Analysis of Polyphenols from Polygala major Jacq.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Polyphenols
2.3. Analysis of Total Phenolics
2.4. Analysis of Total Flavonoids
2.5. Analysis of Antioxidant Capacity with the DPPH Method
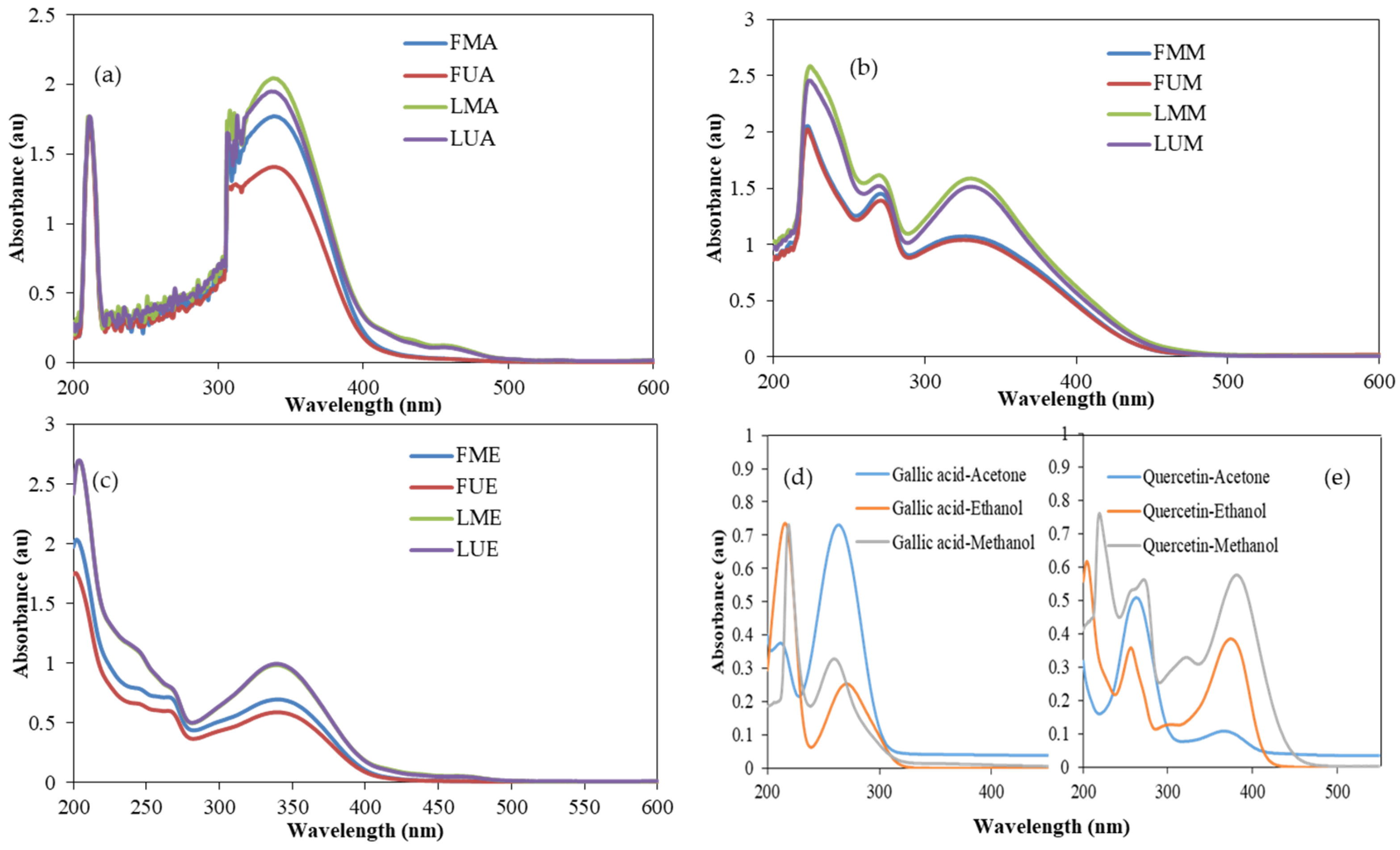
2.6. UV–VIS Spectral Analysis
2.7. FTIR Analysis
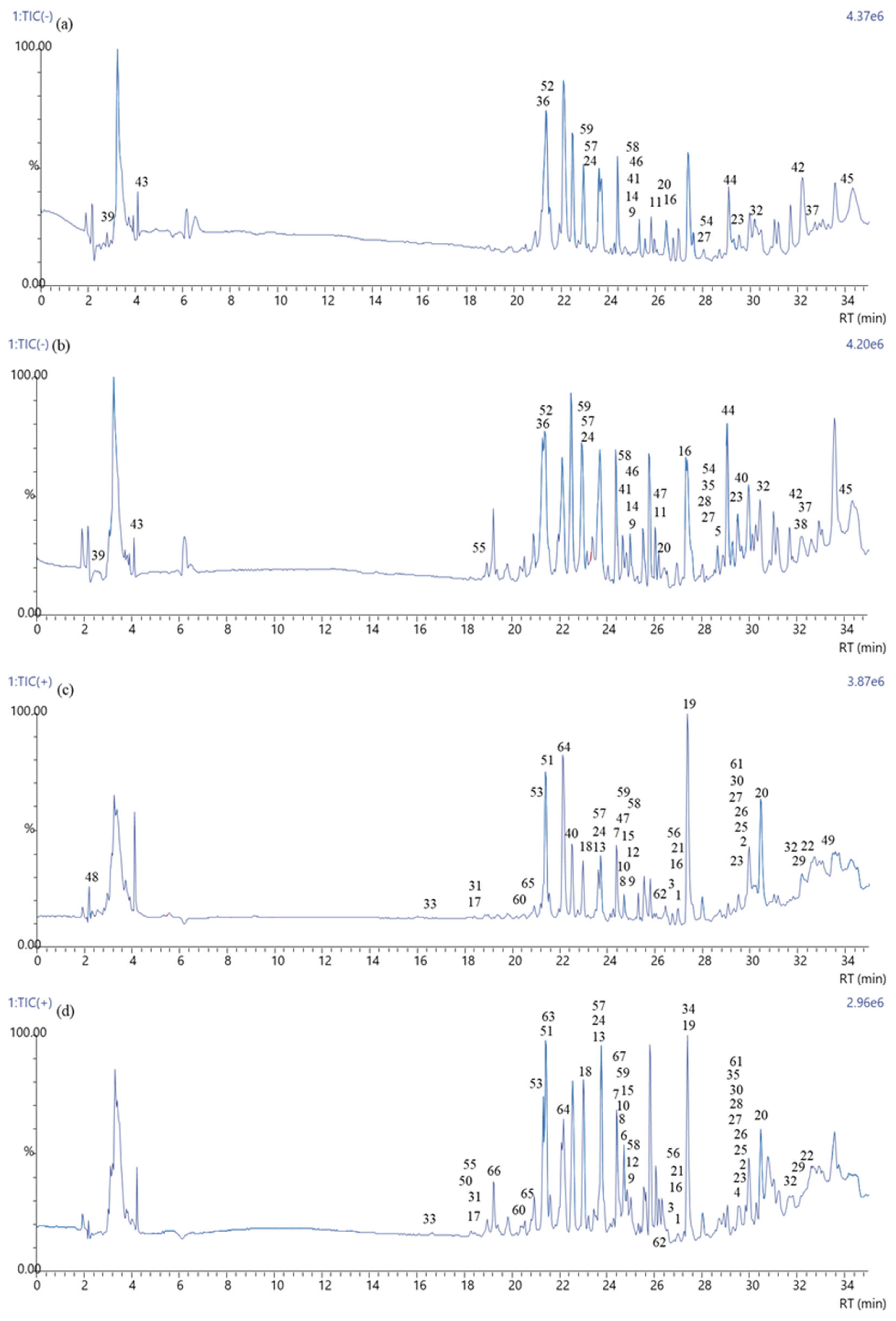
2.8. LC–MS Analysis
2.9. Determination of Antibacterial Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
3.2. Total Phenolic Contents
3.3. Total Flavonoid Contents
3.4. Antioxidant Activity
3.5. Antimicrobial Activity
3.6. UV–VIS Spectra
3.7. FTIR Analysis
3.8. LC–MS Analysis of Polyphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kou, J.; Si, M.; Dai, G.; Lin, Y.; Zhu, D. Antiinflammatory activity of Polygala japonica extract. Fitoterapia 2006, 77, 411–415. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Y.; Wu, P.; Zhao, P.; Zhou, Q.; Zhang, Z.; Wang, Y.; Zhang, X. Polygalae Radix: A review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 2020, 147, 104759. [Google Scholar] [CrossRef] [PubMed]
- Donmez, E.O.; Aydin, Z.U.; Donmez, A.A. Pollen morphology of Polygala L.(Polygalaceae) from Turkey and its taxonomic implications. Acta Biol. Cracoviensia. Ser. Bot. 2023, 65, 21–47. [Google Scholar] [CrossRef]
- Johann, S.; Mendes, B.G.; Missau, F.C.; Resende, M.A.D.; Pizzolatti, M.G. Antifungal activity of five species of Polygala. Braz. J. Microbiol. 2011, 42, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Potor, D.C.; Georgescu, M.I. Morphological characters variabilities of some populations of species Polygala major Jacq. Sci. Pap.-Ser. A Agron. 2011, 54, 463–468. [Google Scholar]
- Altemimi, A.; Choudhary, R.; Watson, D.G.; Lightfoot, D.A. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason. Sonochem. 2015, 24, 247–255. [Google Scholar] [CrossRef]
- Avcı, A.; Cerit, İ.; Hamk, M.; Keskin, S.Y. Improved Extraction of Bioactive Compounds From The Pollens of Typha Domingensis wıth Sequential Conventional and Ultrasound Treatment. Gıda 2023, 48, 256–270. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Study of continuous flow ultrasonication to improve total phenolic content and antioxidant activity in sorghum flour and its comparison with batch ultrasonication. Ultrason. Sonochem. 2021, 71, 105402. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical analysis of green extraction techniques used for botanicals: Trends, priorities, and optimization strategies—A review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green extraction techniques of bioactive compounds: A state-of-the-art review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green extraction of bioactive compounds from plant biomass and their application in meat as natural antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Naveira-Pazos, C.; Veiga, M.C.; Mussagy, C.U.; Farias, F.O.; Kennes, C.; Pereira, J.F. Carotenoids production and extraction from Yarrowia lipolytica cells: A biocompatible approach using biosolvents. Sep. Purif. Technol. 2024, 343, 127136. [Google Scholar] [CrossRef]
- Bergeron, C.; Marston, A.; Wolfender, J.L.; Mavi, S.; Rogers, C.; Hostettmann, K. Isolation of polyphenols from Polygala gazensis and liquid chromatography–mass spectrometry of related African Polygala species. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 1997, 8, 32–36. [Google Scholar]
- De Leo, M.; Peruzzi, L.; Granchi, C.; Tuccinardi, T.; Minutolo, F.; De Tommasi, N.; Braca, A. Constituents of Polygala flavescens ssp. flavescens and their activity as inhibitors of human lactate dehydrogenase. J. Nat. Prod. 2017, 80, 2077–2087. [Google Scholar] [CrossRef]
- Li, C.J.; Yang, J.Z.; Yu, S.S.; Zhao, C.Y.; Peng, Y.; Wang, X.L.; Zhang, D.M. Glomexanthones A–C, three xanthonolignoid C-glycosides from Polygala glomerata Lour. Fitoterapia 2014, 93, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Zhou, L.Y.; Wang, J.M.; Li, Q.; Geng, Y.Y.; Liu, H.Y.; Hua, Y. Two New Flavonol Glycosides from Polygala sibirica L. var megalopha Fr. Molecules 2015, 20, 21494–21500. [Google Scholar] [CrossRef]
- Cervellati, R.; Innocenti, G.; Dall’Acqua, S.; Costa, S.; Sartini, E. Polyphenols from Polygala spp. and their antioxidant activity. Chem. Biodivers. 2004, 1, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Zeleke, B.; Mekonnen, Z.; Bireda, M.; Yitbarek, M.; Dendir, A. Phytochemical screening and antimicrobial activity of Polygala sadebeckiana Gürke extracts on bacterial isolates from Wound samples of patients with “Shimetere”. BMC Complement. Med. Ther. 2024, 24, 72. [Google Scholar] [CrossRef]
- Liu, J.; Liu, A.; Mao, F.; Zhao, Y.; Cao, Z.; Cen, N.; Li, S.; Li, L.; Ma, X.; Sui, H. Determination of the active ingredients and biopotency in Polygala tenuifolia Willd. and the ecological factors that influence them. Ind. Crops Prod. 2019, 134, 113–123. [Google Scholar] [CrossRef]
- Yılmazer Keskin, S.; Avcı, A.; Kurnia, H.F.F. Analyses of phytochemical compounds in the flowers and leaves of Spiraea japonica var. fortunei using UV-VIS, FTIR, and LC-MS techniques. Heliyon 2024, 10, e25496. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Eghdami, A.; Sadeghi, F. Determination of total phenolic and flavonoids contents in methanolic and aqueous extract of Achillea millefolium. Org. Chem. J. 2010, 2, 81–84. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol.-Lebensm.-Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Avcı, A.; Kamiloğlu, A.; Dönmez, S. Efficient production of acetone butanol ethanol from sole fresh and rotten potatoes by various Clostridium strains. Biomass Convers. Biorefinery 2023, 13, 4161–4169. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Simultaneous effect of vacuum and ultrasound assisted enzymatic extraction on the recovery of plant protein and bioactive compounds from sesame bran. J. Food Compos. Anal. 2020, 87, 103424. [Google Scholar] [CrossRef]
- Bauer, A. Antibiotic Susceptibility Testing by a StandardizedSingle Disc Method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Hamk, M.; Akçay, F.A.; Avcı, A. Green synthesis of zinc oxide nanoparticles using Bacillus subtilis ZBP4 and their antibacterial potential against foodborne pathogens. Prep. Biochem. Biotechnol. 2023, 53, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug. Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Kozłowska, H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Doan, H.T.; Vu, T.V.; Pham, Q.T.; Khoi, N.M.; Huu, T.N.; Thuong, P.T. Oligosaccharide and glucose esters from the roots of Polygala arillata. Nat. Prod. Res. 2020, 34, 2900–2906. [Google Scholar] [CrossRef]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.E.; Lovillo, M.P.; El Farissi, H.; Oufdou, H.; Brigui, J. Extraction, analysis of polyphenols and antioxidant properties of morrocan barley seed extracts (Hordeum vulgare L.). Mater. Today Proc. 2021, 43, 1896–1902. [Google Scholar] [CrossRef]
- Irfan, S.; Ranjha, M.; Nadeem, M.; Safdar, M.N.; Jabbar, S.; Mahmood, S.; Murtaza, M.A.; Ameer, K.; Ibrahim, S.A. Antioxidant activity and phenolic content of sonication-and maceration-assisted ethanol and acetone extracts of Cymbopogon citratus leaves. Separations 2022, 9, 244. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- El Guiche, R.; Tahrouch, S.; Amri, O.; El Mehrach, K.; Hatimie, A. Antioxidant activity and total phenolic and flavonoid contents of 30 medicinal and aromatic plants located in the South of Morocco. Int. J. New Technol. Res. 2015, 1, 263695. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Li, M.; Pare, P.W.; Zhang, J.; Kang, T.; Zhang, Z.; Delong, Y.; Wang, K.; Xing, H. Antioxidant capacity connection with phenolic and flavonoid content in Chinese medicinal herbs. Rec. Nat. Prod. 2018, 12, 239. [Google Scholar] [CrossRef]
- Gurav, S.; Deshkar, N.; Gulkari, V.; Duragka, N.; Patil, A. Free radical scavenging activity of Polygala chinensis Linn. Pharmacol. Line 2007, 2, 245–253. [Google Scholar]
- Anokwuru, C.P.; Anyasor, G.N.; Ajibaye, O.; Fakoya, O.; Okebugwu, P. Effect of extraction solvents on phenolic, flavonoid and antioxidant activities of three nigerian medicinal plants. Nat. Sci. 2011, 9, 53–61. [Google Scholar]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Mane, M.P.; Patil, R.S.; Magdum, A.B.; Narayankar, C.U.; Nimbalkar, M.S. Evaluation of antioxidant and pancreatic lipase inhibitory potential of Polygala glaucoides L. and Polygala erioptera DC. Int. J. Bot. Stud. 2021, 6, 1259–1264. [Google Scholar]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 389103. [Google Scholar] [CrossRef]
- Alhajali, O.; Adnan, A.N. Phytochemical Screening and Antibacterial Activity of Pistacia atlantica and Pinus canariensis Extracts. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 403–418. [Google Scholar] [CrossRef]
- Subhadradevi, V.; Asokkumar, K.; Umamaheswari, M.; Sivashanmugam, A.T.; Ushanandhini, J.R.; Jagannath, P. Antimicrobial activity of leaves and flowers of Cassia auriculata Linn. Bangladesh J. Sci. Ind. Res. 2011, 46, 513–518. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Khalid, S.; Almaary, K.S.; Dawoud, T.M.; Essam, N.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Abdullah, E.; Raus, R.A.; Jamal, P. Extraction and evaluation of antibacterial activity from selected flowering plants. Am. Med. J. 2012, 3, 27–32. [Google Scholar]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of Flavonoid Subgroups and Hydroxy Substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef]
- Arora, S.; Itankar, P. Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts. J. Tradit. Complement. Med. 2018, 8, 476–482. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, 15th ed.; Meyers, R.A., Ed.; John Wiley and Sons: New York, NY, USA, 2000; Volume 12, pp. 10815–10837. [Google Scholar]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Domenicotti, C. Development of a Fast, Low-Cost, Conservative and Ecological Method for Quantifying Gallic Acid in Polymeric Formulations by FTIR Spectroscopy in Solution. ChemistrySelect 2020, 5, 4381–4388. [Google Scholar] [CrossRef]
- Naz, S.; Khaskheli, A.R.; Aljabour, A.; Kara, H.; Talpur, F.N.; Sherazi, S.T.H.; Khaskheli, A.A.; Jawaid, S. Synthesis of highly stable cobalt nanomaterial using gallic acid and its application in catalysis. Adv. Chem. 2014, 2014, 686925. [Google Scholar] [CrossRef]
- Tousi, M.S.; Sepehri, H.; Khoee, S.; Farimani, M.M.; Delphi, L.; Mansourizadeh, F. Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines. J. Pharm. Anal. 2021, 11, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Han Jie, L.; Jantan, I.; Yusoff, S.D.; Jalil, J.; Husain, K. Sinensetin: An insight on its pharmacological activities, mechanisms of action and toxicity. Front. Pharmacol. 2020, 11, 553404. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef] [PubMed]
- Nooreen, Z.; Singh, S.; Singh, D.K.; Tandon, S.; Ahmad, A.; Luqman, S. Characterization and evaluation of bioactive polyphenolic constituents from Zanthoxylum armatum DC., a traditionally used plant. Biomed. Pharmacother. 2017, 89, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Basque, A.; Touaibia, M.; Martin, L.J. Sinapic and ferulic acid phenethyl esters increase the expression of steroidogenic genes in MA-10 tumor Leydig cells. Toxicol. Vitr. 2023, 86, 105505. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.N.; Ayyadurai, N.; Anandasadagopan, S.K. Synergistic effect of vancomycin and gallic acid loaded MCM-41 mesoporous silica nanoparticles for septic arthritis management. J. Drug Deliv. Technol. 2023, 82, 104353. [Google Scholar] [CrossRef]
- Ito, S.; Sasaki, H.; Gotow, T.; Suetake, I.; Nagai, K. Soy isoflavone daidzein protects Neuro2a cells from NO stress via activation of AMPK-PGC1α pathway followed by mitochondrial enhancement. PharmaNutrition 2023, 24, 100337. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; Sabet, S.; El-Shorbagy, H.M.; Abdelhamid, I.A.; Ibrahim, S.A. Chalcones: Promising therapeutic agents targeting key players and signaling pathways regulating the hallmarks of cancer. Chem.-Biol. Interact. 2023, 369, 110297. [Google Scholar] [CrossRef] [PubMed]
- Olawale, F.; Olofinsan, K.; Iwaloye, O. Biological activities of Chromolaena odorata: A mechanistic review. S. Afr. J. Bot. 2022, 144, 44–57. [Google Scholar] [CrossRef]
- Saquib, Q.; Ahmed, S.; Ahmad, M.S.; Al-Rehaily, A.J.; Siddiqui, M.A.; Faisal, M.; Ahmad, J.; Alsaleh, A.N.; Alatar, A.A.; Al-Khedhairy, A.A. Anticancer efficacies of persicogenin and homoeriodictyol isolated from Rhus retinorrhoea. Process Biochem. 2020, 95, 186–196. [Google Scholar] [CrossRef]
- Li, K.; Li, M.; Luo, Z.; Mao, Y.; Yu, Y.; He, Y.; Zhou, J.; Fei, Y.; Pei, Y.; Cai, K. Overcoming the hypoxia-induced drug resistance in liver tumor by the concurrent use of apigenin and paclitaxel. Biochem. Biophys. Res. Commun. 2020, 526, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Oksbjerg, N.; Young, J.F.; Jeppesen, P.B. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes. Metab. 2014, 16, 602–612. [Google Scholar] [CrossRef]
- Murillo, J.I.; Encarnación-Dimayuga, R.; Malmstrøm, J.; Christophersen, C.; Franzblau, S.G. Antimycobacterial flavones from Haplopappus sonorensis. Fitoterapia 2003, 74, 226–230. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, G. An insight into the role of citrus bioactives in modulation of colon cancer. J. Funct. Foods 2015, 13, 239–261. [Google Scholar] [CrossRef]

| Solvent, 80% | Sample | Extraction Efficiency (%) | |
|---|---|---|---|
| Macerated Extraction | Ultrasonic Extraction | ||
| Acetone | Flower | 36.72 bA* ± 1.82 | 36.80 bA* ± 0.84 |
| Leaf | 27.52 cB ± 2.36 | 30.20 cB ± 1.13 | |
| Ethanol | Flower | 28.64 cA ± 2.12 | 28.80 cA ± 0.82 |
| Leaf | 21.72 dB* ± 1.78 | 22.90 dB* ± 0.94 | |
| Methanol | Flower | 42.44 aA* ± 2.44 | 44.02 aA* ± 0.83 |
| Leaf | 28.44 cB ± 2.48 | 31.38 cB ± 1.06 | |
| Solvent, 80% | Sample | Total Phenolic Content (mg GAE/g Dry Matter) | |
|---|---|---|---|
| Macerated Extraction | Ultrasonic Extraction | ||
| Acetone | Flower | 37.81 cB* ± 2.00 | 29.04 cdC* ± 2.93 |
| Leaf | 45.38 abA* ± 2.67 | 42.18 bA* ± 1.29 | |
| Ethanol | Flower | 35.71 cdB* ± 0.90 | 26.69 dC* ± 1.35 |
| Leaf | 47.81 aA* ± 1.53 | 48.51 aA* ± 1.35 | |
| Methanol | Flower | 32.99 dB* ± 1.65 | 31.57 cB* ± 0.96 |
| Leaf | 43.30 bA* ± 1.42 | 44.27 bA* ± 2.45 | |
| Solvent, 80% | Sample | Total Flavonoid Content (mg QE/g Dry Matter) | |
|---|---|---|---|
| Macerated Extraction | Ultrasonic Extraction | ||
| Acetone | Flower | 5.14 dC* ± 0.13 | 5.48 dC* ± 0.22 |
| Leaf | 16.56 aA* ± 0.72 | 15.14 aB* ± 0.95 | |
| Ethanol | Flower | 4.47 dC* ± 0.08 | 4.27 eC* ± 0.18 |
| Leaf | 10.97 bA* ± 0.52 | 9.95 bB* ± 0.24 | |
| Methanol | Flower | 4.17 dB* ± 0.39 | 5.02 deB* ± 0.40 |
| Leaf | 7.93 cA* ± 0.61 | 7.48 cA* ± 0.22 | |
| Solvent, 80% | Sample | DPPH Scavenging Activity (mgTE/g Dry Matter) | |
|---|---|---|---|
| Macerated Extraction | Ultrasonic Extraction | ||
| Acetone | Flower | 0.74 bB* ± 0.00 | 0.74 bA* ± 0.00 |
| Leaf | 0.66 dC* ± 0.01 | 0.65 dC* ± 0.02 | |
| Ethanol | Flower | 0.72 bB* ± 0.01 | 0.74 bA* ± 0.00 |
| Leaf | 0.69 cC* ± 0.01 | 0.70 cC* ± 0.01 | |
| Methanol | Flower | 0.80 aB* ± 0.00 | 0.77 bC* ± 0.00 |
| Leaf | 0.81 bD* ± 0.01 | 0.86 aA* ± 0.00 | |
| Bacteria | Zone of Inhibition (mm) | |
|---|---|---|
| Leaves | Flowers | |
| Bacillus cereus | 10.00 ± 0.0 | - |
| Staphylococcus aureus | 9.75 ± 0.5 | - |
| Pseudomonas aeruginosa | 10.50 ± 0.6 | - |
| Escherichia coli O157:H7 | - | - |
| P. major Jacq. | O-H Stretching (cm−1) | C-H Stretching (cm−1) | C=O Stretching (cm−1) | C=C Stretching (cm−1) | C-O Stretching (cm−1) | O-H Bending (cm−1) | C-O-C Stretching (cm−1) | |
|---|---|---|---|---|---|---|---|---|
| Flowers | 3296 | 2916, 2817 | 1760 | 1615 | 1238, 1022 | 1366 | 1002 | |
| Leaves | 3296 | 2916, 2817 | 1733 | 1620 | 1240, 1020 | 1363 | 1000 | |
|
Macerated Extraction | Flowers–acetone | 3296 | 2876 | 1651 | 1592 | 1264, 1038 | 1363 | 990 |
| Flowers–ethanol | 3296 | 2876 | 1661 | 1592 | 1264, 1038 | 1363 | 990 | |
| Flowers–methanol | 3296 | 2876 | 1697 | 1592 | 1264, 1038 | 1390 | 990 | |
| Leaves–acetone | 3296 | 2896 | 1713 | 1599 | 1261, 1039 | 1366 | 990 | |
| Leaves–ethanol | 3296 | 2896 | 1707 | 1602 | 1261, 1039 | 1366 | 990 | |
| Leaves–methanol | 3296 | 2896 | 1697 | 1580 | 1261, 1039 | 1388 | 990 | |
| Ultrasonic Extraction | Flowers–acetone | 3285 | 2896 | 1648 | 1599 | 1265, 1036 | 1367 | 990 |
| Flowers–ethanol | 3285 | 2896 | 1648 | 1599 | 1265, 1036 | 1367 | 990 | |
| Flowers–methanol | 3285 | 2896 | 1661 | 1599 | 1265, 1036 | 1386 | 990 | |
| Leaves–acetone | 3289 | 2892 | 1648 | 1599 | 1265, 1036 | 1361 | 987 | |
| Leaves–ethanol | 3289 | 2892 | 1648 | 1599 | 1265, 1036 | 1361 | 987 | |
| Leaves–methanol | 3289 | 2892 | 1661 | 1599 | 1265, 1036 | 1395 | 987 | |
| No | Compound | MW, (amu) | Flower and /or Leaf | RT * (min) | (M+) | (M+H)+ | (M-H)− |
|---|---|---|---|---|---|---|---|
| 1 | Eupatorin (3′,5-dihydroxy-4′,6,7-trimethoxyflavone) | 344.30 | F-L | 27.39 | 344 | - | - |
| 2 | Sinensetin (3′,4′,5,6,7- pentamethoxyflavone) | 372.36 | F-L | 29.96 | 372 | - | - |
| 3 | Apigenin (5,7,4′- trihydroxyflavone) | 270.24 | F-L | 26.97 | - | 271 | - |
| 4 | Naringenin (4′,5,7-trihydroxyflavanone) | 272.25 | L | 28.90 | - | 273 | - |
| 5 | 3,5,7-Trihydroxy-2-phenylchroman-4-one | 272.25 | L | 28.87 | - | - | 271 |
| 6 | Quersetin (3,5,7,3′,4′-pentahydroxyflavone) | 302.24 | L | 24.83 | - | 303 | - |
| 7 | Acacetin (5,7-dihydroxy-4′-methoxyflavone) | 284.26 | F-L | 24.27 | - | 285 | - |
| 8 | 3,5,7-Trihydroxy-4′-methoxyflavone | 300.26 | F-L | 24.46 | - | 301 | - |
| 9 | 5,7-Dihydroxy-3′,4′-dimethoxyflavone | 314.29 | F-L | 25.54 | - | 315 | - |
| 9 | 5,7-Dihydroxy-3′,4′-dimethoxyflavone | 314.29 | F-L | 25.51 | - | - | 313 |
| 10 | 5-Hydroxy-6,7,4′-trimethoxyflavanone | 330.33 | F-L | 24.46 | - | 331 | - |
| 11 | Ombuin(3,3′,5-trihydroxy-7,4′-dimethoxyflavone) | 330.29 | F-L | 26.39 | - | - | 329 |
| 12 | 6-Methoxyluteolin | 316.26 | F-L | 25.54 | 316 | - | - |
| 13 | Subscandenin(5,7-dihydroxy-8,4′-dimethoxyflavanone) | 316.31 | F-L | 23.76 | - | 317 | - |
| 14 | Persikogenin(5,3′-dihydroxy-7,4′-dimethoxyflavanone) | 316.31 | F-L | 25.51 | - | - | 315 |
| 15 | Isoquercitrin (quercetin-3-O-glucopyranoside) | 464.38 | F-L | 24.83 | - | 465 | - |
| 16 | Odoratin(2-hydroxy-4′,5′,6,4-tetramethoxy chalcone) | 344.36 | F-L | 27.39 | 344 | - | - |
| 16 | Odoratin(2-hydroxy-4′,5′,6,4-tetramethoxy chalcone) | 344.36 | F-L | 27.37 | - | - | 343 |
| 17 | 3,5-Dihydroxy-7-methoxy-flavanone | 286.28 | F-L | 18.41 | - | 287 | - |
| 18 | Luteolin (5, 7, 3′,4′-tetrahydroxy flavone) | 286.24 | F-L | 23.53 | - | 287 | - |
| 19 | 4′,5,6,7-tetramethoxyflavone(scutellarein tetramethyl ether) | 342.34 | F-L | 27.82 | - | 343 | - |
| 20 | 2′,4′,6′,3,4-Pentahydroxychalcone) | 288.25 | F-L | 30.45 | 288 | - | - |
| 20 | 2′,4′,6′,3,4-Pentahydroxychalcone | 288.25 | F-L | 26.93 | - | - | 287 |
| 21 | Quercetin-7,3′,4′-trimethylether | 344.32 | F-L | 27.39 | 344 | - | - |
| 22 | Mirisetin | 318.24 | F-L | 32.62 | 318 | - | - |
| 23 | Chalcone | 208.26 | F-L | 29.57 | - | 209 | - |
| 23 | Chalcone | 208.26 | F-L | 29.50 | - | - | 207 |
| 24 | Quercetin 3,7-diglucoside | 626.52 | F-L | 23.76 | 626 | - | - |
| 24 | Quercetin 3,7-diglucoside | 626.52 | F-L | 23.69 | - | - | 625 |
| 25 | Luteolin-7-O-glucuronide | 462.36 | F-L | 28.90 | - | 463 | - |
| 26 | Quercetin 3-O-β-D-glucopyranoside | 464.38 | F-L | 28.90 | 464 | - | - |
| 27 | Daidzein (7,4′-dihydroxyisoflavone) | 254.24 | F-L | 28.69 | - | 255 | - |
| 27 | Daidzein (7,4′-dihydroxyisoflavone) | 254.24 | F-L | 28.64 | - | - | 253 |
| 28 | (+)-Catechin | 290.27 | L | 28.69 | - | 291 | - |
| 28 | (+)-Catechin | 290.27 | L | 28.50 | - | - | 289 |
| 29 | Gallic acid | 170.12 | F-L | 32.25 | 170 | - | - |
| 30 | Syringic acid | 198.17 | F-L | 28.69 | - | 199 | - |
| 31 | Protocatechuic acid | 154.22 | F-L | 18.41 | 154 | - | - |
| 32 | Ferulic acid | 194.18 | F-L | 31.81 | 194 | - | - |
| 32 | Ferulic acid | 194.18 | F-L | 30.42 | - | - | 193 |
| 33 | Veratric acid | 182.17 | F-L | 16.62 | 182 | - | - |
| 34 | Benzenepropanoic acid | 150.17 | L | 27.82 | - | 151 | - |
| 35 | 5-Hydroxyferulic acid | 210.18 | L | 29.52 | 210 | - | - |
| 35 | 5-Hydroxyferulic acid | 210.18 | L | 28.64 | - | - | 209 |
| 36 | Eriocitrin (Eriodictyol 7-O-rutinoside) | 596.17 | F-L | 21.25 | - | - | 595 |
| 37 | Apigenin-7,4′-dimethyl ether | 298.29 | F-L | 33.04 | - | - | 297 |
| 38 | 4-Methoxycinnamic acid | 178.18 | L | 32.60 | - | - | 177 |
| 39 | Caffeic acid | 180.16 | F-L | 3.05 | - | - | 179 |
| 40 | Sinapic acid | 224.21 | L | 29.67 | - | - | 223 |
| 40 | Sinapic acid | 224.21 | F | 22.498 | - | 225 | - |
| 41 | Myricetin 3-β-D-glucopyranoside | 480.38 | F-L | 25.27 | - | - | 479 |
| 42 | Kaempferol-4′-glucoside | 448.38 | F-L | 32.18 | - | - | 447 |
| 43 | p-Coumaric acid | 164.16 | F-L | 4.10 | - | - | 163 |
| 44 | p-Hydroxybenzoic acid | 138.12 | F-L | 29.27 | - | - | 137 |
| 45 | 3-phenyl-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one | 256.25 | F-L | 34.31 | - | - | 255 |
| 46 | AgestricinC(6-hydroxy-5,7,3′,4′-tetramethoxyflavanone) | 360.36 | F-L | 25.51 | - | - | 359 |
| 47 | Vanilic acid | 168.18 | L | 26.39 | - | - | 167 |
| 47 | Vanillic acid | 168.18 | F | 25.390 | - | 169 | - |
| 48 | 5-hydroxy-6,7,3′,4′-tetramethoxyflavone | 358.30 | F | 2.175 | 358 | - | - |
| 49 | Cinnamic acid | 148.16 | F | 33.713 | - | 149 | - |
| 50 | Nevadensin(5,7-dihydroxy-6,8,4′-trimethoxyflavone) | 344.32 | L | 18.26 | 344 | - | - |
| 51 | Hesperitin (hesperetin-7-rutinoside) | 610.60 | F-L | 21.41 | - | 611 | - |
| 52 | Quercetin-3-O-α-L-rhamnopyranosyl-(1–6)-β-D-galactopyranoside | 610.52 | F-L | 21.40 | - | - | 609 |
| 53 | Vicenin-2 (apigenin-6,8-di-C-glucoside) | 594.50 | F-L | 22.15 | - | 595 | - |
| 54 | Padmatin (taxifolin-7-methyl ether) | 318.28 | F-L | 28.69 | - | - | 319 |
| 55 | Apigenin-5-O-β-D-glucopyranoside | 432.40 | L | 18.96 | - | 433 | - |
| 55 | Apigenin-5-O-β-D-glucopyranoside | 432.40 | L | 18.30 | - | - | 431 |
| 56 | Quercetin-3-O-β-D-glucuronide | 478.36 | F-L | 26.97 | - | 479 | - |
| 57 | Narcissin (isorhamnetin-3-rutinoside) | 624.54 | F-L | 23.76 | - | 625 | - |
| 57 | Narcissin (isorhamnetin-3-rutinoside) | 624.54 | F-L | 23.69 | - | - | 623 |
| 58 | Quercetin 3-O-α-L-arabinoside | 434.35 | F-L | 25.30 | - | 435 | - |
| 58 | Quercetin 3-O-α-L-arabinoside | 434.35 | F-L | 25.27 | - | - | 433 |
| 59 | Quercetin-3-O-rutinoside-7-O-glycoside | 772.66 | F-L | 24.71 | 772 | - | - |
| 59 | Quercetin-3-O-rutinoside-7-O-glycoside | 772.66 | F-L | 23.38 | - | - | 771 |
| 60 | Spireasalicin (Kuersetin-3-O-[6″-(4′′′-hidroksi-2′′′-metilenbutirol)]- β-D-glukopiranosid) | 562.48 | F-L | 20.42 | - | 563 | - |
| 61 | (+)-Catechin-7-α-L-arabinofuranoside | 422.38 | F-L | 28.90 | - | 423 | - |
| 62 | 3,5-Dicaffeoylquinic acid | 516.45 | F-L | 26.53 | 516 | - | - |
| 63 | Cichoric acid | 474.37 | L | 21.60 | - | 475 | - |
| 64 | 5-O-feruloylquinic acid | 368.30 | F-L | 22.55 | - | 369 | - |
| 65 | Apigenin-7-O-glucuronide | 446.36 | F-L | 20.93 | 446 | - | - |
| 66 | Amentoflavone | 538.45 | L | 19.22 | 538 | - | - |
| 67 | Hiperosit (Quercetin-3-galactoside) | 464.38 | L | 24.83 | - | 465 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmazer Keskin, S.; Avcı, A.; Arif Ali Ali, L.; Keskin, C.S. Analysis of Polyphenols from Polygala major Jacq. Antioxidants 2025, 14, 153. https://doi.org/10.3390/antiox14020153
Yılmazer Keskin S, Avcı A, Arif Ali Ali L, Keskin CS. Analysis of Polyphenols from Polygala major Jacq. Antioxidants. 2025; 14(2):153. https://doi.org/10.3390/antiox14020153
Chicago/Turabian StyleYılmazer Keskin, Semra, Ayşe Avcı, Lana Arif Ali Ali, and Can Serkan Keskin. 2025. "Analysis of Polyphenols from Polygala major Jacq." Antioxidants 14, no. 2: 153. https://doi.org/10.3390/antiox14020153
APA StyleYılmazer Keskin, S., Avcı, A., Arif Ali Ali, L., & Keskin, C. S. (2025). Analysis of Polyphenols from Polygala major Jacq. Antioxidants, 14(2), 153. https://doi.org/10.3390/antiox14020153






