Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic Review
Abstract
:1. Introduction
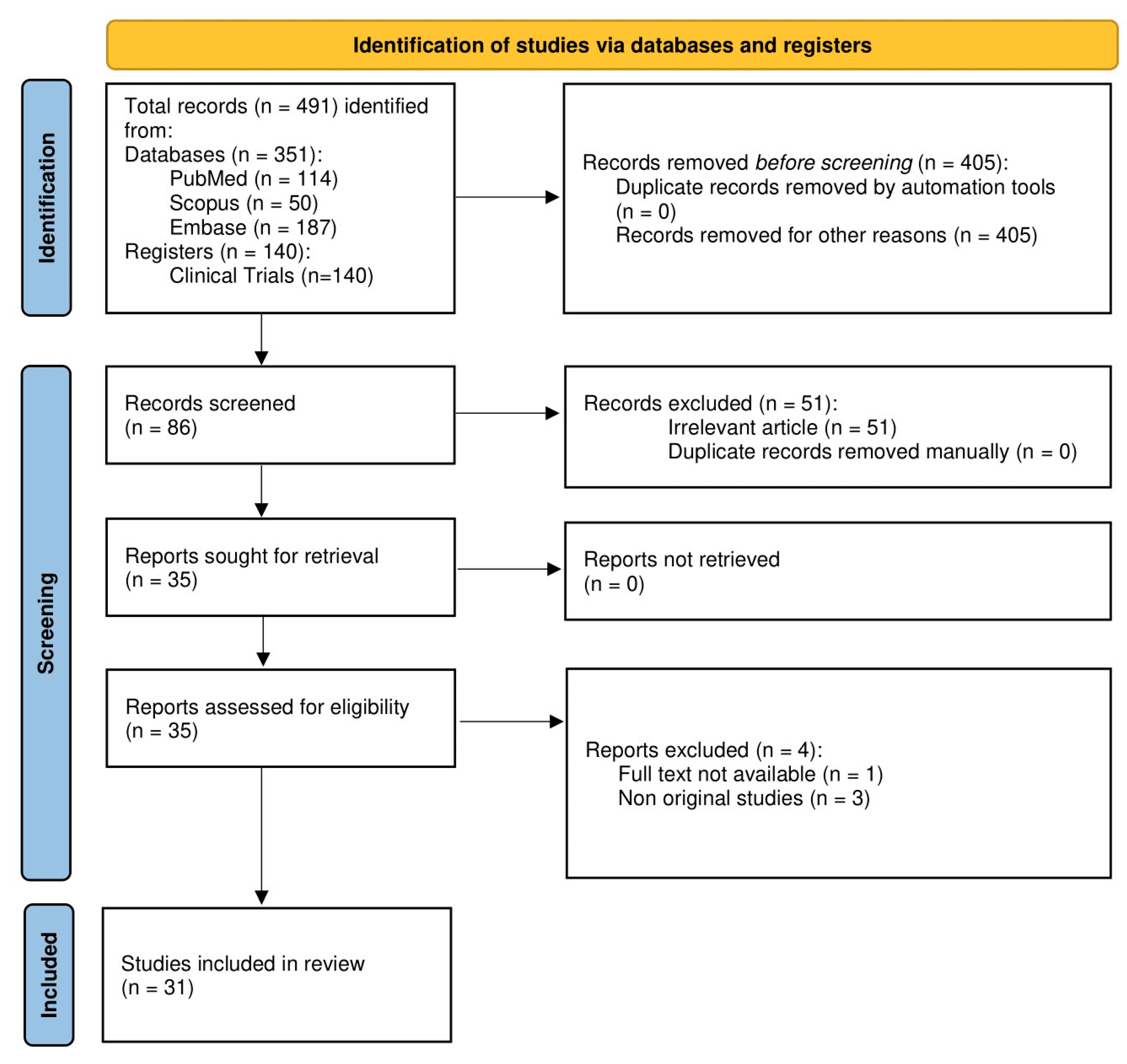
2. Methodology
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Items
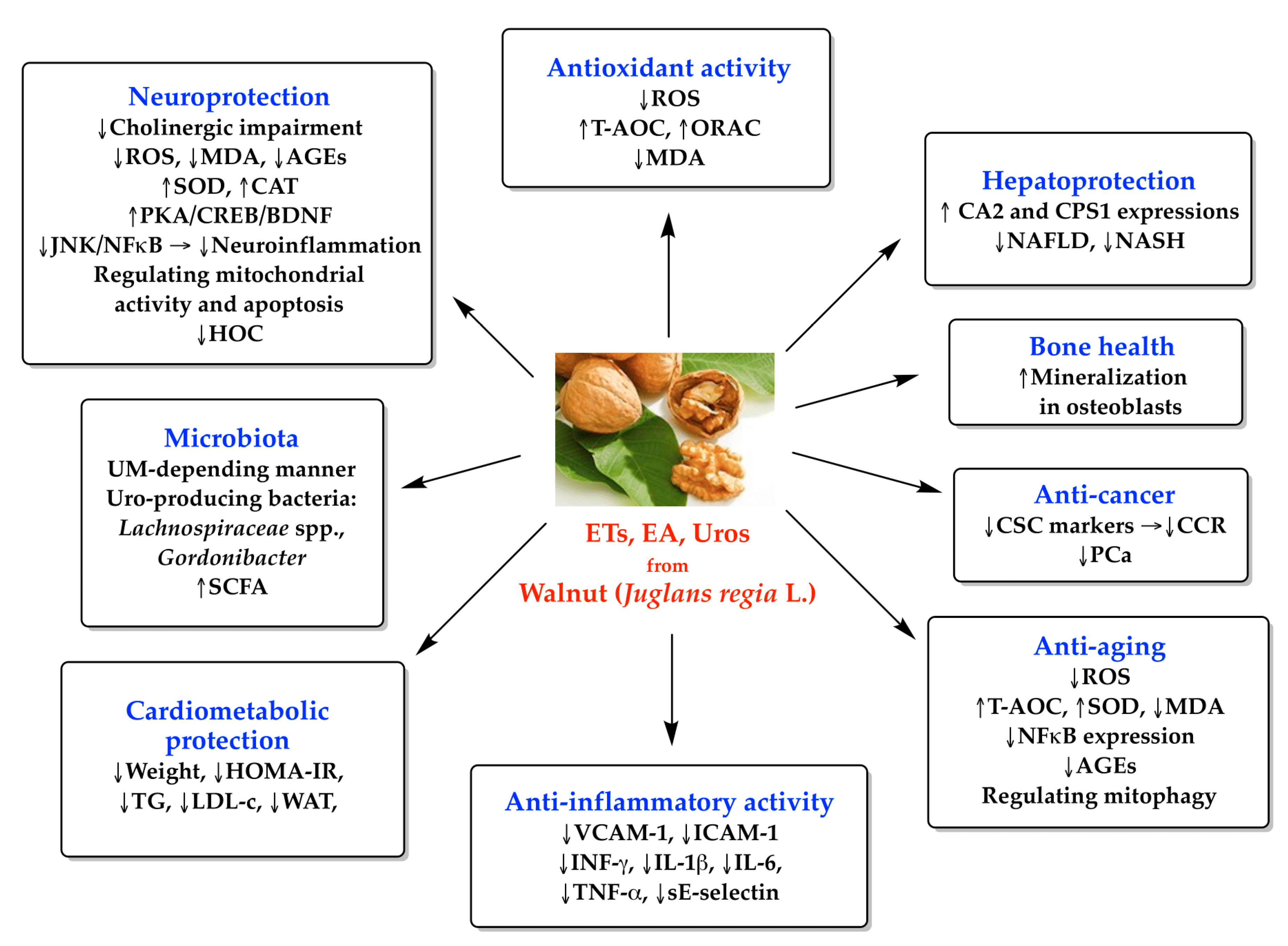
3. Results and Discussion
3.1. Phytochemical Composition
3.1.1. Walnut Sample Preparation and ETs and EA Extraction
3.1.2. Separation and Characterization of Walnut ETs and EA
3.1.3. Walnut ETs and EA Derivatives
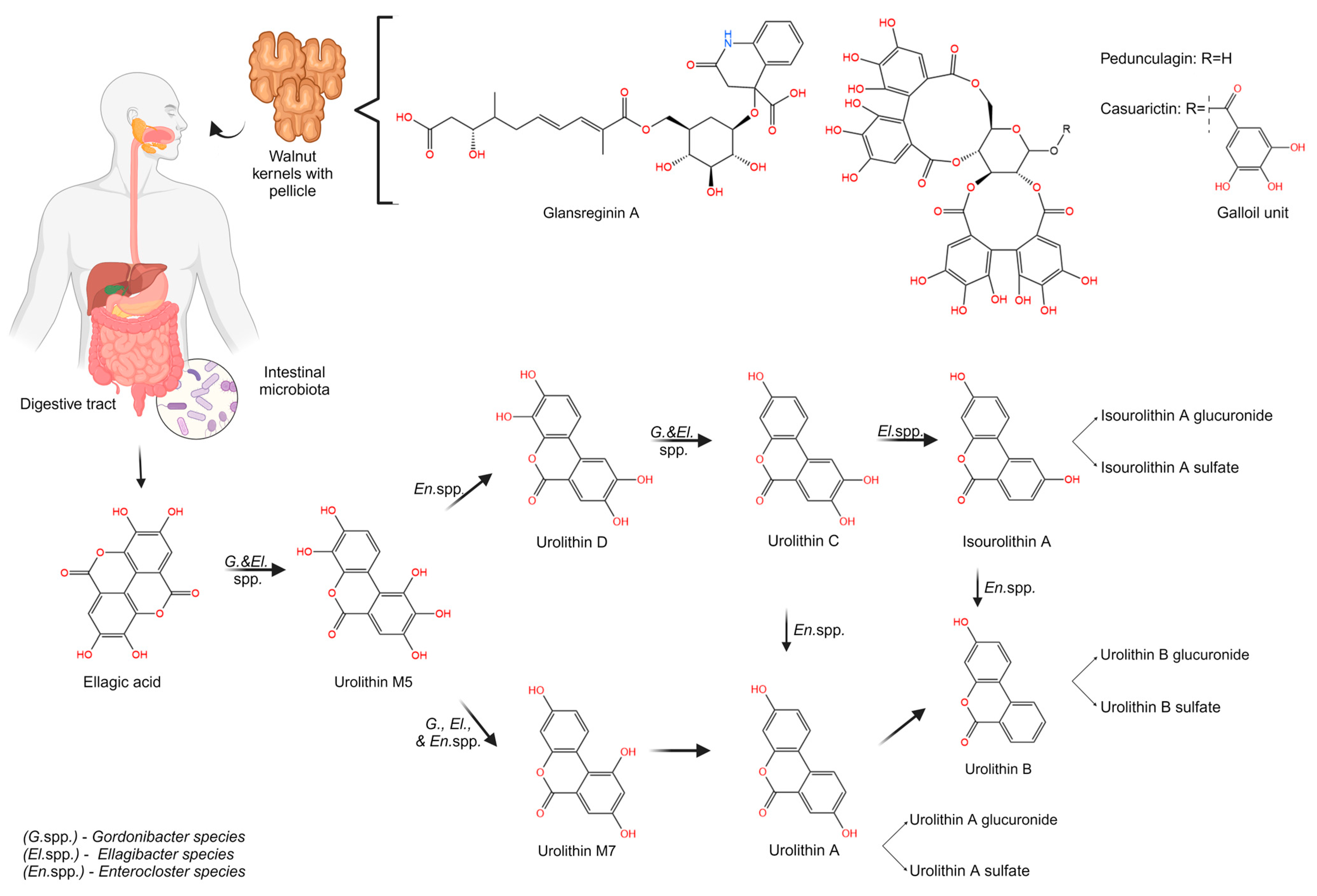
3.2. Metabolism of Walnut ETs and EA
3.2.1. Urolithin Biosynthesis from ETs and EA Metabolism
3.2.2. Influence of Individual Metabotype and GM on Urolithin Biosynthesis
3.2.3. Bioavailability of Urolithins and Their Metabolites after Walnut Intake
3.2.4. Pharmacokinetics of Uro-A and Uro-B after Walnut Intake
3.2.5. Metabolic Compounds Derived from Walnuts
3.3. Antioxidant Activity of ETs and Their Metabolites
3.4. Anti-Inflammatory Activity of ETs and Their Metabolites
3.4.1. Preclinical Studies
3.4.2. Clinical Studies
3.5. Cardiometabolic Activity of ETs and Their Metabolites
3.5.1. Weight, Waist Circumference, Visceral Adiposity
3.5.2. Gut Health
3.6. Neuroprotection Activity of ETs and Their Metabolites
3.6.1. Preclinical Studies
3.6.2. Clinical Studies
3.7. Antitumoral Potential of ETs and Their Metabolites
3.7.1. Preclinical Studies
3.7.2. Clinical Studies
3.8. Other Potential Therapeutic Effects of ETs and Their Metabolites
3.8.1. Hepatoprotective Effects
3.8.2. Bone Health
3.8.3. Anti-Aging Effects
Preclinical Studies
Clinical Studies
3.8.4. Antimicrobial Activity
4. Strength, Limitations, and Future Prospects
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative Stress, Nutritional Antioxidants and Beyond. Sci. China Life Sci. 2020, 63, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Bigman, G.; Ryan, A.S.; Popa, D.-S. Investigating the Effects and Mechanisms of Combined Vitamin D and K Supplementation in Postmenopausal Women: An Up-to-Date Comprehensive Review of Clinical Studies. Nutrients 2024, 16, 2356. [Google Scholar] [CrossRef] [PubMed]
- Shadfar, S.; Parakh, S.; Jamali, M.S.; Atkin, J.D. Redox Dysregulation as a Driver for DNA Damage and Its Relationship to Neurodegenerative Diseases. Transl. Neurodegener. 2023, 12, 18. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Medic, A.; Jakopic, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and Quantification of the Major Phenolic Constituents in Juglans Regia L. Peeled Kernels and Pellicles, Using HPLC–MS/MS. Food Chem. 2021, 352, 129404. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut–Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive Identification of Walnut Polyphenols by Liquid Chromatography Coupled to Linear Ion Trap–Orbitrap Mass Spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. Phytochemical Profiling and Biological Activities of Quercus Sp. Galls (Oak Galls): A Systematic Review of Studies Published in the Last 5 Years. Plants 2023, 12, 3873. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Mantzourani, C.; Kakouri, E.; Palikaras, K.; Tarantilis, P.A.; Kokotou, M.G. Urolithins and Their Precursors Ellagic Acid and Ellagitannins: Natural Sources, Extraction and Methods for Their Determination. Separations 2024, 11, 174. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Miereles, M.; Ascacio-Valdés, J.A.; Aguilera-Carbo, A.; Sepúlveda, L.; Contreras-Esquivel, J.; Rodríguez-Herrera, R.; Aguilar, C.N. Enzymatic Biotransformation of Pomegranate Ellagitannins: Initial Approach to Reaction Conditions. Iran. J. Biotechnol. 2020, 18, 30–36. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on Their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Iglesias-Aguirre, C.E.; Marín, A.; Romo-Vaquero, M.; Vallejo, F.; Espín, J.C.; Victoria Selma, M. Urolithin A Production Drives the Effects of Pomegranate on the Gut Microbial Metabolism of Bile Acids and Cholesterol in Mild Dyslipidaemic Overweight and Obese Individuals. Food Funct. 2024, 15, 2422–2432. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The Total Antioxidant Content of More than 3100 Foods, Beverages, Spices, Herbs and Supplements Used Worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Bitok, E.; Rajaram, S.; Jaceldo-Siegl, K.; Oda, K.; Sala-Vila, A.; Serra-Mir, M.; Ros, E.; Sabaté, J. Effects of Long-Term Walnut Supplementation on Body Weight in Free-Living Elderly: Results of a Randomized Controlled Trial. Nutrients 2018, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, N.; Molyneux, R.J. Phytochemical Inhibition of Aflatoxigenicity in Aspergillus Flavus by Constituents of Walnut (Juglans Regia). J. Agric. Food Chem. 2004, 52, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of Antioxidant and Chemopreventive Ellagitannins from Strawberries, Raspberries, Walnuts, and Oak-Aged Wine in Humans: Identification of Biomarkers and Individual Variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of Urolithin A as a Metabolite Produced by Human Colon Microflora from Ellagic Acid and Related Compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic Acids, Syringaldehyde, and Juglone in Fruits of Different Cultivars of Juglans Regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Zhu, H.; Young, J.C. Polyphenolic Profiles and Antioxidant Activities of Heartnut (Juglans Ailanthifolia Var. Cordiformis) and Persian Walnut (Juglans Regia L.). J. Agric. Food Chem. 2006, 54, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Caboni, M.F.; Fernández-Gutiérrez, A. Development of a Rapid Method to Determine Phenolic and Other Polar Compounds in Walnut by Capillary Electrophoresis–Electrospray Ionization Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2008, 1209, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut Extract (Juglans Regia L.) and Its Component Ellagic Acid Exhibit Anti-Inflammatory Activity in Human Aorta Endothelial Cells and Osteoblastic Activity in the Cell Line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef]
- Anderson, K.C.; Teuber, S.S. Ellagic Acid and Polyphenolics Present in Walnut Kernels Inhibit in Vitro Human Peripheral Blood Mononuclear Cell Proliferation and Alter Cytokine Production. Ann. N. Y. Acad. Sci. 2010, 1190, 86–96. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Álvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A.; et al. Occurrence of Urolithins, Gut Microbiota Ellagic Acid Metabolites and Proliferation Markers Expression Response in the Human Prostate Gland upon Consumption of Walnuts and Pomegranate Juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabaté, J. Effect of a Walnut Meal on Postprandial Oxidative Stress and Antioxidants in Healthy Individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Chromatographic and Spectroscopic Characterization of Urolithins for Their Determination in Biological Samples after the Intake of Foods Containing Ellagitannins and Ellagic Acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.S.; Lee, J.H.; Heo, S.C.; Lee, K.L.; Choi, S.W.; Kim, Y. Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients 2016, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The Gut Microbiota Metabolism of Pomegranate or Walnut Ellagitannins Yields Two Urolithin-Metabotypes That Correlate with Cardiometabolic Risk Biomarkers: Comparison between Normoweight, Overweight-Obesity and Metabolic Syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espín, J.C.; Selma, M.V.; Collado, M.C. Urolithin Metabotypes Can Determine the Modulation of Gut Microbiota in Healthy Individuals by Tracking Walnuts Consumption over Three Days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; García-Villalba, R.; García-Mantrana, I.; Rodríguez-Varela, A.; Romo-Vaquero, M.; Collado, M.C.; Tomás-Barberán, F.A.; Espín, J.C.; Selma, M.V. Urolithins in Human Breast Milk after Walnut Intake and Kinetics of Gordonibacter Colonization in Newly Born: The Role of Mothers’ Urolithin Metabotypes. J. Agric. Food Chem. 2020, 68, 12606–12616. [Google Scholar] [CrossRef] [PubMed]
- Haramiishi, R.; Okuyama, S.; Yoshimura, M.; Nakajima, M.; Furukawa, Y.; Ito, H.; Amakura, Y. Identification of the Characteristic Components in Walnut and Anti-Inflammatory Effect of Glansreginin A as an Indicator for Quality Evaluation. Biosci. Biotechnol. Biochem. 2020, 84, 187–197. [Google Scholar] [CrossRef]
- Tian, W.; Wu, B.; Sun, L.; Zhuang, Y. Protective Effect against D-Gal-Induced Aging Mice and Components of Polypeptides and Polyphenols in Defatted Walnut Kernel during Simulated Gastrointestinal Digestion. J. Food Sci. 2021, 86, 2736–2752. [Google Scholar] [CrossRef]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic Profiles and Antioxidant Activities of Free, Esterified and Bound Phenolic Compounds in Walnut Kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Zelicha, H.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Levakov, G.; Prager, O.; Salti, M.; Yovell, Y.; Ofer, J.; et al. The Effect of a High-Polyphenol Mediterranean Diet (Green-MED) Combined with Physical Activity on Age-Related Brain Atrophy: The Dietary Intervention Randomized Controlled Trial Polyphenols Unprocessed Study (DIRECT PLUS). Am. J. Clin. Nutr. 2022, 115, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, J.M.; Lee, U.; Kang, J.Y.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Go, M.J.; Kim, H.J.; Park, H.W.; et al. Walnut Prevents Cognitive Impairment by Regulating the Synaptic and Mitochondrial Dysfunction via JNK Signaling and Apoptosis Pathway in High-Fat Diet-Induced C57BL/6 Mice. Molecules 2022, 27, 5316. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.M.; Zhang, Q.Z.; Jiang, M.; Chen, M.L.; Xu, X.J.; Wang, D.M.; Pan, Y.N.; Liu, X.Q. Systematic Characterization of the Metabolites of Defatted Walnut Powder Extract in Vivo and Screening of the Mechanisms against NAFLD by UPLC-Q-Exactive Orbitrap MS Combined with Network Pharmacology. J. Ethnopharmacol. 2022, 285, 114870. [Google Scholar] [CrossRef] [PubMed]
- Romo-Vaquero, M.; Fernández-Villalba, E.; Gil-Martinez, A.L.; Cuenca-Bermejo, L.; Espín, J.C.; Herrero, M.T.; Selma, M.V. Urolithins: Potential Biomarkers of Gut Dysbiosis and Disease Stage in Parkinson’s Patients. Food Funct. 2022, 13, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Zelicha, H.; Kloting, N.; Kaplan, A.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Chassidim, Y.; Bluher, M.; Ceglarek, U.; Isermann, B.; et al. The Effect of High-Polyphenol Mediterranean Diet on Visceral Adiposity: The DIRECT PLUS Randomized Controlled Trial. BMC Med. 2022, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Li, M.; Zou, C.; Wang, K.; Zhang, W.; Huang, X.; Wang, Y. Walnut Polyphenols and the Active Metabolite Urolithin A Improve Oxidative Damage in SH-SY5Y Cells by up-Regulating PKA/CREB/BDNF Signaling. Food Funct. 2023, 14, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gu, F.; Yang, T.; Shao, Z.; Zhang, Q.; Zhu, J.; Wang, F. Quantitative Conversion of Free, Acid-Hydrolyzable, and Bound Ellagic Acid in Walnut Kernels during Baking. Food Chem. 2023, 400, 134070. [Google Scholar] [CrossRef] [PubMed]
- Yaskolka Meir, A.; Keller, M.; Hoffmann, A.; Rinott, E.; Tsaban, G.; Kaplan, A.; Zelicha, H.; Hagemann, T.; Ceglarek, U.; Isermann, B.; et al. The Effect of Polyphenols on DNA Methylation-Assessed Biological Age Attenuation: The DIRECT PLUS Randomized Controlled Trial. BMC Med. 2023, 21, 364. [Google Scholar] [CrossRef]
- Wu, S.; Mo, R.; Wang, R.; Li, Q.; Shen, D.; Liu, Y. Identification of Key Antioxidants of Free, Esterified, and Bound Phenolics in Walnut Kernel and Skin. Foods 2023, 12, 825. [Google Scholar] [CrossRef]
- Xu, X.; Song, Y.; Jiang, M.; Liu, M.; Zhang, X.; Wang, D.; Pan, Y.; Ren, S.; Liu, X. Screening of the Active Substances for the Assessment of Walnut Kernel in the Treatment of Scopolamine-Induced AD Animals. Mol. Nutr. Food Res. 2024, 68, e2200816. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from Different Agricultural Residues: Extraction, Identification and Their Antioxidant Properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Canto-Pinto, J.C.; Cuevas-Glory, L.F.; Sauri-Duch, E.; Pérez-Pacheco, E.; Betancur-Ancona, D. Effect of Extraction Solvent on the Phenolic Compounds Content and Antioxidant Activity of Ramon Nut (Brosimum Alicastrum). Chem. Pap. 2019, 73, 1647–1657. [Google Scholar] [CrossRef]
- Itoh, H.; Sakuma, H. Dielectric Constant of Water as a Function of Separation in a Slab Geometry: A Molecular Dynamics Study. J. Chem. Phys. 2015, 142, 184703. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins Extraction: A Key Point for Their Valorization and Cleaner Production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Perez, J.L.; Lombardini, L.; Cornacchia, R.; Cisneros-Zevallos, L.; Braford, J. Phenolic Compounds and Fatty Acid Composition of Organic and Conventional Grown Pecan Kernels. J. Sci. Food Agric. 2009, 89, 2207–2213. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Chavan, Y.; Singhal, R.S. Ultrasound-Assisted Extraction (UAE) of Bioactives from Arecanut (Areca Catechu L.) and Optimization Study Using Response Surface Methodology. Innov. Food Sci. Emerg. Technol. 2013, 17, 106–113. [Google Scholar] [CrossRef]
- Berkani, F.; Serralheiro, M.L.; Dahmoune, F.; Ressaissi, A.; Kadri, N.; Remini, H. Ultrasound Assisted Extraction of Phenolic Compounds from a Jujube By-Product with Valuable Bioactivities. Processes 2020, 8, 1441. [Google Scholar] [CrossRef]
- Setyawan, H.Y.; Subagyo, A.; Balbeid, M.; Sunyoto, N.M.S.; Nizori, A.; Chomsri, N.; Choirun, A. Ultrasonic Extraction of Betara’s Areca Nuts’ Antioxidants. Adv. Eng. Res. 2021, 212, 102–106. [Google Scholar] [CrossRef]
- Ren, J.; Zheng, Y.; Lin, Z.; Han, X.; Liao, W. Macroporous Resin Purification and Characterization of Flavonoids from Platycladus Orientalis (L.) Franco and Their Effects on Macrophage Inflammatory Response. Food Funct. 2017, 8, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Pycia, K.; Kapusta, I.; Jaworska, G. Impact of the Degree of Maturity of Walnuts (Juglans Regia L.) and Their Variety on the Antioxidant Potential and the Content of Tocopherols and Polyphenols. Molecules 2019, 24, 2936. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Groothuis, S.F.; Prencipe, F.P.; Amir, R.; Benvenuti, S.; Pellati, F. Metabolite Fingerprinting of Punica Granatum L. (Pomegranate) Polyphenols by Means of High-Performance Liquid Chromatography with Diode Array and Electrospray Ionization-Mass Spectrometry Detection. J. Chromatogr. A 2017, 1480, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Akande, T.; Khatib, M.; Ola Salawu, S.; Afolabi Akindahunsi, A.; Di Cesare Mannelli, L.; Ghelardini, C.; Balli, D.; Cecchi, L.; Mulinacci, N. 1H NMR and HPLC-DAD-MS for the Characterization of Ellagitannins and Triterpenoids of Less Investigated Anogeissus Leiocarpus DC (Combretaceae) Stem Bark. Food Chem. 2022, 375, 131813. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.M.; Ferreira, G.d.A.; Campos, M.M.; Teixeira, A.M.; Costa, F.d.N.; das Chagas, F.O.; Colonna, M. NMR as a Tool for Compound Identification in Mixtures. Phytochem. Anal. 2023, 34, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Spisso, A.; Gomez, F.J.V.; Fernanda Silva, M. Determination of Ellagic Acid by Capillary Electrophoresis in Argentinian Wines. Electrophoresis 2018, 39, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and Quantification of Phenolic Compounds in Kernels, Oil and Bagasse Pellets of Common Walnut (Juglans Regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Medic, A.; Kunc, P.; Zamljen, T.; Hudina, M.; Veberic, R.; Solar, A. Identification and Quantification of the Major Phenolic Constituents in Castanea Sativa and Commercial Interspecific Hybrids (C. Sativa x C. Crenata) Chestnuts Using HPLC–MS/MS. Int. J. Mol. Sci. 2023, 24, 13086. [Google Scholar] [CrossRef]
- Jia, X.; Luo, H.; Xu, M.; Zhai, M.; Guo, Z.; Qiao, Y.; Wang, L. Dynamic Changes in Phenolics and Antioxidant Capacity during Pecan (Carya Illinoinensis) Kernel Ripening and Its Phenolics Profiles. Molecules 2018, 23, 435. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. HPLC-MSn Identification and Quantification of Phenolic Compounds in Hazelnut Kernels, Oil and Bagasse Pellets. Food Res. Int. 2014, 64, 783–789. [Google Scholar] [CrossRef]
- Ho, K.-V.; Hsieh, H.Y.; Roy, A.; Foote, S.; McDonald, P.; Coggeshall, M.V.; Ito, H.; Lei, Z.; Sumner, L.W.; Stewart, G.C.; et al. Quantification and Characterization of Biological Activities of Glansreginin A in Black Walnuts (Juglans Nigra). Sci. Rep. 2023, 13, 18860. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Fusco, J.L.; Rosenberg, D.W. Antioxidant and Anti-Inflammatory Properties of Walnut Constituents: Focus on Personalized Cancer Prevention and the Microbiome. Antioxidants 2023, 12, 982. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Bakkalbași, E.; Menteș, O.; Artik, N. Food Ellagitannins–Occurrence, Effects of Processing and Storage. Crit. Rev. Food Sci. Nutr. 2008, 49, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Isenring, J.; Bircher, L.; Geirnaert, A.; Lacroix, C. In Vitro Human Gut Microbiota Fermentation Models: Opportunities, Challenges, and Pitfalls. Microbiome Res. Rep. 2023, 2, 2. [Google Scholar] [CrossRef]
- Villalba, K.J.O.; Barka, F.V.; Pasos, C.V.; Rodríguez, P.E. Food Ellagitannins: Structure, Metabolomic Fate, and Biological Properties. In Tannins—Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Kujawska, M.; Jodynis-Liebert, J. Potential of the Ellagic Acid-Derived Gut Microbiota Metabolite—Urolithin A in Gastrointestinal Protection. World J. Gastroenterol. 2020, 26, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberan, F.A.; Espín, J.C.; García-Conesa, M.T. Bioavailability and Metabolism of Ellagic Acid and Ellagitannins. In Chemistry and Biology of Ellagitannins; Quideau, S., Ed.; World Scientific Publishing Company: Singapore, 2009; pp. 273–297. [Google Scholar]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The Dietary Hydrolysable Tannin Punicalagin Releases Ellagic Acid That Induces Apoptosis in Human Colon Adenocarcinoma Caco-2 Cells by Using the Mitochondrial Pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef]
- Harper, P. A Review of the Dietary Intake, Bioavailability and Health Benefits of Ellagic Acid (EA) with a Primary Focus on Its Anti-Cancer Properties. Cureus 2023, 15, e43156. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the Rescue of “Old” Metabolites to Understand a “New” Concept: Metabotypes as a Nexus among Phenolic Metabolism, Microbiota Dysbiosis, and Host Health Status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Aguirre, C.E.; García-Villalba, R.; Beltrán, D.; Frutos-Lisón, M.D.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Gut Bacteria Involved in Ellagic Acid Metabolism To Yield Human Urolithin Metabotypes Revealed. J. Agric. Food Chem. 2023, 71, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-De-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The Gut Microbiota Urolithin Metabotypes Revisited: The Human Metabolism of Ellagic Acid Is Mainly Determined by Aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef]
- Hu, J.; Mesnage, R.; Tuohy, K.; Heiss, C.; Rodriguez-Mateos, A. (Poly)Phenol-Related Gut Metabotypes and Human Health: An Update. Food Funct. 2024, 15, 2814–2835. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.; González-Paramás, A.M.; Heleno, S.A.; Calhelha, R.C. The Role of Gut Microbiota in the Etiopathogenesis of Multiple Chronic Diseases. Antibiotics 2024, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Mena, P.; Dall’Asta, M.; Calani, L.; Brighenti, F.; Del Rio, D. Gastrointestinal Stability of Urolithins: An in Vitro Approach. Eur. J. Nutr. 2017, 56, 99–106. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-Extractable Polyphenols Produce Gut Microbiota Metabolites That Persist in Circulation and Show Anti-Inflammatory and Free Radical-Scavenging Effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; Núñez-Sánchez, M.Á.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. Phase-II Metabolism Limits the Antiproliferative Activity of Urolithins in Human Colon Cancer Cells. Eur. J. Nutr. 2014, 53, 853–864. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s Disease Drug Development Pipeline: 2024. Alzheimer's Dis. Transl. Res. Clin. Interv. 2024, 10, e12465. [Google Scholar] [CrossRef] [PubMed]
- Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New Insights into Oxidative Stress and Inflammatory Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Najda, A.; Michalak, I. Investigation of Chemical Constituents and Antioxidant Activity of Biologically Active Plant-Derived Natural Products. Molecules 2023, 28, 5572. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Simedrea, R.; Gheldiu, A.M.; Mocan, A.; Vlase, L.; Popa, D.S.; Ferreira, I.C.F.R. Benefits of Tree Nut Consumption on Aging and Age-Related Diseases: Mechanisms of Actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Health Benefits of Walnut Polyphenols: An Exploration beyond Their Lipid Profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Vinson, J.A.; Cai, Y. Nuts, Especially Walnuts, Have Both Antioxidant Quantity and Efficacy and Exhibit Significant Potential Health Benefits. Food Funct. 2012, 3, 134–140. [Google Scholar] [CrossRef]
- Fumagalli, M.; Sangiovanni, E.; Vrhovsek, U.; Piazza, S.; Colombo, E.; Gasperotti, M.; Mattivi, F.; De Fabiani, E.; Dell’Agli, M. Strawberry Tannins Inhibit IL-8 Secretion in a Cell Model of Gastric Inflammation. Pharmacol. Res. 2016, 111, 703–712. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus Berries for the Control of Gastric Inflammation: In Vitro and In Vivo Studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Fumagalli, M.; Pozzoli, C.; Maranta, N.; Giavarini, F.; Colombo, L.; Nicotra, G.; Vicentini, S.F.; Genova, F.; et al. Ellagitannins from Castanea Sativa Mill. Leaf Extracts Impair H. Pylori Viability and Infection-Induced Inflammation in Human Gastric Epithelial Cells. Nutrients 2023, 15, 1504. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muñoz, C.; Vaillant, F. Metabolic Fate of Ellagitannins: Implications for Health, and Research Perspectives for Innovative Functional Foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The Influence of Pomegranate By-Product and Punicalagins on Selected Groups of Human Intestinal Microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; López, V. The Metabolite Urolithin-a Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Vodovotz, Y.; Arciero, J.; Verschure, P.F.M.J.; Katz, D.L. A Multiscale Inflammatory Map: Linking Individual Stress to Societal Dysfunction. Front. Sci. 2024, 1, 1239462. [Google Scholar] [CrossRef]
- Dick, T.E.; Molkov, Y.I.; Nieman, G.; Hsieh, Y.H.; Jacono, F.J.; Doyle, J.; Scheff, J.D.; Calvano, S.E.; Androulakis, I.P.; An, G.; et al. Linking Inflammation, Cardiorespiratory Variability, and Neural Control in Acute Inflammation via Computational Modeling. Front. Physiol. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Thompson, A.S.; Tresserra-Rimbau, A.; Karavasiloglou, N.; Jennings, A.; Cantwell, M.; Hill, C.; Perez-Cornago, A.; Bondonno, N.P.; Murphy, N.; Rohrmann, S.; et al. Association of Healthful Plant-Based Diet Adherence with Risk of Mortality and Major Chronic Diseases among Adults in the UK. JAMA Netw. Open 2023, 6, e234714. [Google Scholar] [CrossRef]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial Effects of Walnut Consumption on Human Health: Role of Micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef]
- Cofán, M.; Rajaram, S.; Sala-Vila, A.; Valls-Pedret, C.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.M.; Bitok, E.; Sabaté, J.; Ros, E. Effects of 2-Year Walnut-Supplemented Diet on Inflammatory Biomarkers. J. Am. Coll. Cardiol. 2020, 76, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- Mateș, L.; Popa, D.S.; Rusu, M.E.; Fizeșan, I.; Leucuța, D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Stanisławska, I.; Granica, S.; Stefanska, J.; Kiss, A.K. Phase II Conjugates of Urolithins Isolated from Human Urine and Potential Role of β-Glucuronidases in Their Disposition. Drug Metab. Dispos. 2017, 45, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Kang, D.R.; Kim, J.Y.; Koh, K.K.; on behalf of the Taskforce Team of the Metabolic Syndrome Fact Sheet of the Korean Society of Cardiometabolic Syndrome. Metabolic Syndrome Fact Sheet 2021: Executive Report. CardioMetabolic Syndr. J. 2021, 1, 125–134. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Neri-Numa, I.A.; de Oliveira, W.Q.; de Araújo, F.F.; Pastore, G.M. Dietary Polyphenols and Their Relationship to the Modulation of Non-Communicable Chronic Diseases and Epigenetic Mechanisms: A Mini-Review. Food Chem. Mol. Sci. 2023, 6, 100155. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.; Vahid, F.; Devaux, Y.; Bohn, T. Biomarkers of Food Intake and Their Relevance to Metabolic Syndrome. Food Funct. 2024, 15, 7271. [Google Scholar] [CrossRef] [PubMed]
- Dal, N.; Bilici, S. Dietary Modulations in Preventing Cardiometabolic Risk in Individuals with Type 2 Diabetes. Curr. Nutr. Rep. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut Microbiota: A New Path to Treat Obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Y.; Chen, M.; Xue, L.; Wang, J.; Ding, Y.; Gu, Q.; Zhang, J.; Zhao, H.; Xie, X.; et al. Therapeutic Applications of Gut Microbes in Cardiometabolic Diseases: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2024, 108, 156. [Google Scholar] [CrossRef] [PubMed]
- Looi, D.; Moorthy, M.; Chaiyakunapruk, N.; Devi Palanisamy, U. Impact of Ellagitannin-Rich Fruit Consumption on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2022, 99, 105320. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Gevers, D.; Knight, R.; Petrosino, J.F.; Huang, K.; McGuire, A.L.; Birren, B.W.; Nelson, K.E.; White, O.; Methé, B.A.; Huttenhower, C. The Human Microbiome Project: A Community Resource for the Healthy Human Microbiome. PLoS Biol. 2012, 10, e1001377. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wang, J.; Guo, Y. Polysaccharides Influence Human Health via Microbiota-Dependent and -Independent Pathways. Front. Nutr. 2022, 9, 1030063. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, J.; Cheng, H.; Zhang, D.; Tan, Y.; Peng, C. Dietary Compounds in Modulation of Gut Microbiota-Derived Metabolites. Front. Nutr. 2022, 9, 939571. [Google Scholar] [CrossRef]
- Tan, B.; Wang, Y.; Zhang, X.; Sun, X. Recent Studies on Protective Effects of Walnuts against Neuroinflammation. Nutrients 2022, 14, 4360. [Google Scholar] [CrossRef]
- McQuade, A.; Blurton-Jones, M. Microglia in Alzheimer’s Disease: Exploring How Genetics and Phenotype Influence Risk. J. Mol. Biol. 2019, 431, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Denis Alexander, H.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.F.; Martins, A.; Majolo, F.; Contini, V.; Laufer, S.; Goettert, M.I. Neural Regeneration Research Model to Be Explored: SH-SY5Y Human Neuroblastoma Cells. Neural Regen. Res. 2023, 18, 1265–1266. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Walnut Diet Reduces Accumulation of Polyubiquitinated Proteins and Inflammation in the Brain of Aged Rats. J. Nutr. Biochem. 2013, 24, 912–919. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Sun, Y.; Zhang, W.; Huang, X.; Xue, R.; Zhang, Y.; Wang, Y. Walnut Diets Up-Regulate the Decreased Hippocampal Neurogenesis and Age-Related Cognitive Dysfunction in d-Galactose Induced Aged Rats. Food Funct. 2018, 9, 4755–4762. [Google Scholar] [CrossRef] [PubMed]
- Shui, G. Development of In Vitro Neural Models for Drug Discovery and Toxicity Screening. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Oxford, UK, 2011; Volume 5, pp. 565–572. ISBN 9780080885049. [Google Scholar]
- Lim, J.; Bang, Y.; Kim, K.M.; Choi, H.J. Differentiated HT22 Cells as a Novel Model for in Vitro Screening of Serotonin Reuptake Inhibitors. Front. Pharmacol. 2023, 13, 1062650. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; De La Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.; Cerquera-Cleves, C.; Kekenadze, M.; Wild Crea, P.; Singleton, A.B.; Bandres-Ciga, S. Towards a Global View of Parkinson’s Disease Genetics. Ann. Neurol. 2024, 95, 831–842. [Google Scholar] [CrossRef]
- Long, J.; Ji, Z.; Yuan, P.; Long, T.; Liu, K.; Li, J.; Cheng, L. Nut Consumption and Risk of Cancer: A Meta-Analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2020, 29, 565–573. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and Number of Cancer Cases and Deaths Attributable to Potentially Modifiable Risk Factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Culp, M.B.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Nevalainen, J.; Raitanen, J.; Natunen, K.; Kilpeläinen, T.; Rannikko, A.; Tammela, T.; Auvinen, A. Early Detection of Clinically Significant Prostate Cancer: Protocol Summary and Statistical Analysis Plan for the ProScreen Randomised Trial. BMJ Open 2024, 14, e075595. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.R.; Oyekunle, T.; Howard, L.E.; Wiggins, E.K.; Freedland, S.J.; Allott, E.H. Family History of Prostate Cancer and Prostate Tumor Aggressiveness in Black and Non-Black Men; Results from an Equal Access Biopsy Study. Cancer Causes Control. 2021, 32, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Mashchak, A.D.; Jaceldo-Siegl, K.; Utt, J.T.; Knutsen, S.F.; Sveen, L.E.; Fraser, G.E. Dairy Foods, Calcium Intakes, and Risk of Incident Prostate Cancer in Adventist Health Study–2. Am. J. Clin. Nutr. 2022, 116, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.P.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.H.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global Incidence and Prevalence of Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Smirne, C.; Croce, E.; Di Benedetto, D.; Cantaluppi, V.; Comi, C.; Sainaghi, P.P.; Minisini, R.; Grossini, E.; Pirisi, M. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. Livers 2022, 2, 30–76. [Google Scholar] [CrossRef]
- Kothe, B.; Klein, S.; Petrosky, S.N. Urolithin A as a Potential Agent for Prevention of Age-Related Disease: A Scoping Review. Cureus 2023, 15, e42550. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The Mitophagy Activator Urolithin A Is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef]
- Dubey, A.; Park, D.W.; Kwon, J.E.; Jeong, Y.J.; Kim, T.; Kim, I.; Kang, S.C.; Chi, K.-W. Investigation of the Biological and Anti-Cancer Properties of Ellagic Acid-Encapsulated Nano-Sized Metalla-Cages. Int. J. Nanomed. 2015, 10, 227–240. [Google Scholar] [CrossRef]
- Yu, C.; Naeem, A.; Liu, Y.; Guan, Y. Ellagic Acid Inclusion Complex-Loaded Hydrogels as an Efficient Controlled Release System: Design, Fabrication and In Vitro Evaluation. J. Funct. Biomater. 2023, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S. Enhanced Activity of Ellagic Acid in Lipid Nanoparticles (EA-Liposomes) against Acinetobacter Baumannii in Immunosuppressed Mice. Saudi J. Biol. Sci. 2023, 30, 103707. [Google Scholar] [CrossRef] [PubMed]
| Reference/ Country/ Study Type | Study Purpose/ Design | Type of Extracts/Biological Systems (Cell Lines, Animal Model, Clinical Trial)/Doses | Analysis Methods/ Tests/Biomarkers | Significantly Outcomes |
|---|---|---|---|---|
| [23] USA Analytical/ In vitro | WP phytochemical composition: GA, EA | WPE—MeOH, HCl | HPLC-UV | EA/GA ratio in WP: 4.4—Tulare cv. vs. 6.1—Chico cv. |
| WP aflatoxigenicity inhibition potential | A. flavus NRRL 25347 spore suspension (aflatoxin B1); treatment: GA, EA, TA, WPE (hexane, acetone, MeOH, H2O) | HPLC-FD: Aflatoxin B1 | WPE inhibit aflatoxigenesis ↓ Aflatoxin levels: GA to 4% of control (day 6) vs. EA to 84% of control (same day) | |
| [24] Spain Clinical | Colonic microflora Uro-A production from EA, punicalagin, and ETs rich WE | Fecal samples (6 healthy donors); Treatment: EA, punicalagin, WKE | HPLC-DAD-MS/MS: 0, 5, 24, 48, 72 h | Identified: Uro-A in all cultures No correlation between daidzein and EA metabolisms by fecal microflora |
| [25] Spain Analytical/Clinical | ET composition of WK | WKE—80% MeOH | HPLC-DAD-ESI-MS/MS | Identified: 3 ETs: pedunculagin, valoneic acid dilactone, casuarictin |
| ETs metabolism | Healthy volunteers (n = 40), WK: 35 g/day, single dose | HPLC-MS/MS, UV Urine: MeOH fractions (F1-F5) | Uro-B glucuronides: all urine fractions F3-F5 ETs, EA: ND Metabolite excretion: 16.6% | |
| [26] Slovenia Analytical | Phenolic composition: WKs vs. WP | WKE—MeOH WPE—MeOH | HPLC-DAD | Syringic acid, juglone, EA (WKE, WPE): predominant—all cvs. WP: most important source of walnut phenolics |
| [27] China, Canada Analytical | Polyphenolic profiles, AAs: J. ailanthifolia L., J. regia L. | WKEs—80% MeOH WKEs—FPA, AHPA, and BPA fractions | HPLC-DAD, LC-ESI-MS Quantification: TPC AAs: FRAP, PCL assays | EA (FPA, AHPA, BPA) and valoneic acid dilactone (AHPA, BPA): Combe, Lake vars. TPC (FPA, AHPA): J. regia > J. a. L. FRAP and PCL values (FPA, AHPA, BPA): J. r. L. > J. a. L. |
| [28] Spain, Italy Analytical | CE-MS method developed to identify and quantify phenolic and related polar compounds in WK | WKE—80% MeOH | CE–ESI-TOF-MS | New ET: (2E,4E)-8-hydroxy-2,7-dimethyl-2,4-decadiene-1,10-dioic acid 6′-O-β-D-glucopiranosyl ester Gla A, Gla B, and the new ET: 72–86% of TAPC |
| [29] Greece Analytical/ In vitro | WE phytochemical composition | PWKE—MeOH | HPLC-DAD, TLC, NMR Quantification: TPC | Identified: EA, GA, catechin, caffeic acid, and coumaric acid TPC: 16.9 ± 0.8 μM MC/g dw |
| AI activity in HAEC and osteoblastic activity in KS483 cells | HAEC cultures marked with TNF-α +/− WE (10, 50, 200 µg/mL) or EA (10−7–10−5 µM) 2KS483 osteoblastic cell cultures marked with ascorbic acid +/− WE (10, 25, 50 μg/mL) or EA (10−9–10−6 M) | Quantification: VCAM-1, ICAM-1 (ELISA) Cell viability: MTT Mineralized nodules: light microscopy | ↓ VCAM-1 and ICAM-1 expressions vs. control WE and EA: ↑ nodule formation in KS483 osteoblasts | |
| [30] USA In vitro | WKPhs and EA ability have to modulate cytokine levels and the cellular proliferation of stimulated human PBMCs | WKPhs: CE (24 h, 4 °C) HE (24 h, 56 °C) PBMC stimulation agents: PHA, α-CD3, or PMA/ionomycin | Cytokine levels: IL-2, IL-4, IL-13, TNF-α (ELISA kits) Proliferation assay: [3H]TdR incorporation, 24 h | Cytokine production from PHA-stimulated PBMCs: EA: ↑ IL-2 EA, CE, HE: ↓ IL-13 CE, HE: ↓ TNF-α IL-4: no changed WKPhs and EA: ↓ stimulated [PHA, α-CD3 or PMA/ionomycin] PBMC proliferation in a dose-dependent manner |
| [31] Spain Analytical/Clinical | ET composition of PWK | PWKE—80% MeOH | HPLC-DAD-MS/MS | Identified: EA and 10 ETs |
| ETs, EA, and Uros identifying and quantifying in human prostate gland | PCa and BPH male patients (n = 14), PWK: 35 g/day, 3 days | HPLC-DAD-MS/MS: urine, plasma, prostate | Identified: Uro-A glucuronide, Uro-B glucuronide (traces), dimethyl ellagic acid Small number of prostates containing metabolites | |
| ETs, EA, and Uros effect on gene expression | Expression levels of CDKN1A, MKi-67 and c-Myc: prostate | Walnut ETs: no apparent effect on the expression of p21, c-Myc or MKi-67 in the prostate gland | ||
| [32] USA Clinical | Effect of walnut meal on metabolic profile | Healthy volunteers (n = 16), WK: 90 g/day, single dose | HPLC-UV, HPLC-MS: urine TPC: plasma | Uro-A (urine): ↑ following walnut meal GCG, ECG, EGCG (plasma): ↑ at 1 h |
| Effect of a walnut meal on postprandial OS and antioxidants | AAs: FRAP, ORAC: plasma Lipid oxidation: MDA, oxidized LDL: plasma Lipidic profile, uric acid: serum | AUC0–5 h: ↓ MDA ↑ hydrophilic and lipophilic ORAC No change: total phenols, FRAP, uric acid ↓ Oxidized LDL at 2 h | ||
| [11] Spain Analytical | Screening the complete profile of WPhs | WKE—60% acetone | LC-ESI-LTQ-Orbitrap-MS Quantification: TPC AAs: ABTS+, DPPH assays | Identified: 120 compounds: hydrolysable/condensed tannins, flavonoids, phenolic acids (8 new walnut ETs) TPC: 2,464 ± 22 mg GAE/100 g ABTS+: 21.4 ± 2.0 mmol TE/100 g DPPH: 25.7 ± 2.1 mmol TE/100 g |
| [33] Spain Clinical | ETs and EA metabolism by human GM; urolithin phenotypes | Healthy volunteers (n = 20), WK: 30 g/day, 3 days | HPLC-DAD-ESI-IT-MS/MS: urine | Phenotype A: 65% Phenotype B: 20% Phenotype 0: 15% |
| [34] Spain Clinical | Uros chromatographic and spectroscopic characterization after ET and EA food intake | Healthy volunteers (n = 10), WK: 30 g/day, 3 days | HPLC-DAD-ESI-Q (MS) UPLC-ESI-QqQ (MS/MS) UPLC-ESI-QTOF(MS/MS) UV Urine, feces | Uros characterization: LC coupled to DAD and/or MS detectors (QqQ, QTOF) UV RRFs of different Uros compared with UV spectrum of Uro-A and EA: relevant for Uro quantification and identification |
| [35] Korea Analytical/ In vitro | WPhE: phytochemical composition | WPhE—50% MeOH | HPLC-PDA | Identified/quantified: EA, GA, (+)-catechin, chlorogenic acid |
| Anti-CSC potential evaluation of WPhE and its bioactive compounds | CD133+CD44+ isolated from HCT116 cells and incubated with WPhE (0, 10, 20, and 40 μg/mL), (+)-catechin, chlorogenic acid, EA, and GA | Cell proliferation assay: MTT RT-PCR Western blot: protein expressions Clonogenic assay Sphere formation assay | WPhE: ↓ Cell growth, ↑ cytokeratin 20 (CK20) expression ↓ CD133, CD44, DLK1, Notch1, β-catechin, and p-GSK3β expressions, ↓ self-renewal CSCs capacity: colony formation and non-adherent spheroid formation | |
| [36] Spain, Turkey Clinical | UMs identification | Healthy normoweight volunteers (n = 20), WK: 30 g/day, 3 days | UPLC-ESI-qToF-MS: urine | UM-A: 70% UM-B: 20% |
| UMs and CMR factors | Lipidic/glycemic profile: plasma Bacterial DNA extraction, real-time qPCR, Gordonibacter spp.: feces | CMR factors and Uros: no correlations Fecal Gordonibacter correlations: Uro-A: positive Isouro-A + Uro-B: negative | ||
| [37] Spain, Belgium Clinical | UMs identification | Healthy volunteers (n = 27), PWK: 33 g/day, 3 days | UPLC-ESI-QTOF-MS: urine | Metabotype stratification: UM-A: Uro-A UM-B: Uro-B, IsoUro-A, Uro-A UM-0—no Uros |
| UMs microbiota modulation | GM composition: 16S RNA illumina sequencing and qPCRs Microbial activity: SCFA analysis: feces | UM-B GM: sensitive to walnut intervention Blautia, Bifidobacterium, Coriobacteriaceae fam. members ↑ in UM-B Lachnospiraceae fam. members ↓ in UM-A Coprococcus and Collinsella ↑ in UM-A and UM-B Walnut: modulates GM in a UM-depending manner and ↑ SCFA production | ||
| [38] Spain Analytical/ Clinical | Free EA quantification in PWK | PWK—acid hydrolysis | HPLC-DAD-MS/MS | Identified/quantified: EA, primary precursor of Uros |
| Uros identification in human breast milk | Healthy postpartum women (n = 27), PWK: 30 g/day, 3 days | HPLC-DAD-ESI-Q-MS: urine UPLC-ESI-QTOF MS: breast milk | Mothers UMs (urine): UM-A (44%), UM-B (55%); governed the breast milk urolithin profile Total Uros (breastmilk): 8.5–176.9 nM | |
| Kinetics of Gordonibacter colonization in newly born babies | Babies (n = 30) stool samples at 1, 4, 6, and 12 months | qPCR: Gordonibacter: breast milk, infant feces | Fecal Gordonibacter ↑ to 78% in 4-month-old babies from UM-A mothers, ↓ for 6-month-old babies from UM-B mothers Pattern of Gordonibacter in babies: conditioned by their mother’s UM | |
| [39] Japan Analytical | Identification of characteristic component(s) in walnuts: quality evaluation | WKE—80% MeOH | NMR, LC-HR-ESI-MS/MS | Identified: 30 compounds New: Gla C, EA 4-O-(3′-O-galloyl)-β-D-xyloside, platycaryanin A methyl ester |
| [9] Slovenia Analytical | Identification and quantification of major phenolic constituents in WP and PWK | WPE—MeOH PWKE—MeOH | HPLC–MS/MS Quantification: TPC | Identified/quantified: 56 compounds WPE: 19 ETs, 12 EA derivatives, 4 anthocyanins, 5 other phenols (14 new) PWKE: 5 ETs, 10 dicarboxylic acid derivatives, 1 phenol (13 new) TPC: WP (~1000-fold) > PWK TPC intake/1 WK: highest Franquette, Rubina cvs.; lowest Krka cv. |
| [40] China Analytical/ In vivo | Identification of polypeptides and polyphenols in defatted WK | WK WKH: 1 M HCl, hydrolyzed: pepsin (E/S: 6/100, w/w), 37 °C for 1.0 h, tripsin (E/S: 1:25, w/w), 37 °C for 2.0 h | UPLC-Q-Orbitrap-MS Quantification: TPC (WK, WKH) | Identified 42 compounds: 13 ETs, 10 EA derivatives, 5 gallotannins, 1 ketone,1 flavanone, 2 esters, 2 flavonoids, 5 organic acids, 3 simple phenolic acids The major polyphenols: ETs TPC: WK: 4.90 mg GAE/g WKH: 40.17 mg GAE/g |
| Protective effect of defatted walnut kernel hydrolysates (WKH, obtained by simulated GI digestion) in mice with d-gal-induced aging | SD mice, male—5 groups: normal (NG), model (MG), low-dose (WKH-L), medium-dose (WKH-M), and high-dose (WKH-H) groups Doses: 300 mg d-gal/kg bw/day (i.p.); WKH: 75, 150, and 300 mg/kg bw/day (i.g.) for 6 weeks | Biomarkers of OS: SOD, T-AOC, MDA in serum, liver, kidney, and brain tissues Histopathological analysis: liver and kidney Immunohistochemistry: TNF-α, IL-1β, and IL-6—liver | WKHs: recover T-AOC and SOD activity, and ↓ MDA in tissues and serum in d-gal-induced aging mice. WKH: protect the tissue structure of the liver and kidney and reduce the inflammatory biomarker expressions (TNF-α, IL-1β, and IL-6) in liver of mice with d-gal-induced aging | |
| [41] China Analytical | Phenolic profiles and AAs of free, esterified and bound phenolic compounds in WK | PWKE—70% MeOH, PWKE—70% EtOH PWKE—70% acetone | UPLC-ESI-MS/MS Quantification: TPC, TFC AAs: DPPH, ABTS, TAC assays | EA, GA, ferulic acid, sinapic acid, caffeic acid: all forms EA: the major constituent: free form (7 times) > esterified form Walnut phenolics: free: 51.1–68.1%; bound: 21.0–38.0%; esterified: 9.7–18.7% Free phenolics: highest radical scavenging activity (IC50: DPPH, 15.5 µg/mL; ABTS, 13.6 µg/mL) |
| [42] Israel Clinical | WK metabolic profile | Healthy volunteers (n = 284), WK—28 g/day, 18 months (MED) | HPLC-QTOF: urine | Identified: Uro-A, tyrosol |
| Effect of MED diet combined with physical activity on age-related brain atrophy | Lipidic profile, glycemia a jeun, HOMA-IR: plasma MRI-derived brain anatomical parameters: HOC and LVV | Participants ≥ 50 y of age: weight loss, lower HOMA-IR, and lower TG conc.: associated with lower decline in HOC. ↑ walnut consumption: associated with lower decline in HOC | ||
| [43] Korea Analytical/ In vitro/ In vivo | WKE phytochemical profile | WKE—80% EtOH | UPLC Q-TOF/MS | Identified: 2 EA derivatives, 4 ETs, and 1 flavanol |
| Neuroprotective effect of WKE from GC on: (1) neuronal PC12 and hippocampal HT22 cell lines exposed to H2O2 or high glucose concentrations | PC12 and HT22 cell lines; H2O2: 200 μM; Glucose: 50 μM; WKE: 20 μg/mL and 50 μg/mL | Cell viability: MTT Intracellular ROS content: DCF-DA method | GC (20 and 50 μg/mL): - ↑ cell viability and ↓ ROS production in both cell lines | |
| (2) cognitive impairment in an animal model of HFD-induced diabetes | Male C57BL/6 mice—4 groups (n = 8): NC group (normal diet), HFD group (HFD for 12 weeks), GC20 and GC50 groups (HFD for 12 weeks + 20 and 50 mg/kg bw, respectively, orally, for 4 weeks) | Behavioral tests: Y-Maze, passive avoidance, and Morris Water Maze (MWM) tests Biochemical tests: LDH, TG, TC, HDL-c, LDL-c, HDL-c/TC ratio (HTR) OS biomarkers: FRAP and AGEs (serum) MDA (brain and liver) Cerebral cholinergic system: ACh level, AChE activity Mitochondrial activity in brain: ROS, MMP Western blot: protein expressions in brain | GC20 and GC50 restored the HFD-altered behavior: the ability, the step-through latency, the escape latency time, and the time in the W zone GC improved lipidic profile: ↓ total WAT and liver fat mass, ↓ LDL-c, ↓ LDH and TG vs. HFD GC50: - ↑ serum AA in FRAP assay and ↓ AGEs vs. HFD GC20 and GC50 attenuated cholinergic system impairment: ↑ ACh level, ↓ AChE activity,↓ AChE/β-actine, and ↑ ChAT/β-actine relative expressions vs. HFD GC20 and GC50: - ↓ mitochondrial ROS production and ↑ MMP levels in cerebral tissues vs. HFD - synergically regulated the p-JNK, p-Akt, p-tau, IDE, Aβ, BAX, and caspase-3 expressions vs. HFD ↓ neuroinflammation: ↓ protein expression of TNF-α, IL-1β, p-NFκB, caspase-1, and ↑ HO-1 expressions (p < 0.05 for all proteins for both GC20 and GC50 vs. HFD) | |
| [44] China Analytical/ In silico/ In vitro/ In vivo | DWPE phytochemical profile | DWPE—80% EtOH | UPLC-Q-Exactive Orbitrap MS | Identified: 36 compounds: 9 new derivatives (dicarboxylic acid glycosides) |
| Identification of the DWPE metabolites in rats | Male SD rats (n = 12): 10 g DWPE/kg (i.g.) | Metabolite profile: in rat plasma, bile, urine, and feces samples by UPLC-Q-Exactive Orbitrap MS | 52 metabolites of DWPE identified in vivo (26 in plasma, 24 in bile, 36 in urine, and 13 in feces), derived from GA, EA and Gla A | |
| Pathway mechanism screening of DWPE metabolites against NAFLD and NASH by network pharmacology | Network pharmacology: Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways | 11 potential pathways identified, including inflammation, PPAR signaling, and nitrogen metabolism pathways | ||
| Protective effect of DWPE against NAFLD: (1) on Oleic acid (OA)-induced HepG2 cells; | OA: 0.25 mM; DWPE: 25–200 μg/mL; L-ornitine L-aspartate (LOLA): 50 μM | Cell viabilities: at 25, 50, 100 and 200 μg/mL of DWPE; OA-induced cellular steatosis by Oil Red O staining; Western blot: protein expressions | DWPE (100 μg/mL): - ↓ intracellular lipid accumulation - ↓ intracellular ammonia concentration - ↑ CA2 and CPS1 expressions | |
| (2) in HFD-induced mice | Male C57BL/6 mice—5 groups (n = 8): (1) ND (normal diet); (2) HFD (HFD for 12 weeks); (3) DWPE-H and (4) DWPE-L (HFD + 1.2 g or 0.6 g DWEP/kg, respectively, orally, for 12 weeks); (5) LOLA (HFD + LOLA granules, 1.35 g/kg, orally, for 12 weeks) | Biochemical tests: TG, TC, ammonia levels (serum) Western blot: protein expressions in liver | - ↓ TG and TC levels in serum in DWPE-H group - ↓ ammonia concentrations in serum - ↑ CA2 and CPS1 expressions in liver | |
| [45] Spain Clinical | UMs identification | PD patients (n = 52) and healthy controls (HC) (n = 117), WK: 30 g/day, 3 days | UPLC-ESI-QTOF-MS: urine | UM-A: PD—45%, HC—57% UM-B: PD—27%, HC—34% UM-0: PD—27%, HC—9% Significant ↑ of UM-0 as the disease severity ↑ |
| Uros as biomarkers of gut dysbiosis and stage disease in PD patients | GM composition, UMs: feces | GM of patients with UM-0 and highest severity PD: ↑ Enterobacteriaceae ↓ Lachnospiraceae members and Gordonibacter | ||
| [46] Israel Clinical | WK metabolic profile | Obese volunteers, BMI = 31.2 kg/m2 (n = 294) WK: 28 g/day, 18 months | HPLC-QTOF: urine | Identified: Uro-A |
| Effect of MED diet on visceral adiposity | Magnetic resonance imaging (MRI)—to quantify the abdominal adipose tissues | MED diet: moderate weight loss (−2.7%), WC loss (−4.7%) MED diet VAT loss (−6.0%) Vs. green-MED-diet (−14.1%; p < 0.05) ↑ total plasma polyphenols (hippuric acid), Uro-A (urine)—significantly related to greater VAT loss (p < 0.05) | ||
| [47] China Analytical/ In vitro | WPhE phytochemical profile | WPhE—75% EtOH | UPLC-QTOF MS/MS | Identified: 13 phenolic compounds, of which 10 ETs (61.8% of TAPC, w/w) |
| The neuroprotective effect of WPhE and Uro-A on H2O2-induced damage in SH-SY5Y cells and the mechanisms involving CREB signaling pathways | Human neuroblastoma SH-SY5Y cell cultures pretreated with WPhE (50–150 μg/mL) or Uro-A (2.5–20 μM) for 12 h and then exposed to H2O2 (200 μM) | Cell viability: MTT Cell apoptosis: by Hoechst 33342 staining Biochemical analysis: Extracellular LDH activity, intracellular Ca level, ROS, SOD, CAT Western blot analysis: protein expressions in the absence and in the presence of H89, a PKA inhibitor (pretreatment with 10 μM for 1 h) | Pretreatment with WPhE or Uro A: - protect SH-SY5Y cells viability against H2O2 damage: completely by >75 μg/mL WPhE; in a reversed ”U”-shape manner by Uro-A, with a maximum effect at 10 μM - ↓ number of apoptotic cells and normalize the nuclear chromatin morphology - ↓ (prevents) extracellular LDH leakage and intracellular Ca overload as well as ROS level - ↑ (prevents) SOD and CAT activities - ↑ cAMP-dependent PKA activity, pCREB (Ser133) and BDNF expressions PKA inhibitor H89 pretreatment: abolished the protective effects of WPhE and Uro-A | |
| [48] China Analytical | Conversion of ETs into free EA in WKs during baking: investigating the EAC in the FPA, AHPA, and BPA fractions | WKE—60% acetone | LC-MS Quantification: EAC, ETC, and TPC | 8 ETs: main precursors of EA in WK EAC in FPA: max. (5.17 ± 0.30 mg/g dw) after baking at 165 C for 30 min; ↑ by 99.52% compared to control. ETC in AHPA and BPA: ↓ by 89.14%, and 26.08% TPC: max. (102.29 ± 7.75 mg GAE/g dw) after baking at 150 °C for 30 min Baking: conversion of ETs in AHPA and BPA to EA in FPA |
| [49] Israel Clinical | WK metabolic profile | Healthy volunteers, abdominal obesity or dyslipidemia (n = 256), WK: 28 g/day, 8 months (MED) | HPLC-QToF: urine | Identified: Uro-A, Uro-C and hydroxytyrosol |
| Effect of polyphenols on DNA methylation-assessed biological age attenuation | Biological aging epigenetic clocks: DNA methylation (Illumina EPIC array): blood | All interventions: did not differ in terms of changes between mAge clocks MED inversely associated with biological aging | ||
| [50] China Analytical | Identification of key antioxidants in free, esterified, and bound forms in WKs and WP | PWKE: 70% acetone WPE: 70% acetone | Phytochemical profile: UPLC-MS/MS Quantification: TPC AA: DPPH assay | Identified/quantified (PWKE, WPE): 31 phenolic compounds: phenolic acids, flavonoids, and 1 proanthocyanidin EA: the most abundant component in WKs (62.9%), and in WP (68.0%) BPA forms: WPE > PWKE TPC levels of all forms: positively correlated with AAs (R: 0.76–0.94, p < 0.05) |
| [51] China In vivo/ Molecular docking | Active fractions and substances of walnut kernel in a scopolamine-induced AD animal model | Male ICR mice—9 groups (n = 10): control, model (scopolamine, 3 mg/kg/day for 10 consecutive days), donepezil (positive, 0.65 mg/kg/day), WK, DWP, WO, WKP, WKOA, and WKPS (i.g. for 56 days, equivalent dose of 15.6 g crude W/kg) | Morris Water Maze Test Biochemical and ELISA: ACh, MDA, SOD, TNF-α, IL-6, IL-1 Histopathology analysis: hippocampus and cortex tissues Western blot: protein expressions in hippocampus and cortex | - ↓ escape latency time in WO, WKOA, and WKPS groups - ↑ attention time in WK, DWP and WKOA groups - ↑ spatial memory significant grater in WKOA group vs. other groups - WKOA and WKP restored Ach levels in hippocampus vs. model - WO, WKOA and WKP restored ACh levels in cerebral cortex vs. model - ↓ MDA levels in hippocampus and cortex by all WK fractions (but not WK) WKP and WKOA attenuated histopatological damage in scopolamine-induced AD mice brain - ↓ NFκB protein level in hippocampus in WKOA and WO groups as well as in cortex in WKOA group |
| Distribution and metabolism of WKOA in brain tissue in AD model rat | Male SD rats (n = 6): control group and model group (scopolamine for 10 days) received WKOA (20x pharmaco-dynamic dose) on day 10 | Metabolic profile in brain: UPLC-Q-Exactive Orbitrap MS | - 8 metabolites identified in rat brain after WKOA administration: p-hydroxycinnamic acid + 2H + sul; glansreginic acid + 2H + H2O; ellagic acid 4-O xyloside; ethyl gallate; EA; glansreginic acid; Gla A; ethyl gallate + sul | |
| Structure–activity relationship between the screened active compounds and AChE, BChE, SOD, IL-6, IL-1β, and TNF-α | Molecular docking: Surflex-Dock Geom (SFXC) | Main function: Gla A, glansreginic acid, and glansreginic acid + 2H + H2O as AChE and BChE inhibitors; EA and ellagic acid 4-O xyloside as OS and neuroinflammation inhibitors |
| Ref. | Extract | Analytical Method | Compounds | Amount | |||
|---|---|---|---|---|---|---|---|
| [23] | Methanolic HCl extract of WP—8 walnut varieties: | HPLC-DAD | |||||
| Chandler | Ellagic acid | 10.0 ± 0.50 g/100 g dw | |||||
| Chico | Ellagic acid | 11.0 ± 0.70 g/100 g dw | |||||
| Serr | Ellagic acid | 11.8 ± 0.03 g/100 g dw | |||||
| Payne | Ellagic acid | 12.3 ± 0.10 g/100 g dw | |||||
| Hartley | Ellagic acid | 13.3 ± 0.10 g/100 g dw | |||||
| Tehama | Ellagic acid | 11.0 ± 0.15 g/100 g dw | |||||
| Tulare | Ellagic acid | 14.0 ± 0.15 g/100 g dw | |||||
| Red Zinger | Ellagic acid | 15.9 ± 0.20 g/100 g dw | |||||
| [24] | 80% methanolic extract of WK | HPLC-DAD-ESI-MS/MS | Casuarictin | ||||
| Pedunculagin | NQ | ||||||
| Valoneic acid dilactone | |||||||
| [26] | Methanolic extracts of WKs and WP—10 walnut cultivars: | HPLC-DAD | WK | WP | |||
| Cisco | Ellagic acid | 6.70 ± 0.60 mg/100 g | 128.71 ± 6.73 mg/100 g | ||||
| Fernette | Ellagic acid | 3.26 ± 0.18 mg/100 g | 60.66 ± 6.28 mg/100 g | ||||
| Ferner | Ellagic acid | 4.17 ± 0.35 mg/100 g | 89.34 ± 3.80 mg/100 g | ||||
| Rasna | Ellagic acid | 6.59 ± 0.53 mg/100 g | 124.46 ± 6.05 mg/100 g | ||||
| A-117 | Ellagic acid | 9.77 ± 0.75 mg/100 g | 266.19 ± 10.98 mg/100 g | ||||
| Franquette | Ellagic acid | 8.87 ± 0.91 mg/100 g | 200.08 ± 3.08 mg/100 g | ||||
| Adams | Ellagic acid | 5.75 ± 0.60 mg/100 g | 118.25 ± 1.86 mg/100 g | ||||
| Lara | Ellagic acid | 4.53 ± 0.31 mg/100 g | 94.03 ± 1.97 mg/100 g | ||||
| Chandler | Ellagic acid | 4.30 ± 0.26 mg/100 g | 78.36 ± 2.29 mg/100 g | ||||
| Elit | Ellagic acid | 5.09 ± 0.56 mg/100 g | 129.73 ± 3.19 mg/100 g | ||||
| [27] | FPA | AHPA | BPA | ||||
| 80% methanolic extract of defatted WK—Combe variety | HPLC-DAD, LC-ESI-MSn | Ellagic acid | 0.32 mg/g of nut | 1.30 mg/g of nut | 1.21 mg/g of nut | ||
| 80% methanolic extract of defatted WK—lake variety | Ellagic acid | 0.25 mg/g of nut | 1.33 mg/g of nut | 0.64 mg/g of nut | |||
| [28] | 80% ethanolic extract of defatted WK—3 walnut varieties: | CE–ESI-TOF-MS | Ellagic acid derivatives | ||||
| Chandler | Ellagic acid | 24.7 ± 2.1 mg/kg dw | |||||
| Howard | Ellagic acid | 12.4 ± 0.3 mg/kg dw | |||||
| Hartley | Ellagic acid | 6.9 ± 1.3 mg/kg dw | |||||
| Chandler | Ellagic acid pentoside dimer | 36.1 ± 3.6 mg/kg dw | |||||
| Howard | Ellagic acid pentoside dimer | 33.0 ± 3.9 mg/kg dw | |||||
| Hartley | Ellagic acid pentoside dimer | 37.2 ± 3.1 mg/kg dw | |||||
| Ellagitannins | |||||||
| Chandler | Glansreginin A | 76.3 ± 15.6 mg/kg dw | |||||
| Howard | Glansreginin A | 335.6 ± 22.9 mg/kg dw | |||||
| Hartley | Glansreginin A | 76.3 ± 14.3 mg/kg dw | |||||
| Chandler | Glansreginin B | 92.1 ± 30.6 mg/kg dw | |||||
| Howard | Glansreginin B | 35.5 ± 5.5 mg/kg dw | |||||
| Hartley | Glansreginin B | 99.7 ± 0.1 mg/kg dw | |||||
| Chandler | (2E,4E)-8-hydroxy-2,7-dimethyl-2,4-decadiene-1,10-dioic acid 6′-O-β-D-glucopiranosyl ester | 40.8 ± 6.21 mg/kg dw | |||||
| Howard | (2E,4E)-8-hydroxy-2,7-dimethyl-2,4-decadiene-1,10-dioic acid 6′-O-β-D-glucopiranosyl ester | 48.3 ± 4.3 mg/kg dw | |||||
| Hartley | (2E,4E)-8-hydroxy-2,7-dimethyl-2,4-decadiene-1,10-dioic acid 6′-O-β-D-glucopiranosyl ester | 32.3 ± 3.9 mg/kg dw | |||||
| [29] | Methanolic extract of peeled, defatted WK | TLC, NMR, HPLC-DAD | Ellagic acid | NQ | |||
| [31] | 80% methanolic extract of peeled WK | HPLC-DAD-MS/MS | Ellagic acid | NQ | |||
| Ellagitannins | |||||||
| Casuarictin | |||||||
| Glansrin A | |||||||
| Glansrin B | |||||||
| Glansrin C | |||||||
| Pedunculagin | |||||||
| Rugosin | |||||||
| Stenophyllanin A | |||||||
| Tellimagrandin I | |||||||
| Tellimagrandin II | |||||||
| 2,3-hexahydroxydiphenoyl-b-D-glucopyranoside | |||||||
| [11] | 60% acetone–water extract of WK | LC-ESI-LTQ-Orbitrap-MS | Ellagic acid derivatives | NQ | |||
| Ellagic acid | |||||||
| Ellagic acid pentoside isomer | |||||||
| Ellagic acid hexoside (2 isomers) | |||||||
| Ellagitannins | |||||||
| Alienanin B (3 isomers) | |||||||
| Casuarinin/casuarictin (2 isomers) | |||||||
| Euprostin A (2 isomers) | |||||||
| Eucalbanin A/cornusiin B (3 isomers) | |||||||
| Glansreginin A | |||||||
| Glansreginin B | |||||||
| Glansrin B (3 isomers) | |||||||
| Glansrin C (4 isomers) | |||||||
| Glansrin D/degalloyl rugosin F (3 isomers) | |||||||
| Heterophylliin D | |||||||
| Heterophylliin E (2 isomers) | |||||||
| HHDP-glucose (3 isomers) | |||||||
| Malabathrin A isomer | |||||||
| Oenothein B (2 isomers) | |||||||
| Pedunculagin/casuariin (bis-HHDP-glucose) (4 isomers) | |||||||
| Praecoxin A/platycariin isomer (trigalloyl-HHDP-glucose) (5 isomers) | |||||||
| Pterocarinin A (2 isomers) | |||||||
| Pterocarinin B | |||||||
| Reginin A/reginin D isomer (5 isomers) | |||||||
| Rugosin C/platycaryanin A/glansrin A (3 isomers) | |||||||
| Rugosin F | |||||||
| 2′,3′-bis-O-degalloyl rugosin F isomer | |||||||
| 1,2′,3′-tris-O-degalloyl rugosin Fisomer | |||||||
| Stenophyllanin A/B (2 isomers) | |||||||
| Stenophyllanin C (2 isomers) | |||||||
| Strictinin/isostrictinin (galloyl-HHDP-glucose) (6 isomers) | |||||||
| Tellimagrandin I (digalloyl-HHDP-glucose) (5 isomers) | |||||||
| Tellimagrandin II/pterocaryanin C (2 isomers) | |||||||
| Valoneic acid dilactone/sanguisorbic acid dilactone (2 isomers) | |||||||
| [35] | 50% methanolic extract of WK | HPLC-PDA | Ellagic acid | 12.6 mg/100 g | |||
| [38] | Peeled WK | HPLC-DAD-MS/MS | Ellagic acid | 4.1 ± 0.6 mg/g fw | |||
| [39] | 80% methanolic extract of WK | NMR, LC-HR-ESI-MS/MS | Ellagic acid derivatives | NQ | |||
| Ellagic acid | |||||||
| Ellagic acid 4-O-β-D-xyloside | |||||||
| Ellagic acid 4-O-(3′-O-galloyl)-β-D-xyloside | |||||||
| Ellagitannins | |||||||
| Casuarictin | |||||||
| Casuarinin | |||||||
| Euprostin A | |||||||
| Glansreginin A | |||||||
| Glansreginin B | |||||||
| Glansreginin C | |||||||
| Glansreginic acid | |||||||
| Glansreginic acid 8-O-β-D-glucoside | |||||||
| Isostrictinin | |||||||
| Pedunculagin | |||||||
| Platycaryanin A methyl ester | |||||||
| Pterocarinin C | |||||||
| Rugosin C | |||||||
| Rugosin C methyl ester | |||||||
| Strictinin | |||||||
| Tellimagrandin I | |||||||
| Tellimagrandin II | |||||||
| Valoneic acid dilactone methyl ester | |||||||
| [9] | Methanolic extract of WP | HPLC-MS/MS | Ellagic acid derivatives | ||||
| Ellagic acid | 17.5–23.3 mg/g fw | ||||||
| Ellagic acid pentoside | 27.3–37.2 mg/g fw | ||||||
| Galloyl ellagic acid derivative | 10.9–14.1 mg/g fw | ||||||
| Ellagic acid derivative 1 | 6.4–12.0 mg/g fw | ||||||
| Ellagic acid derivative 2 | 15.7–20.3 mg/g fw | ||||||
| Ellagic acid derivative 3 | 24.4–35.9 mg/g fw | ||||||
| Ellagic acid derivative 4 | 9.9–16.6 mg/g fw | ||||||
| Ellagic acid derivative 5 | 12.4–21.0 mg/g fw | ||||||
| Ellagic acid derivative 6 | 27.1–46.3 mg/g fw | ||||||
| Ellagic acid derivative 7 | 13.4–21.1 mg/g fw | ||||||
| Ellagic acid derivative 8 | 11.4–17.7 mg/g fw | ||||||
| Ellagic acid derivative 9 | 7.3–10.5 mg/g fw | ||||||
| Ellagitannins | |||||||
| bis-HHDP-glucose derivative | 20.2–26.1 mg/g fw | ||||||
| Castalagin/vescalagin isomer 1 | 17.8–25.4 mg/g fw | ||||||
| Castalagin/vescalagin isomer 2 | 22.1–35.9 mg/g fw | ||||||
| Castalagin/vescalagin isomer 3 | 9.5–15 mg/g fw | ||||||
| Casuarin/casuarictin isomer (galloyl-bis-HHDP glucose) 1 | 23.9–39.8 mg/g fw | ||||||
| Casuarin/casuarictin isomer (galloyl-bis-HHDP glucose) 2 | 8.6–18.7 mg/g fw | ||||||
| Pedunculagin/casuariin isomer (bis-HHDP-glucose) 1 | 6.5–13.3 mg/g fw | ||||||
| Pedunculagin/casuariin isomer (bis-HHDP-glucose) 2 | 3.1–5.8 mg/g fw | ||||||
| Pterocarinin A isomer | 0.5–2.8 mg/g fw | ||||||
| Strictinin/isostrictinin isomer (galloyl-HHDP-glucose) 1 | 1.9–2.9 mg/g fw | ||||||
| Strictinin/isostrictinin isomer (galloyl-HHDP-glucose) 2 | 7.3–9.6 mg/g fw | ||||||
| Strictinin/isostrictinin isomer (galloyl-HHDP-glucose) 3 | 7.0–9.5 mg/g fw | ||||||
| Tellimagrandin 1 isomer (digalloyl-HHDP-glucose) 1 | 6.2–10.1 mg/g fw | ||||||
| Tellimagrandin 1 isomer (digalloyl-HHDP-glucose) 2 | 3.1–14.1 mg/g fw | ||||||
| Tellimagrandin 1 isomer (digalloyl-HHDP-glucose) 3 | 18.4–27.9 mg/g fw | ||||||
| Trigalloyl-HHDP-glucose isomer 1 | 5.7–7.9 mg/g fw | ||||||
| Trigalloyl-HHDP-glucose isomer 2 | 2.9–4.0 mg/g fw | ||||||
| Trigalloyl-HHDP-glucose isomer 3 | 3.2–4.2 mg/g fw | ||||||
| Trigalloyl-HHDP-glucose isomer 4 | 17.8–27.5 mg/g fw | ||||||
| Methanolic extract of peeled WK | Ellagitannins | ||||||
| Glansreginin A | 103.0–846.7 mg/kg fw | ||||||
| Glansreginin A [M+2H] | 11.4–24.9 mg/kg fw | ||||||
| Glansreginin B | 84.1–175.8 mg/kg fw | ||||||
| Glansreginin B [M+2H] | 4.0–14.1 mg/kg fw | ||||||
| Glansreginin B hexoside | 13.7–32.6 mg/kg fw | ||||||
| [40] | WK hydrolysates | UPLC-Q-Orbitrap-MS | Ellagic acid derivatives | NQ | |||
| Ellagic acid | |||||||
| Ellagic acid pentoside | |||||||
| Ellagic acid hexoside | |||||||
| Ellagic acid-acetylglucoside | |||||||
| Ellagic acid diglycoside | |||||||
| Ellagic rhamnoside (2 isomers) | |||||||
| Methyl ellagic acid glucoside | |||||||
| Dimethyl ellagic acid | |||||||
| 3,4-O, O-methylene-3′, 4′-O-dimethyl ellagic acid | |||||||
| Ellagitannins | |||||||
| Casuarinin/casuarictin | |||||||
| Glansreginin A | |||||||
| Glansreginin B | |||||||
| Glansrin C (2 isomers) | |||||||
| HHDP-glucose | |||||||
| Pedunculagin/casuariin (bis-HHDP-glucose) (3 isomers) | |||||||
| Strictinin/isostrictinin (galloyl-HHDP-glucose) (2 isomers) | |||||||
| Tellimagrandin (digalloyl-HHDP-glucose) (2 isomers) | |||||||
| [41] | UPLC-ESI-MS/MS | Free | Esterified | Bound | |||
| 70% methanol/water extract of peeled, defatted WK | Ellagic acid | 100.085 μg/g dw | 7.518 μg/g dw | 93.275 μg/g dw | |||
| 70% ethanol/water extract of peeled, defatted WK | Ellagic acid | 112.711 μg/g dw | 10.073 μg/g dw | 79.801 μg/g dw | |||
| 70% acetone/water extract of peeled, defatted WK | Ellagic acid | 146.331 μg/g dw | 66.376 μg/g dw | 46.380 μg/g dw | |||
| [43] | 80% ethanolic extract of WK | UPLC IMS Q-TOF/MS | Ellagic acid derivatives | NQ | |||
| Ellagic acid | |||||||
| Ellagic acid-O-pentoside | |||||||
| Ellagitannins | |||||||
| Pedunculagin/casuariin isomer (bis-HHDP-glucose) I | |||||||
| Pedunculagin/casuariin isomer (bis-HHDP-glucose) II | |||||||
| Strictinin | |||||||
| Tellimagrandin I (digalloyl-HHDP-glucopyranose) | |||||||
| [44] | 80% ethanolic extract of defatted WK | UPLC-Q-Exactive Orbitrap MS | Ellagic acid derivatives | NQ | |||
| Ellagic acid | |||||||
| Ellagic acid hexoside | |||||||
| Ellagic acid 4-O-xyloside | |||||||
| 3-O-methylellagic acid-pentoside | |||||||
| Ellagitannins | |||||||
| Glansreginin A | |||||||
| Glansreginin A+2H | |||||||
| Glansreginin A-H2O | |||||||
| Glansreginin A-H2O-2H | |||||||
| Glansreginin A+H2O+2H | |||||||
| Glansreginin A+Glc | |||||||
| Glansreginin B | |||||||
| Glansreginin B+2H | |||||||
| Glansreginin B-H2O | |||||||
| Glansreginin C | |||||||
| Glansreginic acid+2H | |||||||
| Glansreginic acid 8-O-β-D-glucoside | |||||||
| HHDP-glucose (2 isomers) | |||||||
| Pedunculagin/casuariin | |||||||
| Strictinin/isostrictinin (2 isomers) | |||||||
| Valoneic acid dilactone | |||||||
| [47] | 75% ethanolic extract of defatted WK | Ellagic acid | NQ | ||||
| UPLC-Q-TOF MS/MS | Ellagitannins | ||||||
| Casuarinin/casuarictin isomer (2 isomers) | |||||||
| Glansrin B isomer | |||||||
| Glansrin C isomer | |||||||
| Pedunculagin/casuariin (2 isomers) | |||||||
| Praecoxin A/platycariin isomer | |||||||
| Sanguisorbic acid dilactone (2 isomers) | |||||||
| Strictinin/isostrictinin (2 isomers) | |||||||
| Tellimagrandin I (4 isomers) | |||||||
| Valoneic acid dilactone (2 isomers) | |||||||
| [48] | 60% acetone–water extracts defatted WK | LC-MS | Ellagic acid derivatives | NQ | |||
| Ellagic acid | |||||||
| Ellagic acid pentoside isomer | |||||||
| Ellagic acid hexoside (HHDP-hexoside) | |||||||
| Ellagitannins | |||||||
| Casuarinin/casuarictin (galloyl-bis-HHDP-glucose) (2 isomers) | |||||||
| Glansrin C(trigalloyl-HHDP-glucose) (2 isomers) | |||||||
| HHDP-glucose (2 isomers) | |||||||
| Pedunculagin/casuariin (bis-HHDP-glucose) (2 isomers) | |||||||
| Praecoxin A/platycariin (trisgalloyl-HHDP-glucose) (2 isomers) | |||||||
| Strictinin/isostrictinin isomer (galloyl-HHDP-hexoside) | |||||||
| [50] | Free | Esterified | Bound | Total | |||
| 70% acetone–water extracts of peeled, defatted WKs | UPLC-MS/MS | Ellagic acid | 56.49–164.95 μg/g dw | 5.83–28.10 μg/g dw | 0.82–5.58 μg/g dw | 109.88 μg/g dw | |
| 70% acetone–water extracts of WS | Ellagic acid | 448.15–929.34 μg/g dw | 600.99–724.70 μg/g dw | 254.32–602.69 μg/g dw | 1666.90 μg/g dw | ||
| Study Type/ Reference | Study Design/Biological Cultures, Animal Model, Participants | Walnut Treatment/ Control | Biological Material Analyzed/ Samples Extracts Type | Analytical Method | Compounds/ Amount/ Metabotypes |
|---|---|---|---|---|---|
| In vitro [25] | 6 healthy donors feces age: 25–30 y | Walnut extracts: EA:10 µg/mL ETs: 110 μg EA Eq/mL Punicalagin: 100 µg/mL Control: Daidzein: 1 g/mL | Feces samples: microflora cultures; diethyl ether | HPLC-DAD-MS/MS | Uro-A (48 h) EA extract: V2: 294.81 µg/100 mL fecal culture V3: 620.65 µg/100 mL fecal culture ET extract: V2: 386.37 µg/100 mL fecal culture V3: 321.55 µg/100 mL fecal culture |
| In vivo [44] | Male SD rats (n = 12), 180–220 g | DWPE: 10 g/kg (i.g.) | Plasma samples: (0, 1, 2, 4, 8, 10, 12, 24, 36, 48 h); MeOH extract Bile samples: (12 h); MeOH, 0.1% formic acid in H2O Urine and feces samples: (12 h), MeOH | UPLC-Q-Exactive Orbitrap MS | Ellagic acid—NQ Glansreginin A—NQ Glansreginic acid—NQ Glansreginic acid 8-O-β-D-glucoside—NQ Uro-M5—NQ Uro-D—NQ Uro-C—NQ |
| In vivo [51] | Male ICR mice (n = 10), 9 groups | WK, DWP, WO, WKP, WKOA, and WKPS: 15.6 g kg/day (i.g.), 56 days Control model: Scopolamine: 3 mg/kg/day, 10 days Donepezil: 0.65 mg/kg/day | Brain samples: 0.1% ACN, MeOH | UPLC-Q-Exactive Orbitrap MS | WKOA: p-Hydroxycinnamic acid + 2H + sul—NQ Glansreginic acid + 2H + H2O—NQ Ellagic acid 4-O xyloside—NQ Ethyl gallate—NQ EA—NQ Glansreginic acid—NQ Glansreginin A—NQ Ethyl gallate + sul—NQ |
| Clinical [24] | CT: 40 healthy (20 females), Age (mean): 29 y | WK—35 g/day (191 mg EA), 1 dose Control: ET-free diet | Urine samples: (8, 16, 32, 40, 56 h), fractions F1-F5, MeOH | HPLC-ESI- MS/MS, UV HPLC–APCI–MS/MS, UV | F1→F5: ETs, EA—ND Uro-B gluc: F1: ND F2: <LOQ F3: 8.3 ± 18.4 mg F4: 10.7 ± 20.4 mg F5: 12.6 ± 14.5 mg |
| Clinical [31] | RCT: 14 PCa and BPH male patients, age: 68.9 ±7.6 y (56–90) | PWK—35 g/day, 3 days Control: ET-free diet | Urine samples: | HPLC-DAD-MS/MS, UV | Uro-A gluc: 9 patients > 5 µM, 4 patients < 5 µM, 1 patient <LOQ |
| Plasma samples: MeOH | Uro-A gluc: 0.11±0.05 mM Uro-C gluc—NQ Uro-C methyl ether glucuronide—NQ Dimethyl ellagic acid glucuronide—NQ | ||||
| Prostate samples: MeOH: HCl:H2O (79,9:0,1:20, v:v:v), MeOH | Uro-A gluc: 0.5–2 ng/g Uro-B gluc—NQ Dimethyl ellagic acid glucuronide—NQ | ||||
| Clinical [32] | RCT: 16 healthy (10 females), age: 26 y (23–44) | WK—90 g/day, 1 dose Control: ET-free diet | Urine samples: (0–12 h, 12–24 h), 2% formic acid/MeOH (9:1 v/v); diethyl ether Enzymatic treatement: β-glucoronidase + sulfatase | HPLC-UV HPLC-MS | 0–12 h/12–24 h: 3,4-Dihydroxyphenylacetic acid: 0.375 ±197 mM/0.401 ± 210 mM 4-Hydroxyphenylacetic acid: 48.90 ± 25.37 mM/72.44 ± 36.15 mM 4-Methoxyphenylacetic acid: 4.59 ± 1.82 mM/7.71 ± 1.70 mM (p < 0.05) Uro-A: 20.44 ± 32.18 μM/100.59 ± 114.86 μM |
| Plasma samples: (1 h, 2 h), MeOH | LS Mean, 1 h/2 h: Gallocatechin gallate: 4.63 ng/mL (p < 0.05)/1.33 ng/mL Epicatechin gallate: 12.77 ng/mL (p < 0.05)/6.18 ng/mL Epigallocatechin gallate: 108.6 ng/mL (p < 0.05)/26.60 ng/mL | ||||
| Clinical [33] | CT: 20 healthy (9 females), age: 21–55 y | WK- 30 g/day (162.8 mg EA), 3 days Control: ET-free diet | Urine samples: 0.1% formic acid in H2O | HPLC-DAD-ESI-IT-MS/MS, UV | UM-A—65% UM-B—20% UM-0—15% |
| Clinical [34] | CT: 10 healthy, age: 21–55 y | PWK—30 g/day, (5.1 mg free EA/g), 3 days Control: ET-free diet | Urine samples: 0.1% formic acid in H2O | HPLC-DAD-ESI-Q (MS) UPLC-ESI-QqQ (MS/MS) UPLC-ESI-QTOF (MS/MS) HPLC-UV | Urine a (mean ± SD): Uro-A 3-gluc: 70.0 ± 57.4 mg/24 h IsoUro-A 3-gluc: 34.3 ± 36.3 mg/24 h Uro-B gluc: 88.8 ± 1.2 mg/24 h IsoUro-A: 1.0 ± 0.60 mg/24 h Uro-A: 1.7 ± 1.5 mg/24 h Uro-B: 1.0 ± 0.6 mg/24 h |
| Feces samples: 0.1% HCl in MeOH:H2O (80:20, v/v), MeOH | Feces b (mean ± SD): Uro-D: 6.4 ± 1.4 μg/g Uro-M6: 17.2 ± 7.3 μg/g Uro-C: 74.4 ± 88.0 μg/g Uro-M7: 49.9 ± 38.4 μg/g IsoUro-A: 217.8 ± 291.9 μg/g Uro-A: 121.2 ± 85.0 μg/g Uro-B: 108.6 ± 155.4 μg/g | ||||
| Clinical [36] | CT: 20 healthy normoweight (9 females), age: 33.6 ± 10.2 y | PWK—30 g/day, 3 days Control: Pomegranate extract: 450 mg/day Nuts: 15 g-walnuts, 7.5 g-hazelnuts, 7.5 g-almonds/day | Urine samples: 0.1% formic acid in H2O | UPLC-ESI-QTOF MS | UM-A: normoweight (70%), overweight–obese (57%), MetS (50%) UM-B: normoweight (20%), overweight–obese (31%), MetS (41%) |
| Clinical [37] | CT: 27 healthy, (15 females), age: 39.5 ± 7.3 y | PWK—33 g/day, 3 days Control: ET-free diet | Urine samples: 0.1% formic acid in H2O | UPLC-ESI-QTOF-MS | UM-A—52% UM-B—48% UM-0—0% |
| Clinical [38] | Trial 1—Pilot study: 11 healthy postpartum women, Trial 2—CT: 27 healthy postpartum women | PWK—30 g/day, 3 days Control: ET-free diet | Breast milk samples: (24, 48, 72 h), ACN/formic acid (99:1, v/v), MeOH | UPLC-ESI-QTOF-MS | Trial 1 Uro-A, IsoUro-A, Uro-B, Uro-A gluc, IsoUro-A gluc, Uro-A sulfate, Uro-B gluc, Uro-B sulfate Total urolithins: 8.5–176.9 nM Trial 2 Uro-A gluc—UM-A: 27.6 ± 22.9 nM; UM-B: 31.1 ± 24.8 nM Uro-A sulfate—UM-A: 7.9 ± 1.8 nM; UM-B: 17.2 ± 11.8 nM IsoUro-A gluc—UM-A: <LOD; UM-B: 14.8 ± 8.6 nM Uro-B gluc—UM-A: <LOD; UM-B: 19.8 ± 21.6 nM Uro-B sulfate—UM-A: <LOD; UM-B: <LOD Uro-A—UM-A: 4.7 ± 1.0 nM; UM-B: 3.3 ± 2.6 nM IsoUro-A—UM-A: <LOD; UM-B: 2.7 ± 1.4 nM Uro-B—UM-A: <LOD; UM-B: 4.3 nM |
| Urine samples: 0.1% formic acid in H2O | HPLC-DAD-ESI-Q-MS | Trial 1: UM-A (57%): 28.3 ± 12.1 nM; UM-B (43%): 28.8 ± 14.6 nM; UM-0: 0% Trial 2: UM-A: 44%, UM-B: 55%; UM-0: 0% | |||
| Clinical [42] | RCT, parallel: 284 healthy (33 females), age: 51.1 ± 10.6 y | WK—28 g/day, 18 months MED diet (+440 mg polyphenols/day) Control: HDG diet | Blood and urine samples: 12 h fast, baseline, 18 months | HPLC-QTOF Q-TOF-LC/MS | Urine: Uro-A—NQ |
| Clinical [45] | CT: 52 PD patients (21 females), age: 68 ± 8 y (44–88) 117 healthy (48 females), age: 60 ± 6 y (44–72) | WK—30 g/day, 3 days | Urine samples: 0.1% formic acid in H2O | UPLC-ESI-QTOF-MS | PD patients: UM-A: 45%, UM-B: 27.5%, UM-0: 27.5% Healthy control: UM-A: 57%, UM-B: 34%, UM-0: 9% |
| Clinical [46] | RCT, parallel: 286 obese participants (34 females), BMI = 31.2 kg/m2 VAT = 29%, age: 50.8 ± 10.4 y | WK—28 g/day, 18 months MED diet (+440 mg polyphenols/day) Control: HDG diet | Blood and urine samples: 12 h fast, baseline, 18 months | HPLC-QTOF Q-TOF-LC/MS | Urine: Uro-A—NQ |
| Clinical [49] | RCT, parallel: 256 healthy (28 females), age: 51.3±10.6 y | WK—28 g/day, 18 months MED diet (+440 mg polyphenols/day) Control: HDG diet | Blood and urine samples: 12 h fast, baseline, 18 months | HPLC-QTOF Q-TOF-LC/MS | Urine: Uro-A—NQ Uro-C—NQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateș, L.; Banc, R.; Zaharie, F.A.; Rusu, M.E.; Popa, D.-S. Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic Review. Antioxidants 2024, 13, 974. https://doi.org/10.3390/antiox13080974
Mateș L, Banc R, Zaharie FA, Rusu ME, Popa D-S. Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic Review. Antioxidants. 2024; 13(8):974. https://doi.org/10.3390/antiox13080974
Chicago/Turabian StyleMateș, Letiția, Roxana Banc, Flaviu Andrei Zaharie, Marius Emil Rusu, and Daniela-Saveta Popa. 2024. "Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic Review" Antioxidants 13, no. 8: 974. https://doi.org/10.3390/antiox13080974
APA StyleMateș, L., Banc, R., Zaharie, F. A., Rusu, M. E., & Popa, D.-S. (2024). Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic Review. Antioxidants, 13(8), 974. https://doi.org/10.3390/antiox13080974








