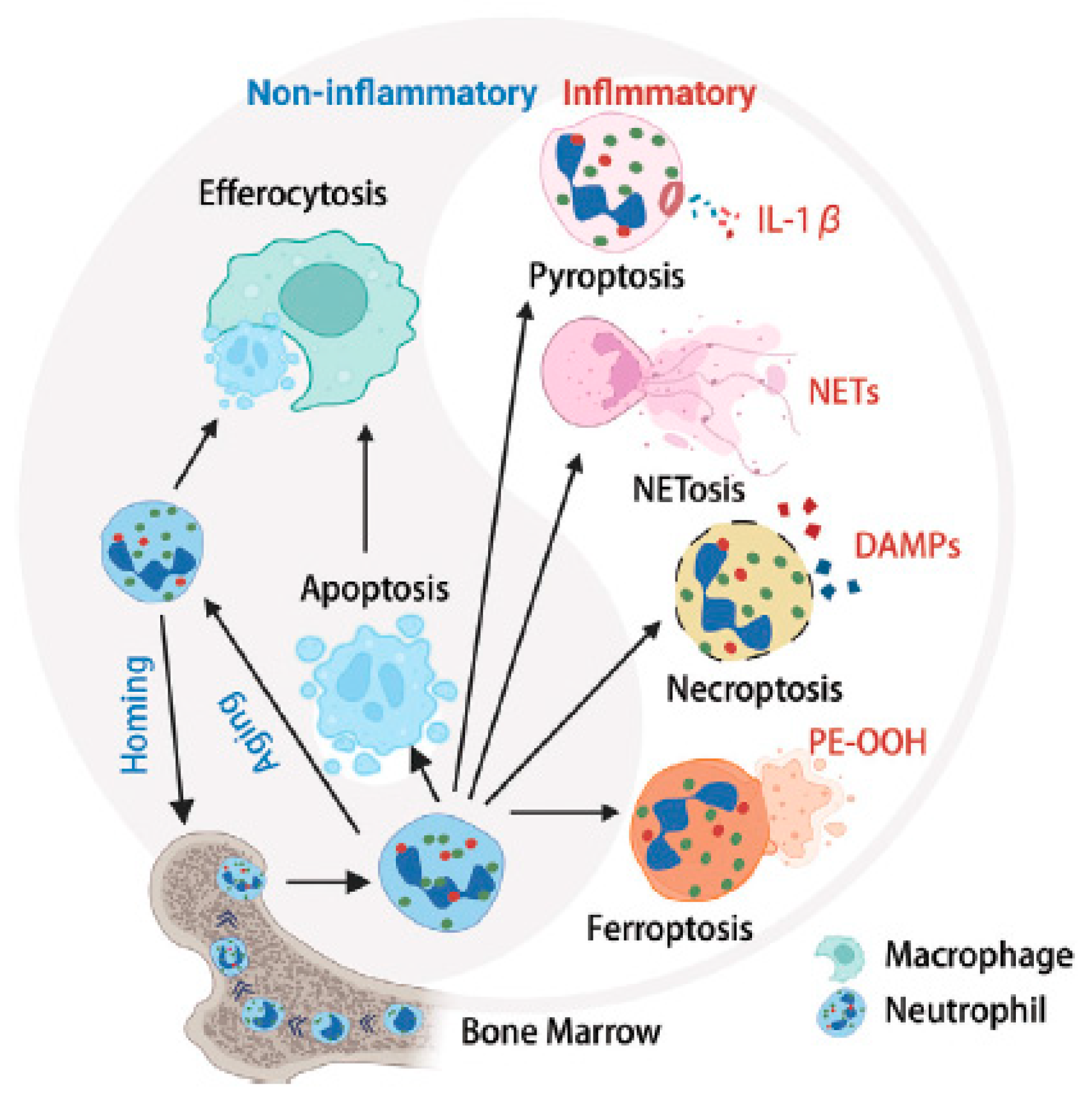
- Tu, H.; Ren, H.; Jiang, J.; Shao, C.; Shi, Y.; Li, P. Dying to Defend: Neutrophil Death Pathways and their Implications in Immunity. Adv. Sci. 2024, 11, e2306457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiang, P.; Guo, S.; Schrodi, S.J.; He, D. Apoptosis, Autophagy, NETosis, Necroptosis, and Pyroptosis Mediated Programmed Cell Death as Targets for Innovative Therapy in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 809806. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, T.; Nurbaeva, K.; Ptashnik, I.; Kudriaeva, A.; Belogurov, A.; Lila, A.; Nasonov, E. Markers of NETosis in Patients with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Int. J. Mol. Sci. 2023, 24, 9210. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Kumar, S. Neutrophil and remnant clearance in immunity and inflammation. Immunology 2022, 165, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.A.E.; Barlous, K.; Hawkins, C.L. Antioxidant Strategies to Modulate NETosis and the Release of Neutrophil Extracellular Traps during Chronic Inflammation. Antioxidants 2023, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Kosonen, J.P.; Eskelinen, A.S.A.; Orozco, G.A.; Nieminen, P.; Anderson, D.D.; Grodzinsky, A.J.; Korhonen, R.K.; Tanska, P. Injury-related cell death and proteoglycan loss in articular cartilage: Numerical model combining necrosis, reactive oxygen species, and inflammatory cytokines. PLoS Comput. Biol. 2023, 19, e1010337. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jian, Z.; Guo, J.; Ning, X. Increased levels of serum myeloperoxidase in patients with active rheumatoid arthritis. Life Sci. 2014, 117, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Talib, J.; Maghzal, G.J.; Cheng, D.; Stocker, R. Detailed protocol to assess in vivo and ex vivo myeloperoxidase activity in mouse models of vascular inflammation and disease using hydroethidine. Free Radic. Biol. Med. 2016, 97, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Agrawal, D.K. Damage-associated molecular patterns in the pathogenesis of osteoarthritis: Potentially novel therapeutic targets. Mol. Cell. Biochem. 2017, 434, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, J.; Caterson, B.; Hughes, C.E. Proteoglycan metabolism, cell death and Kashin-Beck disease. Glycoconj. J. 2012, 29, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leopold, J.; Schiller, J. (Chemical) Roles of HOCl in Rheumatic Diseases. Antioxidants 2024, 13, 921. https://doi.org/10.3390/antiox13080921
Leopold J, Schiller J. (Chemical) Roles of HOCl in Rheumatic Diseases. Antioxidants. 2024; 13(8):921. https://doi.org/10.3390/antiox13080921
Chicago/Turabian StyleLeopold, Jenny, and Jürgen Schiller. 2024. "(Chemical) Roles of HOCl in Rheumatic Diseases" Antioxidants 13, no. 8: 921. https://doi.org/10.3390/antiox13080921




