Characterization of Phenolic Profile and Biological Properties of Astragalus membranaceus Fisch. ex Bunge Commercial Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Materials
2.2. Standards, Solutions, and Reagents
2.3. Sample Preparation
2.4. Phytochemical Composition
2.4.1. Chromatographic Conditions
2.4.2. Total Phenolic Contents (TPCs)
2.4.3. Total Flavonoid Contents (TFCs)
2.4.4. Total Phenolic Acid Contents (TPACs)
2.5. Activity Assays
2.5.1. In Vitro Antioxidant Activities
2.5.2. Antibacterial Activity Assay
2.5.3. Microtiter Assays for AChE/BChE and TYR Inhibition
ChE Inhibition
TYR Inhibition
Data Processing for Enzyme Inhibition Assays
2.6. Statistical Analysis
3. Results
3.1. Analysis of TPCs, TFCs, and TPACs
3.2. Analysis of Individual Phenolic Compounds
3.3. Evaluation of Antioxidant Activity
3.4. Evaluation of Antibacterial Activity
3.5. AChE/BChE and TYR Inhibitory Activity
3.6. Correlation Analysis
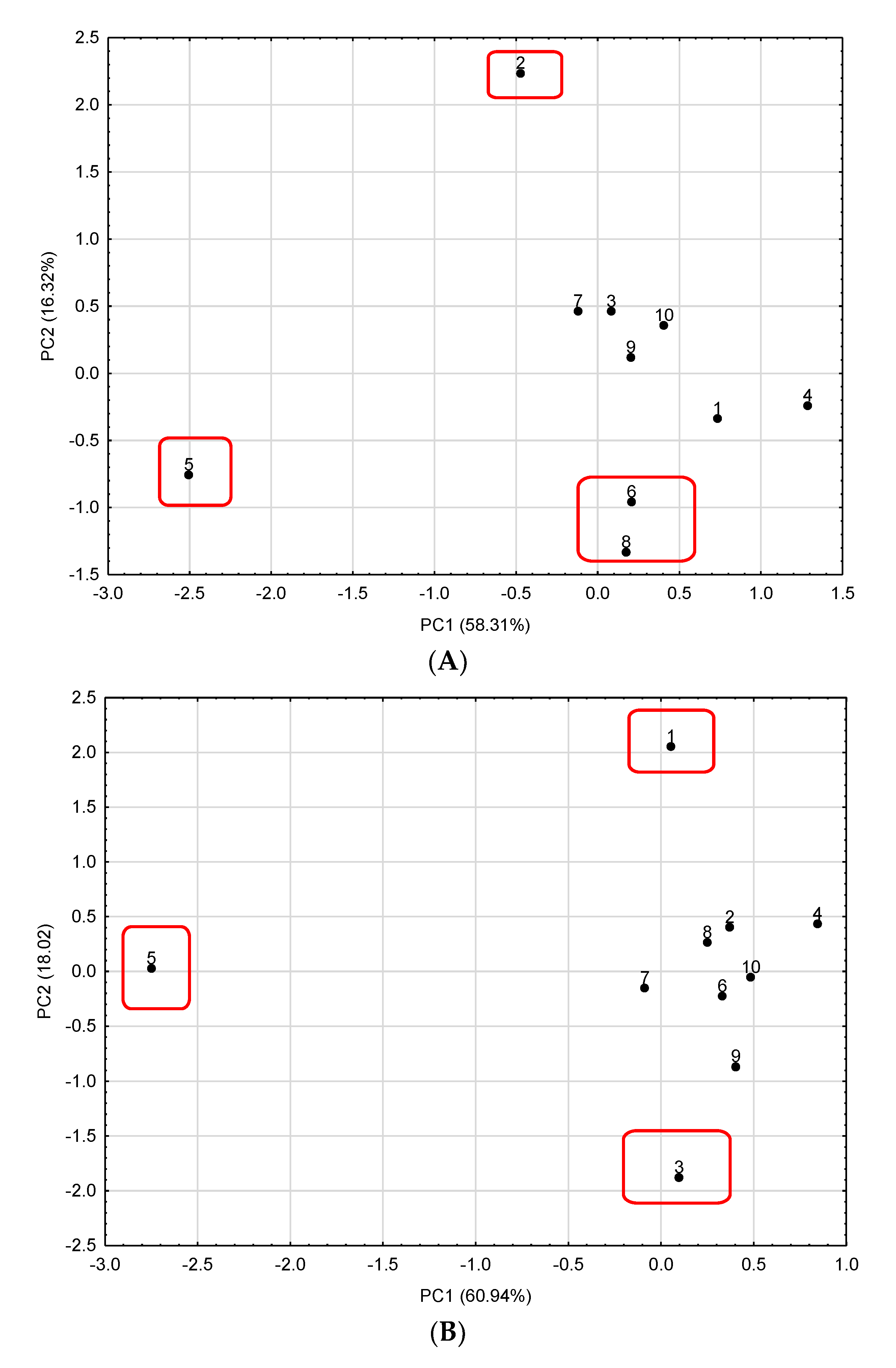
3.7. Discrimination of Astragalus Membranaceus Commercial Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinc, M.; Aytac, Z.; Dogu, S. A new species of Astragalus (Fabaceae) from Turkey. Turk. J. Bot. 2013, 37, 841–846. [Google Scholar] [CrossRef]
- Langran, X.; Podlech, D. Astragalus Linnaeus, flora of China. Cambridge Harvard Univ. Herbaria 2018, 10, 2–453. [Google Scholar]
- Butkute, B.; Dagilyte, A.; Benetis, R.; Padarauskas, A.; Cesviciene, J.; Olsaukaite, V.; Lemeziene, N. Mineral and phytochemical profiles and antioxidant activity of herbal material from two temperate Astragalus species. BioMed Res. Int. 2018, 2018, 6318630. [Google Scholar] [CrossRef] [PubMed]
- Aslanipour, B.; Gulcemal, D.; Nalbantsoy, A.; Yusufoglu, H.; Bedir, E. Cycloartane-type glycosides from Astragalus brachycalyx FISCHER and their effects on cytokine release and hemolysis. Phytochem. Lett. 2017, 21, 66–73. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Bao, H.Y.; Liu, Y. Progress on chemical constituents and pharmacological effects of Astragali Radix in recent five years. Food Drug. 2014, 16, 68–70. [Google Scholar]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Yang, C.M.; Chen, C.H. Effects of physical properties and processing methods on astragaloside IV and flavonoids content in Astragali radix. Molecules 2022, 27, 575. [Google Scholar] [CrossRef]
- Zhou, K.; Mei, X.Y. Study on preventive and therapeutic effects of Astragali radix on denervated tibial muscle atrophy in rats. Zhongguo Zhongyao Zazhi 2014, 39, 1083–1087. [Google Scholar]
- Ma, J.; Qiao, Z.; Xiang, X. Aqueous extract of Astragalus mongholicus ameliorates high cholesterol diet induced oxidative injury in experimental rat models. J. Med. Plants Res. 2011, 5, 855–858. [Google Scholar]
- Zhao, L.H.; Ma, Z.X.; Zhu, J.; Yu, X.H.; Weng, D.P. Characterization of polysaccharide from Astragalus radix as the macrophage stimulator. Cell. Immunol. 2011, 271, 329–334. [Google Scholar] [CrossRef]
- Song, J.Z.; Yiu, H.H.; Qiao, C.F.; Han, Q.B.; Xu, H.X. Chemical comparison and classification of Radix Astragali by determination of isoflavonoids and astragalosides. J. Pharm. Biomed. Anal. 2008, 47, 399–406. [Google Scholar] [CrossRef]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Munch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lin, M.K.; Peng, W.H.; Tseng, C.K.; Lee, M.S.; Yang, B.C.; Chang, W.T. Comparison of the immunomodulatory effect of TCM formulas containing either Astragali radix or with this replaced by Hedysari radix. Nat. Prod. Commun. 2022, 17, 1934578–2211427. [Google Scholar] [CrossRef]
- Kafle, B.; Baak, J.P.A.; Brede, C. Major bioactive chemical compounds in Astragali radix samples from different vendors vary greatly. PLoS ONE. 2021, 16, e0254273. [Google Scholar] [CrossRef]
- Zhang, C.E.; Liang, L.J.; Yu, X.H.; Wu, H.; Tu, P.F.; Ma, Z.J.; Zhao, K.J. Quality assessment of Astragali radix from different production areas by simultaneous determination of thirteen major compounds using tandem UV/charged aerosol detector. J. Pharm. Biomed. Anal. 2019, 165, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Farcas, A.C.; Socaci, S.A.; Tofana, M.; Diaconeasa, Z.M.; Pop, O.L.; Salanta, L.C. An overview of saponins—A bioactive group. Bull. UASVM Food. Sci. Technol. 2020, 77, 25–36. [Google Scholar] [CrossRef]
- Graziani, V.; Scognamiglio, M.; Esposito, A.; Fiorentino, A.; D’Abrosca, B. Chemical diversity and biological activities of the saponins isolated from Astragalus genus: Focus on Astragaloside IV. Phytochem. Rev. 2019, 18, 1133–1166. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. In Advances in Pharmacology; Elsevier Inc.: Amsterdam, The Netherland, 2020; Volume 87, pp. 89–112. [Google Scholar]
- Tan, Y.; Yin, L.; Sun, Z.; Shao, S.; Chen, W.; Man, X.; Du, Y.; Chen, Y. Astragalus polysaccharide exerts anti-Parkinson via activating the PI3K/AKT/mTOR pathway to increase cellular autophagy level in vitro. Int. J. Biol. Macromol. 2020, 153, 349–356. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsay, H.J.; Lu, M.K.; Lin, C.H.; Yeh, C.W.; Liu, H.K.; Shiao, Y.J. Astragalus membranaceus-polysaccharides ameliorates obesity, hepatic steatosis, neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. Int. J. Mol. Sci. 2017, 18, 2746. [Google Scholar] [CrossRef]
- Bi, Y.; Bao, H.; Zhang, C.; Yao, R.; Li, M. Quality control of radix astragali (The root of Astragalus membranaceus var. mongholicus) along its value chains. Front Pharmacol. 2020, 11, 562376. [Google Scholar] [CrossRef]
- Gong, A.G.W.; Duan, R.; Wang, H.Y.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K.; Chan, K. Evaluation of the pharmaceutical properties and value of Astragali radix. Medicines 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, C.; Zhang, Y.; Tsao, R. On-line coupling pressurised liquid extraction with two-dimensional counter current chromatography for isolation of natural acetylcholinesterase inhibitors from Astragalus membranaceus. Phytochem. Anal. 2020, 32, 640–653. [Google Scholar] [CrossRef]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Kuo, L.M.Y.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New isofavonoid glycosides and related constituents from astragali radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Li, S.; Liu, C.; Zhuang, S.; Zhou, X.; Li, Y.; Zhang, Y.; Liang, J. Extraction and preparation of 5-lipoxygenase and acetylcholinesterase inhibitors from Astragalus membranaceus stems and leaves. J. Sep. Sci. 2022, 46, e2200812. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wu, K.X.; Guo, X.R.; Tang, Z.H. A rapid method for sensitive profling of bioactive triterpene and favonoid from Astragalus mongholicus and Astragalus membranaceus by ultrapressure liquid chromatography with tandem mass spectrometry. J. Chromatogr. B 2018, 1085, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profile and antioxidant properties of commercial and wild Fragaria vesca L. roots: A comparison between hydromethanolic and aqueous extracts. Ind. Crops Prod. 2015, 63, 125–132. [Google Scholar] [CrossRef]
- Fernandes, A.; Liberal, A.; Pinela, J.; Finimundy, T.C.; Bancessi, A.; Ćirić, A.; Soković, M.; Catarino, L.; Ferreira, I.C.F.R.; Lillian Barros, L. Compositional features and biological activities of wild and commercial Moringa oleifera leaves from Guinea-Bissau. Food Biosci. 2021, 43, 101300. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Safety of a botanical extract derived from Panax notoginseng and Astragalus membranaceus (AstraGinTM) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, 6099. [Google Scholar]
- Giacobini, E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 920, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Cholinesterases, a target of pharmacology and toxicology. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011, 155, 219–229. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H. Reconsideration of anticholinesterase therapeutic strategies against Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Beschea Chiriac, S.I.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules 2019, 10, 40. [Google Scholar] [CrossRef]
- Mathew, A.; Balaji, E.V.; Pai, S.R.K.; Kishore, A.; Pai, V.; Pemmireddy, R.K.S.C. Current drug targets in Alzheimer’s associated memory impairment: A comprehensive review. CNS Neurol. Disord. Drug Targets 2023, 22, 255–275. [Google Scholar]
- Tan, X.; Song, Y.H.; Park, C.; Lee, K.W.; Kim, J.Y.; Kim, D.W.; Kim, K.D.; Lee, K.W.; Curtis-Long, M.J.; Park, K.H. Highly potent tyrosinase inhibitor, neorauflavane from Campylotropis hirtella and inhibitory mechanism with molecular docking. Bioorg. Med. Chem. 2016, 24, 153–159. [Google Scholar] [CrossRef]
- Vaezi, M. Structure and inhibition mechanism of some synthetic compounds and phenolic derivatives as tyrosinase inhibitors: Review and new insight. J. Biomol. Struct. Dyn. 2023, 41, 4798–4810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, B.; Xing, S.; Chen, Y.; Liao, Q.; Mo, J.; Chen, Y.; Li, Q.; Sun, H. Medicinal prospects of targeting tyrosinase: A feature review. Curr. Med. Chem. 2023, 30, 2638–2671. [Google Scholar] [CrossRef]
- Viapiana, A.; Struck, W.; Konieczynski, P.; Wesolowski, M.; Kaliszan, R. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Matricaria chamomilla L. commercial samples. Front. Plant Sci. 2016, 7, 1561. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela, R.R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- European Pharmacopoeia. In Birkenblätter–Betulae Herba 4.00; Council of Europe: Strasbourg, France, 2002; p. 1308.
- Polish Pharmacopoeia VI; Polish Pharmaceutical Society: Warszawa, Poland, 2002; p. 150.
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessi, M.A. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem. 2010, 12, 1242–1251. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektasoglu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Sheng, Z.; Jiang, Y.; Liu, J.; Yang, B. UHPLC–MS/MS analysis on flavonoids composition in Astragalus membranaceus and their antioxidant activity. Antioxidants 2021, 10, 1852. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Flowers of Astragalus membranaceus var. mongholicus as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Molecules 2019, 24, 434. [Google Scholar] [CrossRef]
- Gebalski, J.; Małkowska, M.; Graczyk, F.; Słomka, A.; Piskorska, E.; Gawenda-Kempczynska, D.; Kondrzycka-Dada, A.; Bogucka-Kocka, A.; Strzemski, M.; Sowa, I.; et al. Phenolic compounds and antioxidant and anti-enzymatic activities of selected adaptogenic plants from South America, Asia, and Africa. Molecules 2023, 28, 6004. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in plants: Structure, biosynthesis, abiotic stress regulation, and practical applications (review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Abdellaoui, R.; Boughalle, F.; Yahia, B.; Mabrouk, M.; Nasri, N. Characterization of lipids, proteins, and bioactive compounds in the seeds of three Astragalus species. Food Chem. 2021, 339, 127824. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhennan, M.; Defu, W.; Yanbing, N. Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves. Ultrason. Sonochem. 2022, 90, 106190. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Li, W.; Zhang, W.; Yin, Y.Y.; Sun, X.X.; Zhou, S.S.; Xu, X.Q.; Tao, C.R. Protective effect of extract of Astragalus on learning and memory impairments and neurons’ apoptosis induced by glucocorticoids in 12-month-old male mice. Anat. Rec. 2011, 294, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Hua, J.; Lin, S.J.; Zheng, H.T.; Wang, J.J.; Li, W.; Ke, J.J.; Cai, H.B. Astragalus polysaccharide alleviates cognitive impairment and β-amyloid accumulation in APP/PS1 mice via Nrf2 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Chen, H.; Li, W.; Li, W.; Zhu, G. Astragaloside IV prevents Aβ1-42 oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020, 1747, 147041. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.M.; Alshehri, S.A.; Almarwani, W.A.; Aljohani, K.K.; Albalawi, A.Z.; Alatawi, A.S.; Al-Atwi, S.M.; Alhwyty, L.S.; Hassan, H.M.; Al-Gayyar, M.M.H. Effects of cycloastragenol on Alzheimer’s disease in rats by reducing oxidative stress, inflammation, and apoptosis. Curr. Alzheimer Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Y.; Yu, C.; Chen, J.; Xu, X.; Zhang, X.; Wang, H.; Zhang, J.; Wang, H.; Dong, J.; et al. Astragaloside protects rat brain from microwave-induced functional injuries via restoring acetylcholine and normalizing electroencephalogram. Environ. Sci. Pollut. Res. Int. 2020, 27, 40787–40794. [Google Scholar] [CrossRef]
- Santoro, V.; Parisi, V.; D’Ambola, M.; Sinisgalli, C.; Monne, M.; Milella, L.; Russo, R.; Severina, L.; De Tomassi, N. Chemical profiling of Astragalus membranaceus (Fish.) Bunge roots herbal preparation and evaluation of its bioactivity. Nat. Prod. Commun. 2020, 15, 1934578X20924152. [Google Scholar] [CrossRef]
- Stępnik, K.; Kulula-Koch, W.; Plazinski, W.; Gawel, K.; Gaweł-Bęben, K.; Boguszewska-Czubara, A. Significance of astragaloside IV from the roots of Astragalus mongholicus as an acetylcholinesterase inhibitor—From the computational and biomimetic analyses to the in vitro and in vivo studies of safety. Int. J. Mol. Sci. 2023, 24, 9152. [Google Scholar] [CrossRef]
- Senol Deniz, F.S.; Eren, G.; Orhan, I.E.; Sener, B.; Ozgen, U.; Aldaba, R.; Calis, I. Outlining in vitro and in silico cholinesterase inhibitory activity of twenty-four natural products of various chemical classes: Smilagenin, kokusaginine, and methyl rosmarinate as emboldening inhibitors. Molecules 2021, 26, 2024. [Google Scholar] [CrossRef]
- Rangani, S.C.; Marapana, R.A.U.J.; Senanayake, G.S.A.; Perera, P.R.D.; Pathmalal, M.M.; Amarasinghe, H.K. Correlation analysis of phenolic compounds, antioxidant potential, oxygen radical scavenging capacity, and alkaloid content in ripe and unripe Areca catechu from major cultivation areas in Sri Lanka. Appl. Food Res. 2023, 3, 100361. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Naeem, M.Y.; Tardugno, R.; Limongelli, F.; Corbo, F. The main phenolic compounds responsible for the antioxidant capacity of sweet cherry (Prunus avium L.) pulp. LWT—Food Sci. Technol. 2023, 185, 115085. [Google Scholar] [CrossRef]
| Sample No. | TPAC [µg CAE/g] | TPC [µg GAE/g] | TFC [µg QE/g] | TPAC [µg CAE/g] | TPC [mg GAE/g] | TFC [µg QE/g] |
|---|---|---|---|---|---|---|
| Hydromethanolic Extracts | Water Extracts | |||||
| 1 | 84.56 ± 2.31 d | 409.60 ± 0.78 a | 69.56 ± 0.45 a | 542.31 ± 2.80 bc | 1.33 ± 0.54 a | 550.70 ± 2.34 bc |
| 2 | 102.53 ± 0.93 e | 478.99 ± 1.02 bc | 75.04 ± 0.52 ab | 574.38 ± 1.56 ab | 1.04 ± 0.34 b | 735.08 ± 1.89 e |
| 3 | 111.38 ± 2.83 f | 576.81 ± 1.48 d | 71.22 ± 0.54 a | 679.12 ± 2.81 d | 1.23 ± 0.25 ac | 849.80 ± 2.33 f |
| 4 | 59.10 ± 1.58 a | 325.31 ± 1.19 e | 59.32 ± 0.56 a | 496.21 ± 1.65 c | 0.96 ± 0.09 b | 414.53 ± 2.82 d |
| 5 | 143.79 ± 3.56 g | 577.70 ± 1.98 d | 170.39 ± 0.55 b | 2139.97 ± 1.26 e | 3.36 ± 0.53 d | 1396.14 ± 2.18 g |
| 6 | 68.55 ± 3.89 bc | 443.92 ± 1.65 ab | 65.94 ± 0.47 a | 727.27 ± 1.48 d | 1.09 ± 0.64 bc | 487.38 ± 2.02 a |
| 7 | 57.80 ± 2.23 a | 480.73 ± 1.31 bc | 68.56 ± 0.81 a | 601.26 ± 2.44 a | 1.24 ± 0.12 ac | 501.65 ± 2.50 ab |
| 8 | 66.81 ± 1.36 bc | 445.49 ± 1.20 ab | 66.49 ± 0.49 a | 591.19 ± 3.96 ab | 1.34 ± 0.23 a | 561.81 ± 1.39 c |
| 9 | 72.47 ± 1.14 c | 507.07 ± 0.83 c | 67.66 ± 0.50 a | 570.80 ± 1.27 ab | 1.31 ± 0.31 a | 513.54 ± 1.53 abc |
| 10 | 64.76 ± 2.13 ab | 412.56 ± 2.6 a | 66.98 ± 1.0 a | 612.65 ± 3.91 a | 1.11 ± 0.10 bc | 522.80 ± 2.75 abc |
| Analytes | Regression Equation | Linearity (µg/mL) | R2 | LOD (µg/mL) | LOQ (µg/mL) | Recovery (%) |
|---|---|---|---|---|---|---|
| GA | y = 20,025x − 29,083 | 56–280 | 0.983 | 3.52 | 10.02 | 96.57 |
| PAT | y = 39,674x + 19,375 | 55.5–276 | 0.983 | 2.46 | 7.04 | 96.49 |
| CNA | y = 35,424x + 14,534 | 48.4–242 | 0.987 | 2.65 | 7.66 | 95.79 |
| VA | y = 30,176x + 56,284 | 53.6–268 | 0.981 | 2.65 | 7.13 | 103.97 |
| FA | y = 22,525x + 17,955 | 52–260 | 0.980 | 3.54 | 9.68 | 91.86 |
| pCA | y = 98,491x + 17,039 | 55.6–278 | 0.979 | 4.54 | 12.53 | 91.89 |
| API | y = 34,257x + 14,385 | 51.4–260 | 0.985 | 3.12 | 8.47 | 95.78 |
| NAR | y = 42,541x + 51,683 | 57.6–288 | 0.998 | 2.38 | 7.14 | 93.88 |
| RUT | y = 13,223x + 29,642 | 46.4–234 | 0.970 | 3.01 | 8.56 | 95.57 |
| Q | y = 17,992x − 35,440 | 42–210 | 0.979 | 5.68 | 12.44 | 96.43 |
| GA | VA | PAT | pCA | FA | CNA | RUT | Q | API | NAR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hydromethanolic extracts (μg/g) | ||||||||||
| 1 | 39.92 ± 4.10 a | ND | ND | ND | ND | ND | 9.18 ± 1.76 a | 43.04 ± 5.20 ab | ND | ND |
| 2 | ND | ND | ND | ND | ND | 69.67 ± 3.57 f | 114.88 ± 3.29 g | 228.34 ± 6.16 g | ND | ND |
| 3 | ND | ND | ND | ND | ND | 31.29 ± 1.14 bc | 12.45 ± 2.03 a | 45.63 ± 1.49 b | ND | ND |
| 4 | 73.32 ± 4.37 b | ND | ND | ND | ND | ND | 13.60 ± 1.77 a | 80.72 ± 5.13 c | ND | 28.55 ± 3.79 a |
| 5 | 705.80 ± 9.35 f | ND | ND | ND | ND | 17.17 ± 0.80 a | 140.74 ± 2.77 h | 83.57 ± 4.43 c | ND | 117.08 ± 6.33 b |
| 6 | 376.60 ± 12.09 e | ND | ND | ND | ND | 35.40 ± 0.24 c | 76.88 ± 1.97 f | 39.86 ± 3.43 a | ND | 828.32 ± 11.45 g |
| 7 | 310.10 ± 8.69 d | ND | ND | ND | ND | 28.43 ± 0.79 a | 68.37 ± 3.72 e | 187.63 ± 4.15 f | ND | 223.62 ± 6.23 e |
| 8 | 263.78 ± 5.61 c | ND | ND | ND | ND | 4.05 ± 0.47 d | 33.89 ± 2.28 c | 44.53 ± 1.49 ab | ND | 689.52 ± 9.60 f |
| 9 | ND | ND | ND | ND | ND | 17.68 ± 1.37 a | 22.82 ± 1.74 b | 93.37 ± 3.44 e | ND | 189.65 ± 6.99 c |
| 10 | ND | ND | ND | ND | ND | 51.67 ± 4.78 e | 43.56 ± 3.75 d | 51.70 ± 4.11 d | ND | 210.61 ± 5.77 d |
| Water extracts (μg/g) | ||||||||||
| 1 | 315.34 ± 6.69 a | ND | ND | ND | ND | ND | ND | 163.95 ± 9.94 f | ND | ND |
| 2 | 363.62 ± 4.70 f | ND | ND | ND | ND | ND | ND | 130.38 ± 7.85 e | ND | ND |
| 3 | 481.40 ± 5.49 g | ND | ND | ND | ND | ND | ND | 105.64 ± 3.52 d | ND | ND |
| 4 | 317.59 ± 5.25 ab | ND | ND | ND | ND | ND | ND | 76.63 ± 2.48 b | ND | ND |
| 5 | 353.83 ± 12.75 c | ND | ND | ND | ND | ND | ND | 77.38 ± 1.29 b | ND | ND |
| 6 | 404.64 ± 9.10 d | ND | ND | ND | ND | ND | ND | 57.86 ± 0.68 a | ND | ND |
| 7 | 352.80 ± 4.12 c | ND | ND | ND | ND | ND | ND | 58.38 ± 0.75 a | ND | ND |
| 8 | 333.20 ± 2.59 e | ND | ND | ND | ND | ND | ND | 56.88 ± 0.26 a | ND | ND |
| 9 | 402.63 ± 6.22 d | ND | ND | ND | ND | ND | ND | 58.75 ± 3.73 a | ND | ND |
| 10 | 323.23 ± 5.99 b | ND | ND | ND | ND | ND | ND | 64.23 ± 3.97 c | ND | ND |
| Sample No. | DPPH (mg TEA/g) | FRAP (mgFe2+/g) | CUPRAC (mg ASA/g) | ABTS (mg TEA/g) |
|---|---|---|---|---|
| Hydromethanolic extracts | ||||
| 1 | 159.47 ± 2.85 ab | 0.74 ± 0.02 e | 2.70 ± 0.05 b | 0.62 ± 0.02 c |
| 2 | 159.71 ± 1.91 ab | 1.11 ± 0.04 ab | 3.27 ± 0.14 a | 0.87 ± 0.01 b |
| 3 | 167.77 ± 1.85 ac | 0.63 ± 0.01 c | 3.17 ± 0.11 a | 0.81 ± 0.02 ae |
| 4 | 126.91 ± 6.58 d | 0.56 ± 0.06 c | 2.22 ± 0.10 d | 0.62 ± 0.03c |
| 5 | 272.96 ± 13.87 e | 1.76 ± 0.02 f | 4.67 ± 0.07 e | 1.17 ± 0.02 f |
| 6 | 167.97 ± 4.12 ac | 0.98 ± 0.02 d | 2.85 ± 0.09 bc | 0.75 ± 0.02 d |
| 7 | 179.24 ± 7.49 c | 1.10 ± 0.03 ab | 3.21 ± 0.14 a | 0.86 ± 0.03 ab |
| 8 | 184.53 ± 1.57 c | 1.13 ± 0.02 a | 3.22 ± 0.09 a | 0.82 ± 0.01 ab |
| 9 | 146.08 ± 4.93 b | 1.15 ± 0.01 a | 3.10 ± 0.14 ac | 0.83 ± 0.04 ab |
| 10 | 157.87 ± 2.65 ab | 1.05 ± 0.04 bd | 2.99 ± 0.97 abc | 0.76 ± 0.08 de |
| Water extracts | ||||
| 1 | 139.89 ± 6.37 e | 7.92 ± 0.21 a | 25.90 ± 0.79 b | 5.48 ± 0.19 de |
| 2 | 205.50 ± 4.58 ab | 6.35 ± 0.16 a | 22.51 ± 0.85 ab | 4.99 ± 0.17 abc |
| 3 | 365.48 ± 7.12 d | 5.93 ± 0.19 a | 20.99 ± 1.09 a | 4.96 ± 0.21 abc |
| 4 | 176.38 ± 5.82 a | 5.86 ± 0.13 a | 21.32 ± 1.18 a | 4.66 ± 0.16 a |
| 5 | 389.64 ± 6.64 d | 10.29 ± 0.85 b | 47.91 ± 3.10 c | 5.76 ± 0.76 e |
| 6 | 178.80 ± 2.82 a | 6.46 ± 0.13 a | 24.10 ± 0.53 ab | 5.26 ± 0.16 cd |
| 7 | 329.22 ± 5.73 f | 7.35 ± 0.17 a | 23.30 ± 1.69 ab | 5.59 ± 0.17 de |
| 8 | 216.70 ± 2.88 b | 6.67 ± 0.10 a | 22.18 ± 0.88 a | 5.23 ± 0.15 bcd |
| 9 | 269.33 ± 8.16 c | 6.36 ± 0.11 a | 21.75 ± 1.20 a | 4.86 ± 0.08 ab |
| 10 | 254.52 ± 3.67 c | 6.02 ± 0.87 a | 23.88 ± 2.08 ab | 4.76 ± 1.09 a |
| Sample No. | Stapphylococcus aureus ATCC6538 | Escherichia coli ATCC8739 |
|---|---|---|
| 1 | 10/10 | nz |
| 2 | 0/0 | nz |
| 3 | 0/0 | nz |
| 4 | 10/10 | nz |
| 5 | 22/15 | nz |
| 6 | 0/0 | nz |
| 7 | 12/10 | nz |
| 8 | 12/10 | nz |
| 9 | 13/10 | nz |
| 10 | 0/0 | nz |
| Sample No. | BChE Inhibition (Inhibition % ± S.D.a) 200 µg/mL b | TYR Inhibition (Inhibition % ± S.D.a) 100 µg/mLb |
|---|---|---|
| 1 | 16.22 ± 0.02 | 5.22 ± 0.12 |
| 2 | 27.31 ± 0.12 | 8.13 ± 0.11 |
| 3 | 31.22 ± 0.17 | 11.02 ± 0.09 |
| 4 | 47.17 ± 1.13 | 19.27 ± 0.12 |
| 5 | 55.17 ± 2.12 (IC50: 42.65 ± 2.5 µg/mL) | 27.16 ± 1.3 |
| 6 | 42.98 ± 0.15 | 7.38 ± 0.83 |
| 7 | 47.15 ± 0.22 | 12.01 ± 0.43 |
| 8 | 13.22 ± 0.15 | 3.12 ± 0.11 |
| 9 | 48.67 ± 0.14 | 6.12 ± 0.01 |
| 10 | 61.14 ± 1.02 (IC50: 32.65 ± 2.78 µg/mL) | 16.12 ± 0.12 |
| References | 66.91 ± 2.32 c (IC50: 119.3 ± 1.05 µg/mL) | 76.58 ± 0.85 d (IC50: 52.42 ± 2.67 µg/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahrivari-Baviloliaei, S.; Erdogan Orhan, I.; Abaci Kaplan, N.; Konopacka, A.; Waleron, K.; Plenis, A.; Viapiana, A. Characterization of Phenolic Profile and Biological Properties of Astragalus membranaceus Fisch. ex Bunge Commercial Samples. Antioxidants 2024, 13, 993. https://doi.org/10.3390/antiox13080993
Shahrivari-Baviloliaei S, Erdogan Orhan I, Abaci Kaplan N, Konopacka A, Waleron K, Plenis A, Viapiana A. Characterization of Phenolic Profile and Biological Properties of Astragalus membranaceus Fisch. ex Bunge Commercial Samples. Antioxidants. 2024; 13(8):993. https://doi.org/10.3390/antiox13080993
Chicago/Turabian StyleShahrivari-Baviloliaei, Saba, Ilkay Erdogan Orhan, Nurten Abaci Kaplan, Agnieszka Konopacka, Krzysztof Waleron, Alina Plenis, and Agnieszka Viapiana. 2024. "Characterization of Phenolic Profile and Biological Properties of Astragalus membranaceus Fisch. ex Bunge Commercial Samples" Antioxidants 13, no. 8: 993. https://doi.org/10.3390/antiox13080993







