Phytochemical Screening, Pharmacognostic Characterization, Antioxidant Activity, and Hepatoprotective Effects of Abroma augustum (L.) L.f. on Human Hepatocellular Carcinoma (HepG2) Cells and Goat Liver Homogenate
Abstract
1. Introduction
2. Materials and Methods
2.1. Phytochemical Screening Using Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HRMS)
2.2. Preparation for Pharmacognostic Studies
2.3. Organoleptic Evaluation
2.4. Macroscopic Evaluation
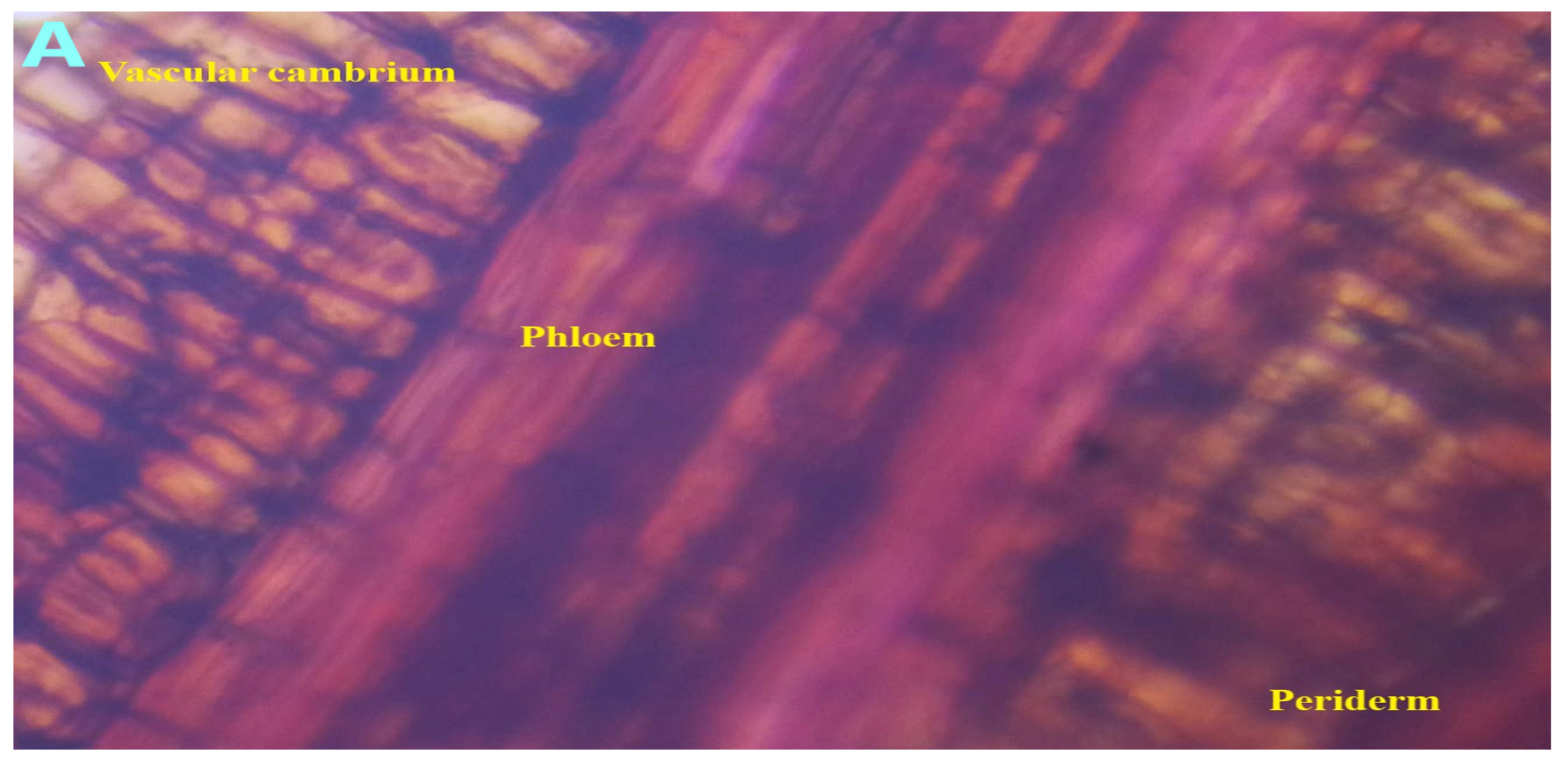
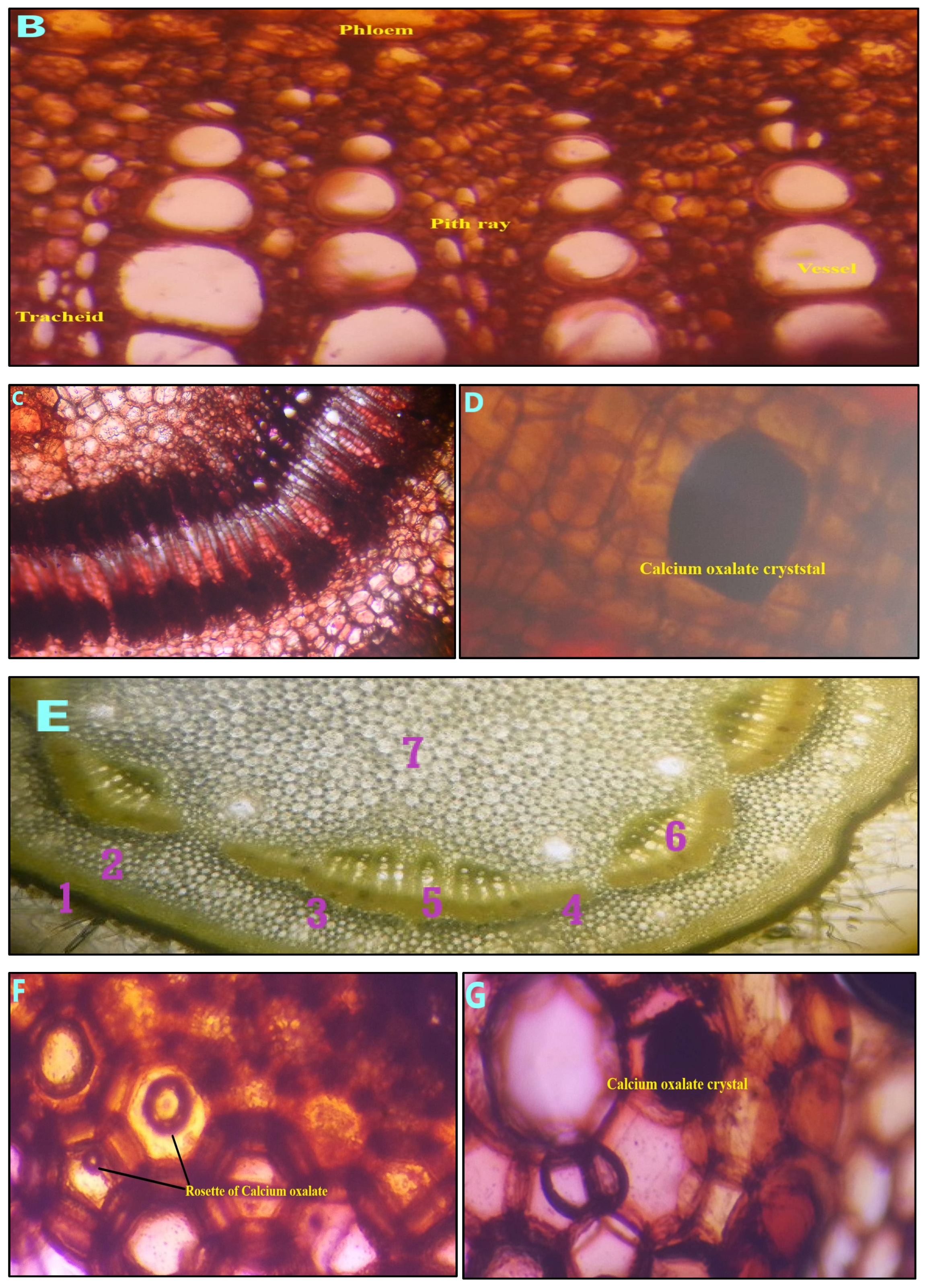
2.5. Microscopic Evaluation
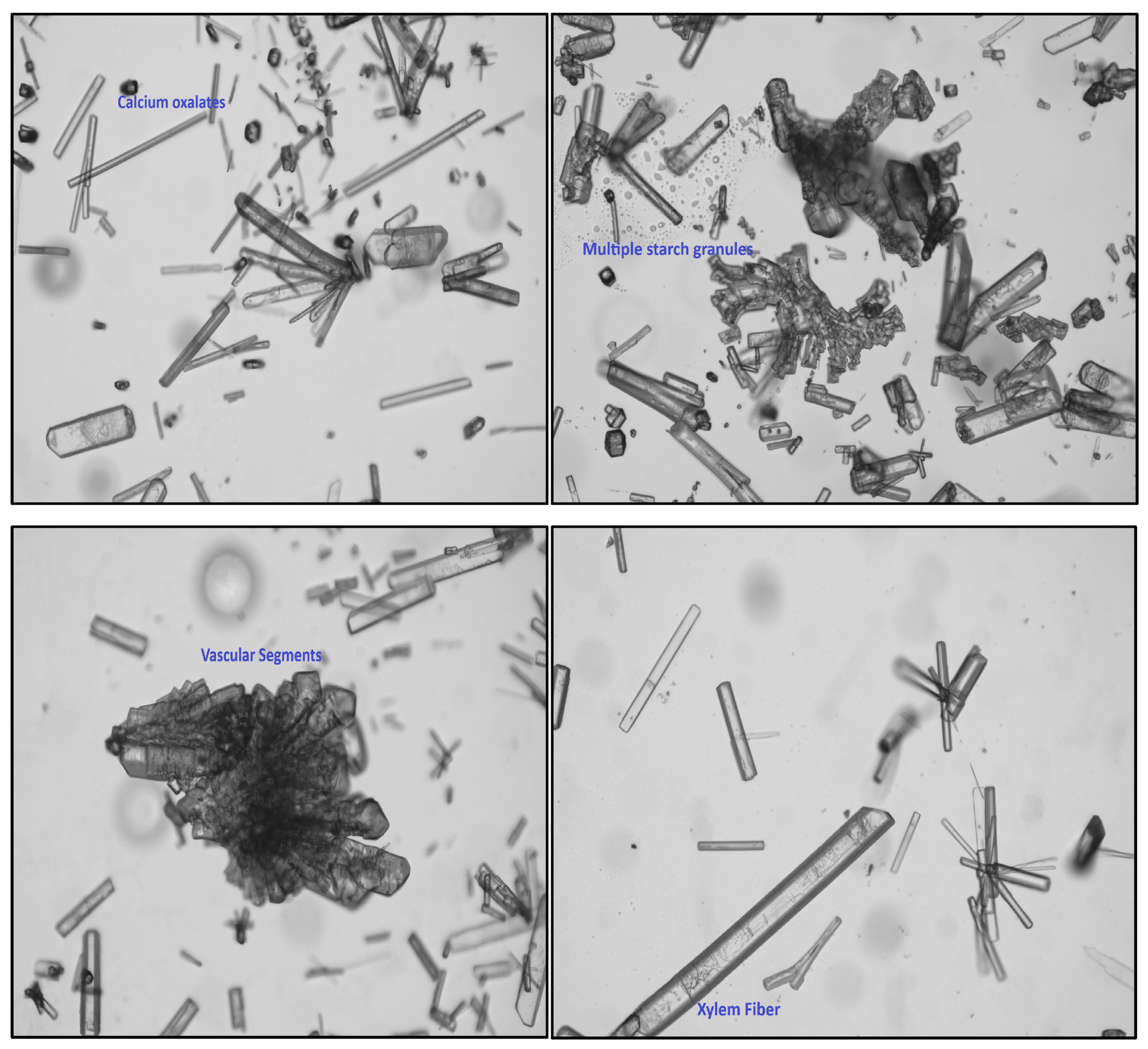
2.6. Powder Microscopic Evaluation
2.7. Histochemical Color Reaction
2.8. Physicochemical Parameters
2.8.1. Total Ash Content
2.8.2. Acid-Insoluble Ash
2.8.3. Water-Soluble Ash
2.8.4. Sulfate Ash
2.8.5. Foreign Matter
2.8.6. Loss of Dying
2.8.7. Foaming Index
2.8.8. Swelling Index
2.8.9. pH of 10%
2.9. Methanolic Stem Bark Extract Preparation
2.10. Qualitative Phytochemical Investigation
2.11. Fluorescence Analysis of Plant Powder with Chemical Reagents
2.12. Quantification of Total Phenolics and Total Flavonoids
2.13. Antioxidants and Radical Scavenging Activity
2.14. In Vitro and Ex Vivo Hepatoprotective Activity
3. Results
3.1. Phytochemical Screening
3.2. Pharmacognostic Standardization and Physicochemical Characterization
3.3. Qualitative Phytochemical Investigation and Fluorescence Analysis
3.4. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.5. Quantification of Antioxidant Properties
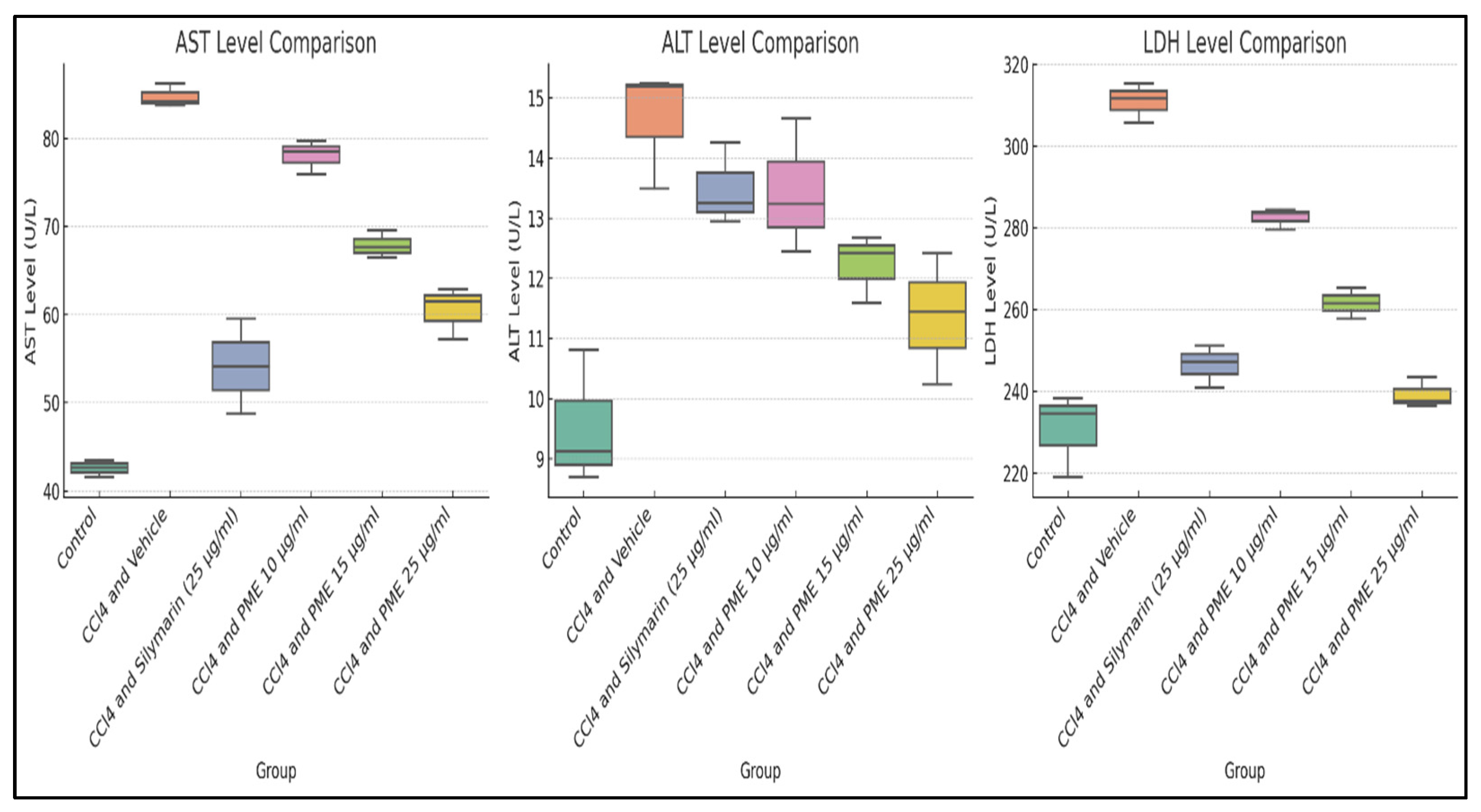
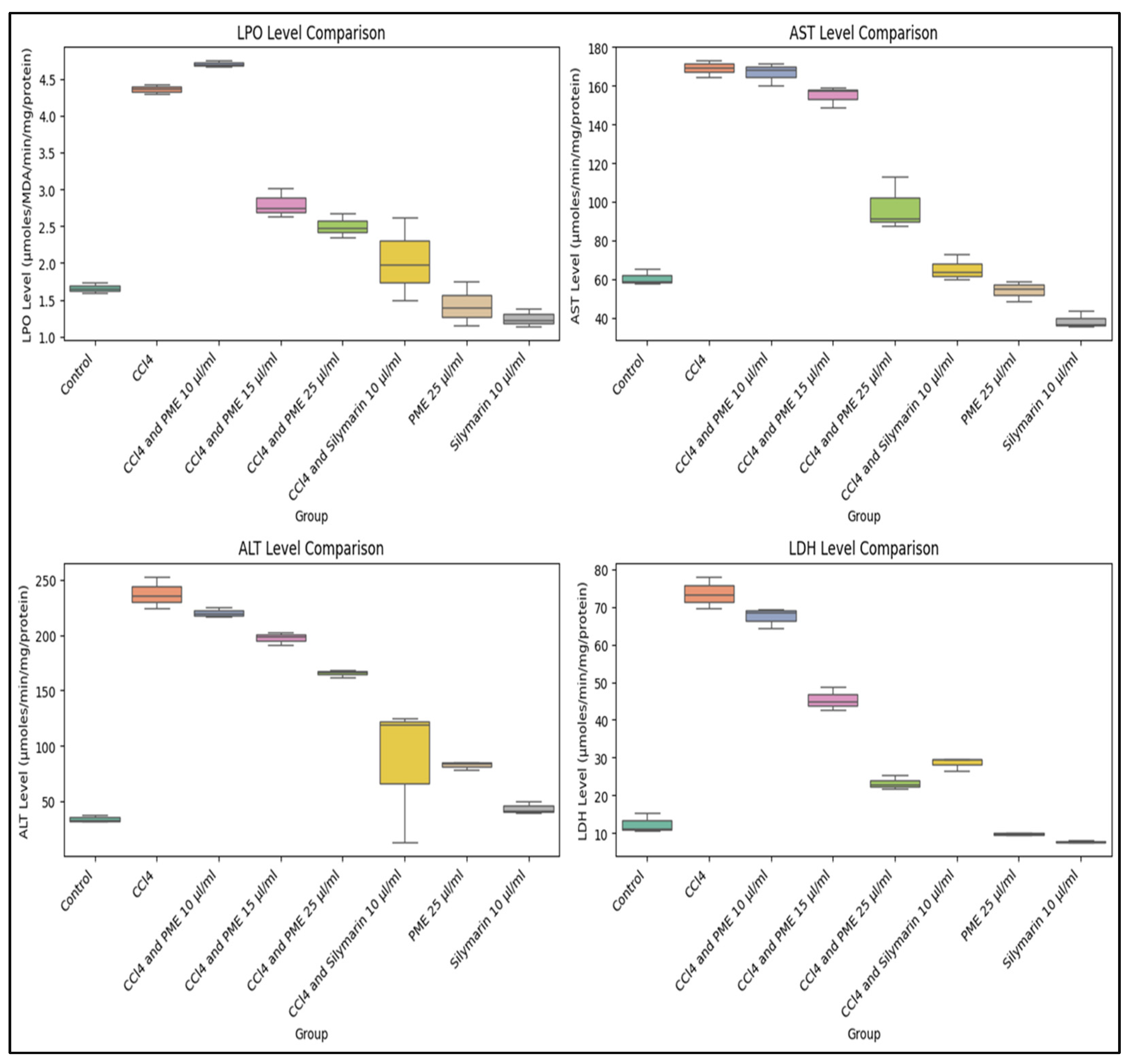
3.6. In Vitro Hepatoprotective Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CCl3+ | Trichloromethyl radicals |
| CCl4 | Carbon tetrachloride |
| CO2 | Carbon dioxide |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| Fe2+ | Ferrous ions |
| Fe3+ | Ferric ions |
| FeCl3 | Ferric chloride |
| FRAP | Ferric-reducing antioxidant power |
| HepG2 | Human hepatocellular carcinoma G2 |
| H2SO4 | Sulfuric acid |
| HNO3 | Nitric acid |
| IC50 | Inhibitory concentration at 50% |
| LC-HRMS | Liquid chromatography–high-resolution mass spectrometry |
| LDH | Lactate dehydrogenase |
| LPO | Lipid peroxidation |
| MDA | Malondialdehyde |
| MTT | 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide |
| Na2CO3 | Sodium carbonate |
| NaOH | Sodium hydroxide |
| PBS | Phosphate-buffered saline |
| PME | Plant methanolic extract |
| PUFA | Polyunsaturated fatty acid |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TS | Transverse section |
| WHO | World Health Organization |
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Saggar, S.; Mir, P.A.; Kumar, N.; Chawla, A.; Uppal, J.; Shilpa, S.; Kaur, A. Traditional and Herbal Medicines: Opportunities and Challenges. Pharmacogn. Res. 2022, 14, 107–114. [Google Scholar] [CrossRef]
- Ho, Y.; Ah, J.; Xuan, N. Phytochemicals and Their Pharmacological Aspects of Acanthopanax Koreanum. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0296-0. [Google Scholar]
- Dahiya, S.; Banerjee, D.N. Complementary and alternative medicine: What is it good for? Bull. Pharm. Res. 2016, 6, 83–92. [Google Scholar] [CrossRef]
- Dekebo, A. Introductory Chapter: Plant Extracts. In Plant Extracts; Dekebo, A., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-83881-888-3. [Google Scholar]
- Shukla, S.K. Conservation of Medicinal Plants: Challenges and Opportunities. J. Med. Bot 2023, 7, 5–10. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L. Traditional Medicines and Globalization: Current and Future Perspectives in Ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef]
- Majid, N.; Nissar, S.; Raja, W.Y.; Nawchoo, I.A.; Bhat, Z.A. Pharmacognostic Standardization of Aralia Cachemirica: A Comparative Study. Futur J. Pharm. Sci. 2021, 7, 33. [Google Scholar] [CrossRef]
- Kasilo, O.M.J.; Nikiema, J. World Health Organization Perspective for Traditional Medicine. In Novel Plant Bioresources; Gurib-Fakim, A., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 23–42. ISBN 978-1-118-46061-0. [Google Scholar]
- Abroma Augustum. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/5/3/5350 (accessed on 20 January 2025).
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Das, U.; Islam, M.S. A Review Study on Different Plants in Malvaceae Family and Their Medicinal Uses. Am. J. Biomed. Sci. Res 2019, 3, 94–97. [Google Scholar]
- Rahman, A.; Gondha, R. Taxonomy and Traditional Medicine Practices on Malvaceae (Mallow Family) of Rajshahi, Bangladesh. Open J. Bot. 2014, 1, 19–24. [Google Scholar]
- Parimelazhagan, T. Pharmacological Assays of Plant-Based Natural Products; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2016; Volume 71, ISBN 978-3-319-26810-1. [Google Scholar]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Kunle Standardization of Herbal Medicines—A Review. Int. J. Biodvers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Cavazos, P.; Gonzalez, D.; Lanorio, J.; Ynalvez, R. Secondary Metabolites, Antibacterial and Antioxidant Properties of the Leaf Extracts of Acacia Rigidula Benth. and Acacia Berlandieri Benth. SN Appl. Sci. 2021, 3, 522. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Ozougwu, J.C. Physiology of the Liver. Int. J. Res. Pharm. Biosci. 2017, 4, 13–24. [Google Scholar]
- Sowunmi, B.O.; Gonzo, M. The Effect of Moringa Oleifera Crude Extract on Liver Cell Line, HepG2. BMC Complement. Med. Ther. 2023, 23, 380. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, B.; Fan, Y.; Wang, M.; Kebebe, D.; Li, J.; Liu, Z. Traditional Chinese Medicine Combined with Hepatic Targeted Drug Delivery Systems: A New Strategy for the Treatment of Liver Diseases. Biomed. Pharmacother. 2019, 117, 109128. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Sapian, S.; Budin, S.B.; Taib, I.S.; Mariappan, V.; Zainalabidin, S.; Chin, K.Y. Role of Polyphenol in Regulating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis in Diabetic Nephropathy. Endocr. Metab. Immune Disord.-Drug Targets 2022, 22, 453–470. [Google Scholar] [CrossRef]
- Gupta, A.; Sheth, N.R.; Pandey, S.; Shah, D.R.; Yadav, J.S. Design and Evaluation of Herbal Hepatoprotective Formulation against Paracetamol Induced Liver Toxicity. J. Young Pharm. 2013, 5, 180–187. [Google Scholar] [CrossRef]
- Ghosh, N.; Ghosh, R.; Mandal, V.; Mandal, S.C. Recent Advances in Herbal Medicine for Treatment of Liver Diseases. Pharm. Biol. 2011, 49, 970–988. [Google Scholar] [CrossRef] [PubMed]
- Redfern, J.; Kinninmonth, M.; Burdass, D.; Verran, J. Using Soxhlet Ethanol Extraction to Produce and Test Plant Material (Essential Oils) for Their Antimicrobial Properties. J. Microbiol. Biol. Educ. 2014, 15, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Kokate, C. Textbook of Pharmaceutical Biotechnology; Elsevier: Kundli, Haryana, India, 2011. [Google Scholar]
- Kumar, S.; Kumar, V.; Prakash, O. Microscopic Evaluation and Physiochemical Analysis of Dillenia Indica Leaf. Asian Pac. J. Trop. Biomed. 2011, 1, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, A.N.M. Microscopy in Pharmacognosy. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2017; Volume 73, pp. 497–513. ISBN 978-3-319-63861-4. [Google Scholar]
- Gurav, S.; Gurav, N. Herbal Drug Microscopy. In Indian Herbal Drug Microscopy; Gurav, S.S., Gurav, N.S., Eds.; Springer: New York, NY, USA, 2014; pp. 15–196. ISBN 978-1-4614-9514-7. [Google Scholar]
- Aeri, V.; Narayana, D.A.; Singh, D. Powdered Crude Drug Microscopy of Leaves and Barks; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Muhamad, I.I.; Hassan, N.D.; Mamat, S.N.H.; Nawi, N.M.; Rashid, W.A.; Tan, N.A. Extraction Technologies and Solvents of Phytocompounds From Plant Materials: Physicochemical Characterization and Identification of Ingredients and Bioactive Compounds From Plant Extract Using Various Instrumentations. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 523–560. ISBN 978-0-12-811521-3. [Google Scholar]
- Feng, W.; Li, M.; Hao, Z.; Zhang, J. Analytical Methods of Isolation and Identification. In Phytochemicals in Human Health; Rao, V., Mans, D., Rao, L., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-587-6. [Google Scholar]
- Kokate, C.K.; Purohit, A.P.; Gokhale, S.B. Pharmacognosy, 55th ed.; Nirali Prakashan: Pune, Maharashtra, India, 2008; ISBN 10: 8196396155. [Google Scholar]
- Stankovic, M.S.; Zia-Ul-Haq, M.; Bojovic, B.M.; Topuzovic, M.D. Total Phenolics, Flavonoid Content and Antioxidant Power of Leaf, Flower and Fruits from Cornelian Cherry (Cornus mas L.). Bulg. J. Agric. Sci. 2014, 20, 358–363. [Google Scholar]
- Pellegrini, N.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F.; Serafini, M. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Wan, C.; Yu, Y.; Zhou, S.; Liu, W.; Tian, S.; Cao, S. Antioxidant Activity and Free Radical-Scavenging Capacity of Gynura Divaricata Leaf Extracts at Different Temperatures. Pharmacogn. Mag. 2011, 7, 40. [Google Scholar]
- Martinez-Morales, F.; Alonso-Castro, A.J.; Zapata-Morales, J.R.; Carranza-Álvarez, C.; Aragon-Martinez, O.H. Use of Standardized Units for a Correct Interpretation of IC50 Values Obtained from the Inhibition of the DPPH Radical by Natural Antioxidants. Chem. Pap. 2020, 74, 3325–3334. [Google Scholar] [CrossRef]
- Thiesen, L.C.; da Silva, L.M.; Santin, J.R.; Bresolin, T.M.B.; de Andrade, S.F.; de Medeiros Amorim, C.; Merlin, L.; de Freitas, R.A.; Niero, R.; Netz, D.J.A. Hepatoprotective Effect of Maytenus Robusta Reiss Extract on CCl4-Induced Hepatotoxicity in Mice and HepG2 Cells. Regul. Toxicol. Pharmacol. 2017, 86, 93–100. [Google Scholar] [CrossRef]
- Nambiar, M.K.G.; Varghese, V. In Vitro Hepatoprotective Activity of Methanolic Leaf Extract of Acalypha Indica Against CCl4 Induced Hepatotoxicity in Goat Liver Slice Culture. Trends Sci. 2023, 20, 4562. [Google Scholar] [CrossRef]
- Alhassan, A.J.; Sule, M.J.; Aliyu, S.A.; Aliyu, M.D. Ideal Hepatotoxicity Model in Rats Using Carbon Tetrachloride (CCl4). Bayero J. Pure Appl. Sci. 2009, 2, 185–187. [Google Scholar]
- Peleman, C.; Francque, S.; Berghe, T.V. Emerging Role of Ferroptosis in Metabolic Dysfunction-Associated Steatotic Liver Disease: Revisiting Hepatic Lipid Peroxidation. EBioMedicine 2024, 102, 105088. [Google Scholar] [CrossRef]
- Gwaltney-Brant, S.M. Nutraceuticals in Hepatic Diseases. In Nutraceuticals; Elsevier: London, UK, 2021; pp. 117–129. [Google Scholar]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Akaberi, T.; Shourgashti, K.; Emami, S.A.; Akaberi, M. Phytochemistry and Pharmacology of Alkaloids from Glaucium spp. Phytochemistry 2021, 191, 112923. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Khadka, D.B.; Cho, W.-J. Pharmacological Effects of Berberine and Its Derivatives: A Patent Update. Expert Opin. Ther. Pat. 2016, 26, 229–243. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, Y.; Wang, M.; Guo, C.; Cao, Z.; Zhang, R.; Peng, C. A Review of Pharmacological and Pharmacokinetic Properties of Stachydrine. Pharmacol. Res. 2020, 155, 104755. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Jeong, W.; Bazer, F.W.; Lee, D.; Song, G. Sideroxylin (Callistemon lanceolatus) Suppressed Cell Proliferation and Increased Apoptosis in Ovarian Cancer Cells Accompanied by Mitochondrial Dysfunction, the Generation of Reactive Oxygen Species, and an Increase of Lipid Peroxidation. J. Cell. Physiol. 2018, 233, 8597–8604. [Google Scholar] [CrossRef]
- Sinha, K.; Ghosh, J.; Sil, P.C. Morin and Its Role in Chronic Diseases. In Anti-inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 453–471. ISBN 978-3-319-41334-1. [Google Scholar]
- Thapa, R.; Afzal, O.; Alfawaz Altamimi, A.S.; Goyal, A.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Jakhmola, V.; Singh, S.K.; Dua, K.; et al. Galangin as an Inflammatory Response Modulator: An Updated Overview and Therapeutic Potential. Chem. -Biol. Interact. 2023, 378, 110482. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef]
- Mochahary, B.; Brahma, S.; Kalita, M.; Goyal, A.K. Characterisation of Indigenous Plants for Herbal Formulations Preparation Based on Pharmacognostic and Physiochemical Data. Plant Sci. Today 2022, 9, 8–17. [Google Scholar] [CrossRef]
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Preparation and Characterization of Mucilage Polysaccharide for Biomedical Applications. Carbohydr. Polym. 2013, 98, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in Plant Life. Front. Plant Sci. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Banjarnahor, S.D.; Artanti, N. Antioxidant Properties of Flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef]
- Al-Nu’airat, J.; Dlugogorski, B.Z.; Gao, X.; Zeinali, N.; Skut, J.; Westmoreland, P.R.; Oluwoye, I.; Altarawneh, M. Reaction of Phenol with Singlet Oxygen. Phys. Chem. Chem. Phys. 2019, 21, 171–183. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez De La Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Chimi, H.; Cillard, J.; Cillard, P.; Rahmani, M. Peroxyl and Hydroxyl Radical Scavenging Activity of Some Natural Phenolic Antioxidants. J. Am. Oil Chem. Soc. 1991, 68, 307–312. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, F. Phenolic Compounds from Plants as Nitric Oxide Production Inhibitors. Curr. Med. Chem. 2011, 18, 1137–1145. [Google Scholar] [CrossRef]
- Pannala, A.S.; Singh, S.; Rice-Evans, C. [19] Flavonoids as Peroxynitrite Scavengers in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 207–235. [Google Scholar]
- Heijnen, C.G.; Haenen, G.R.; Vekemans, J.A.; Bast, A. Peroxynitrite Scavenging of Flavonoids: Structure Activity Relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-919-5. [Google Scholar]
- Adewusi, E.A.; Afolayan, A.J. A Review of Natural Products with Hepatoprotective Activity. J. Med. Plants Res. 2010, 4, 1318–1334. [Google Scholar]
- Gerhäuser, C. Phenolic Beer Compounds to Prevent Cancer. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 669–684. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Protective Effect of Rutin on Paracetamol- and CCl4-Induced Hepatotoxicity in Rodents. Fitoterapia 2002, 73, 557–563. [Google Scholar] [CrossRef]
- Zarezade, V.; Moludi, J.; Mostafazadeh, M.; Mohammadi, M.; Veisi, A. Antioxidant and Hepatoprotective Effects of Artemisia Dracunculus against CCl4-Induced Hepatotoxicity in Rats. Avicenna J. Phytomed. 2018, 8, 51. [Google Scholar]
- Moyo, B.; Oyedemi, S.; Masika, P.J.; Muchenje, V. Polyphenolic Content and Antioxidant Properties of Moringa Oleifera Leaf Extracts and Enzymatic Activity of Liver from Goats Supplemented with Moringa Oleifera Leaves/Sunflower Seed Cake. Meat Sci. 2012, 91, 441–447. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Bradley, G.; Afolayan, A.J. In-Vitro and-Vivo Antioxidant Activities of Aqueous Extract of Strychnos Henningsii Gilg. Afr. J. Pharm. Pharmacol. 2010, 4, 70–78. [Google Scholar]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of Endogenous Antioxidant System by Wine Polyphenols in Human Disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Lala, V.; Zubair, M.; Minter, D. Liver Function Tests; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Raval, N.; Kalyane, D.; Maheshwari, R.; Tekade, R.K. Chapter 17–Surface Modifications of Biomaterials and Their Implication on Biocompatibility. Biomater. Bionanotechnol. 2019, 639–674. [Google Scholar] [CrossRef]
- Moriles, K.; Zubair, M.; Azer, S. Alanine Aminotransferase (ALT) Test; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zhang, W.; Chen, L.; Feng, H.; Wang, W.; Cai, Y.; Qi, F.; Tao, X.; Liu, J.; Shen, Y.; Ren, X.; et al. Rifampicin-Induced Injury in HepG2 Cells Is Alleviated by TUDCA via Increasing Bile Acid Transporters Expression and Enhancing the Nrf2-Mediated Adaptive Response. Free Radic. Biol. Med. 2017, 112, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Boro, H.; Usha, T.; Babu, D.; Chandana, P.; Goyal, A.K.; Ekambaram, H.; Yusufoglu, H.S.; Das, S.; Middha, S.K. Hepatoprotective Activity of the Ethanolic Extract of Morus Indica Roots from Indian Bodo Tribes. SN Appl. Sci. 2022, 4, 49. [Google Scholar] [CrossRef]
- Cui, B.; Liu, S.; Lin, X.; Wang, J.; Li, S.; Wang, Q.; Li, S. Effects of Lycium Barbarum Aqueous and Ethanol Extracts on High-Fat-Diet Induced Oxidative Stress in Rat Liver Tissue. Molecules 2011, 16, 9116–9128. [Google Scholar] [CrossRef]
- Bachar, S.C.; Mahmud, Z.A.; Qais, N. Antioxidant and Hepatoprotective Activities of Ethanolic Extracts of Leaves of Premna Esculenta Roxb. against Carbon Tetrachloride-Induced Liver Damage in Rats. J. Young Pharm. 2012, 4, 228–234. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, X.; Liu, Z.; Song, S.; Xue, P.; Wang, J.; Ruan, J. Ethanol Extract of Adiantum Capillus-Veneris L. Suppresses the Production of Inflammatory Mediators by Inhibiting NF-κB Activation. J. Ethnopharmacol. 2013, 147, 603–611. [Google Scholar] [CrossRef]
- Makhuvele, R.; Foubert, K.; Hermans, N.; Pieters, L.; Verschaeve, L.; Elgorashi, E. Protective Effects of Methanolic Leaf Extracts of Monanthotaxis Caffra against Aflatoxin B1-Induced Hepatotoxicity in Rats. Onderstepoort J. Vet. Res. 2022, 89, 1968. [Google Scholar] [CrossRef]

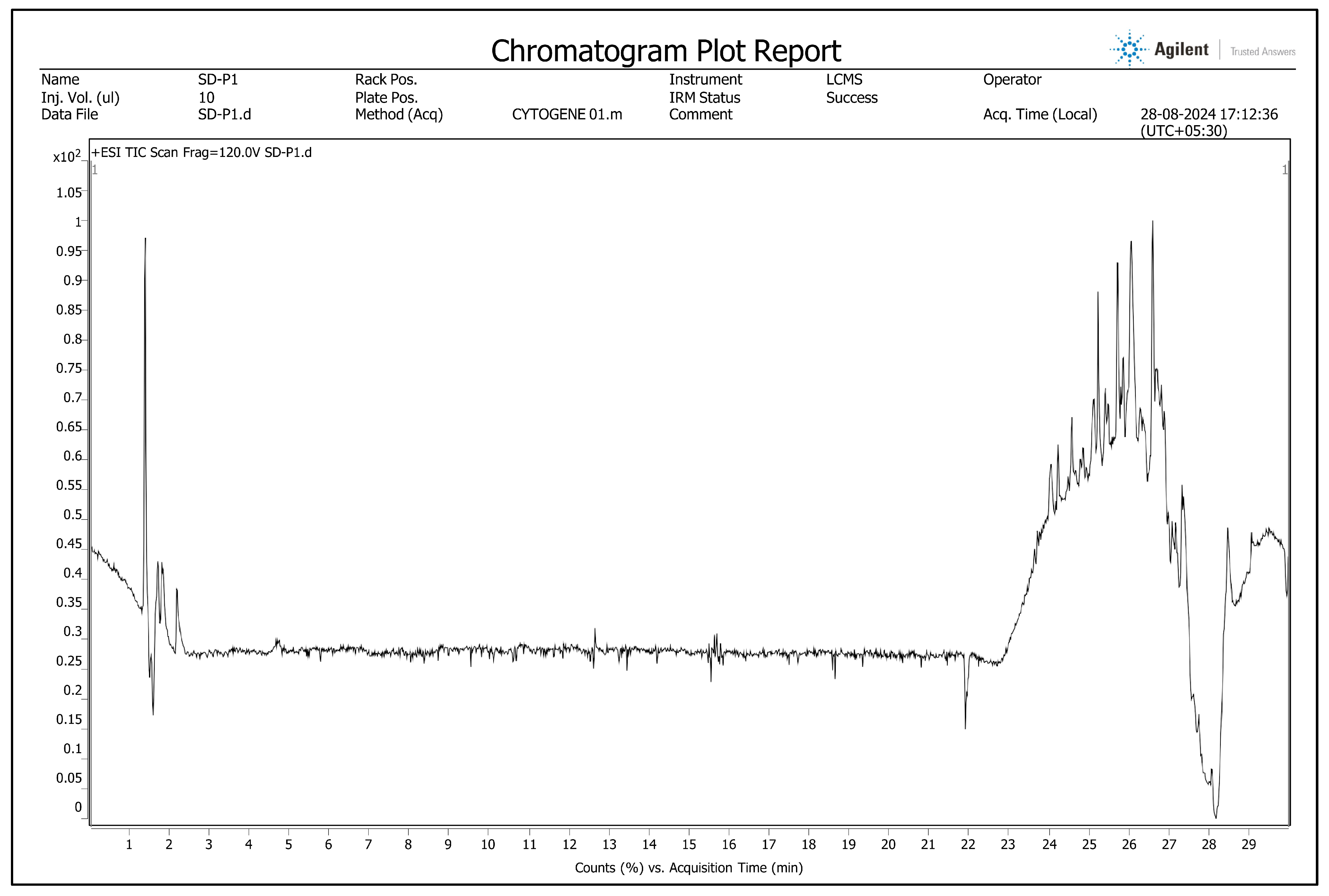
| Sl. No. | Name of the Compound | Possible Nature | Retention Time (minutes) | Adduct | Molecular Weight (g/mol) | Chemical Formula |
|---|---|---|---|---|---|---|
| 1 | Protopine | Alkaloids | 25.99932 | [M + NH4]+ | 353.4 | C20H19NO5 |
| 2 | Stachydrine | 1.8074 | [M + H]+ | 143.18 | C7H13NO2 | |
| 3 | Tsitsikammamine A | 25.86585 | [M + H]+ | 303.3 | C18H13N3O2 | |
| 4 | 3-methyloxindole(oxindoles) | 24.51443 | [M + H]+ | 313.3 | C9H9NO | |
| 5 | Heliamine | 26.68337 | [M + H]+ | 193.24 | C11H15NO2 | |
| 6 | (−)-caaverine | 25.41538 | [M + H]+ | 267.32 | C17H17NO2 | |
| 7 | Berberine | 24.78138 | [M + Na]+ | 336.4 | C20H18NO4+ | |
| 8 | Caffeine | 24.7647 | [M + H]+ | 194.19 | C8H10N4O2 | |
| 9 | Harmine | 24.23082 | [M + H]+ | 212.25 | C13H12N2O | |
| 10 | Theobromine | 25.24853 | [M + H]+ | 180.16 | C7H8N4O2 | |
| 11 | 9-methoxycanthin-6-one | 25.7157 | [M + H]+ | 250.25 | C15H10N2O2 | |
| 12 | Sideroxylin | Flavonoids | 27.1672 | [M + H]+ | 312.3 | C18H16O5 |
| 13 | Acacetin | 27.65103 | [M + Na]+ | 284.26 | C16H12O5 | |
| 14 | Galangin | 28.56867 | [M + H]+ | 270.24 | C15H10O5 | |
| 15 | Morin | 1.723983 | [M + K]+ | 302.23 | C15H10O7 | |
| 16 | Daidzein | 1.406983 | [M + H]+ | 254.24 | C15H10O4 | |
| 17 | Tectochrysin | 24.54782 | [M + H]+ | 268.26 | C16H12O4 | |
| 18 | 6-desmethylsideroxylin | 27.1672 | [M + H]+ | 298.29 | C17H14O5 | |
| 19 | Pinocembrin | 26.11612 | [M + K]+ | 256.25 | C15H12O4 | |
| 20 | Hesperetin | 25.23185 | [M + K]+ | 302.28 | C16H14O6 | |
| 21 | Sanggenon G | 27.1672 | [M + H]+ | 694.7 | C40H38O11 | |
| 22 | Kahweol | Terpenes | 1.8074 | [M + Na]+ | 314.4 | C20H26O3 |
| 23 | Dihydroactinidiolide, (+/−) | 26.016 | [M + H]+ | 180.24 | C11H16O2 | |
| 24 | 8-deoxylactucin | 26.36637 | [M + H]+ | 438.2 | C15H16O4 | |
| 25 | Furanoheliangolide | 25.94927 | [M + H]+ | 360.4 | C19H20O7 | |
| 26 | Nakijiquinone H | 27.71778 | [M + H]+ | 456.6 | C26H40N4O3 | |
| 27 | Loliolide | 24.58118 | [M + H]+ | 196.24 | C11H16O3 | |
| 28 | 12b-O-[deca-2E,4Z-dienoyl]-13a-isobutyl-4b-phorbol | 28.10152 | [2M + Na]+ | 584.7 | C34H48O8 | |
| 29 | Selaginpulvilin M | Phenolics | 27.65103 | [M + Na]+ | 524.6 | C36H28O4 |
| 30 | 3’,4’,5’-trimethoxyacetophenone | 24.54782 | [M + H]+ | 210.23 | C11H14O4 | |
| 31 | (2-Hydroxy-5-methoxyphenyl) (4-hydroxyphenyl)methanone | 26.71673 | [M + Na]+ | 244.24 | C14H12O4 | |
| 32 | 2-acyl-4,6-bisprenylphloroglucinol | 27.2673 | [M + Na]+ | 290.4 | C17H22O4 | |
| 33 | Chlorogenic acid | 26.61663 | [M + H]+ | 354.31 | C16H18O9 | |
| 34 | 3-(8-hydroxyoctyl)phenol | 26.86688 | [M + H]+ | 222.32 | C14H22O2 | |
| 35 | Resveratrol | 25.79912 | [M + H]+ | 228.24 | C14H12O3 | |
| 36 | Cornexistin | 23.98055 | [M + H]+ | 308.33 | C16H20O6 | |
| 37 | Demethoxycurcumin | Coumarins | 26.8502 | [M + H]+ | 338.4 | C20H18O5 |
| 38 | Scopoletin | 12.18487 | [M + Na]+ | 192.17 | C10H8O4 | |
| 39 | Umbelliferone | 1.723983 | [M + H]+ | 162.14 | C9H6O3 | |
| 40 | Urolithin A | 1.406983 | [M + H]+ | 228.20 | C13H8O4 | |
| 41 | Questin | Quinones | 26.16617 | [M + H]+ | 284.26 | C16H12O5 |
| 42 | 2-methoxy-1,4-naphthoquinone | 24.0306 | [M + H]+ | 188.18 | C11H8O3 | |
| 43 | 2-Hydroxy-3-(3-methylbut-2-enyl)-1,4-naphthoquinone | 24.0306 | [M + H]+ | 242.27 | C15H14O3 | |
| 44 | Oxypeucedanin | Furocoumarin | 25.98263 | [M + H]+ | 286.28 | C16H14O5 |
| 45 | 2,4,5-rimethoxybenzoic acid | Benzoic acid | 25.24853 | [M + H]+ | 212.20 | C10H12O5 |
| 46 | Verbascoside | Glycosides | 26.71673 | [2M + Na]+ | 624.6 | C29H36O15 |
| 47 | Gentiopicrin | 27.15052 | [M + Na]+ | 356.32 | C16H20O9 | |
| 48 | Ectoine | Carboxamidine | 1.406983 | [M + 2H]2+ | 142.16 | C6H10N2O2 |
| 49 | Licarin A | Benzofuran | 26.016 | [M + Na]+ | 326.4 | C20H22O4 |
| 50 | 3-Methyladenine | Adenine derivative | 1.8074 | [M + H]+ | 149.15 | C6H7N5 |
| Physicochemical Parameters (%) Except 10% pH | Obtained Values (%) Except 10% pH |
|---|---|
| Total ash | 6.00 |
| Acid insoluble ash | 1.18 |
| Water soluble ash | 1.40 |
| Sulphated ash | 2.1 |
| Foreign matter | 0.00 |
| Loss on drying | 1.46 |
| Foaming index | >100 |
| Swelling index | 100 |
| 10% pH | 6.8 |
| Phytochemical | Reagent/Test | Methanolic Extract | Petroleum Ether | Dichloromethane | Benzene | Acetone |
|---|---|---|---|---|---|---|
| Alkaloids | Dragendorff’s | + | + | + | + | + |
| Mayer’s | + | + | − | + | − | |
| Hager’s | + | + | + | + | − | |
| Wagner’s | + | + | − | + | − | |
| Saponins | Foam | + | + | + | + | + |
| Phenols | Ferric chloride | + | + | − | + | + |
| Lead acetate | + | + | + | + | − | |
| Gelatin | + | − | + | + | − | |
| Mayer’s | + | + | − | + | + | |
| Flavonoids | Shinnoda’s | + | + | − | + | + |
| Lead acetate | + | + | + | + | − | |
| Alkaline reagent | + | + | + | + | − | |
| Tannins | Gelatin’s | + | + | + | + | − |
| Steroids and triterpenoids | Salkowski | + | + | + | + | + |
| Libermann–Burchard | + | + | − | + | + |
| Plant Part | Solvent | Cold Extraction | Hot Extraction |
|---|---|---|---|
| Stem bark | Methanol | 8 | 8.6 |
| Petroleum ether | 7.4 | 7.9 | |
| Dichloromethane | 6.8 | 7.2 | |
| Benzene | 6.7 | 7.4 | |
| Acetone | 7 | 6.9 |
| Chemicals Used | Visible Light | Short UV (254 nm) | Long UV (365 nm) |
|---|---|---|---|
| 1 N NaOH (aq.) | Light yellowish-green | Light green | Purple |
| 1 N NaOH (alc.) | Light pink | Light green | Dark blue |
| 1 N HCl | Pale cream | Pale green | Blue |
| H2SO4 (1:1) | Brown | Light green | Black |
| HNO3 (1:1) | Light yellow | Light green | Black |
| NH3 | Light greenish yellow | Light green | Blue |
| Iodine | Bright yellow | Bright green | Black |
| 5% FeCl3 | Bright yellow | Bright green | Black |
| Acetic acid | Pale green | Light green | Blue |
| Culture Condition. | Concentration (µg/mL) | Cell Viability (%) |
|---|---|---|
| Vehicle (PBS) | - | 97.66 ± 0.33 |
| Silymarin | 10 µg/mL | 94.66 ± 0.33 * |
| PME | 10 µg/mL | 92.66 ± 1.20 * |
| PME | 15 µg/mL | 91.00 ± 0.57 ** |
| PME | 25 µg/mL | 87.66 ± 0.33 ** |
| Parameter | Sum of Squares (Between Groups) | df | F-Value | p-Value |
|---|---|---|---|---|
| LPO | 35.87 | 7 | 81.42 | 2.58 × 10−11 |
| AST | 62,678.20 | 7 | 189.09 | 3.58 × 10−14 |
| ALT | 138,834.63 | 7 | 36.47 | 1.14 × 10−8 |
| LDH | 14,120.11 | 7 | 327.95 | 4.61 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Deb, T.; Mottola, F.; Madhu, N.R.; Revanaiah, Y.; Rosas, I.M.; Ray, S.D.; Roychoudhury, S. Phytochemical Screening, Pharmacognostic Characterization, Antioxidant Activity, and Hepatoprotective Effects of Abroma augustum (L.) L.f. on Human Hepatocellular Carcinoma (HepG2) Cells and Goat Liver Homogenate. Antioxidants 2025, 14, 472. https://doi.org/10.3390/antiox14040472
Das S, Deb T, Mottola F, Madhu NR, Revanaiah Y, Rosas IM, Ray SD, Roychoudhury S. Phytochemical Screening, Pharmacognostic Characterization, Antioxidant Activity, and Hepatoprotective Effects of Abroma augustum (L.) L.f. on Human Hepatocellular Carcinoma (HepG2) Cells and Goat Liver Homogenate. Antioxidants. 2025; 14(4):472. https://doi.org/10.3390/antiox14040472
Chicago/Turabian StyleDas, Sandipan, Tanushree Deb, Filomena Mottola, Nithar Ranjan Madhu, Yogisharadhya Revanaiah, Israel Maldonado Rosas, Sarbani Dey Ray, and Shubhadeep Roychoudhury. 2025. "Phytochemical Screening, Pharmacognostic Characterization, Antioxidant Activity, and Hepatoprotective Effects of Abroma augustum (L.) L.f. on Human Hepatocellular Carcinoma (HepG2) Cells and Goat Liver Homogenate" Antioxidants 14, no. 4: 472. https://doi.org/10.3390/antiox14040472
APA StyleDas, S., Deb, T., Mottola, F., Madhu, N. R., Revanaiah, Y., Rosas, I. M., Ray, S. D., & Roychoudhury, S. (2025). Phytochemical Screening, Pharmacognostic Characterization, Antioxidant Activity, and Hepatoprotective Effects of Abroma augustum (L.) L.f. on Human Hepatocellular Carcinoma (HepG2) Cells and Goat Liver Homogenate. Antioxidants, 14(4), 472. https://doi.org/10.3390/antiox14040472










