A Diet Fortified with Anthocyanin-Rich Extract (RED) Reduces Ileal Inflammation in a Senescence-Prone Mice Model of Crohn’s-Disease-like Ileitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. 16S rRNA Microbiome Analysis
2.3. Metabolomics Sample Preparation and Analysis
2.4. Untargeted Lipidomics and Metabolomics
2.5. Statistical Analysis and Metabolomics Data Processing
2.6. RED Diet Administration
3. Results
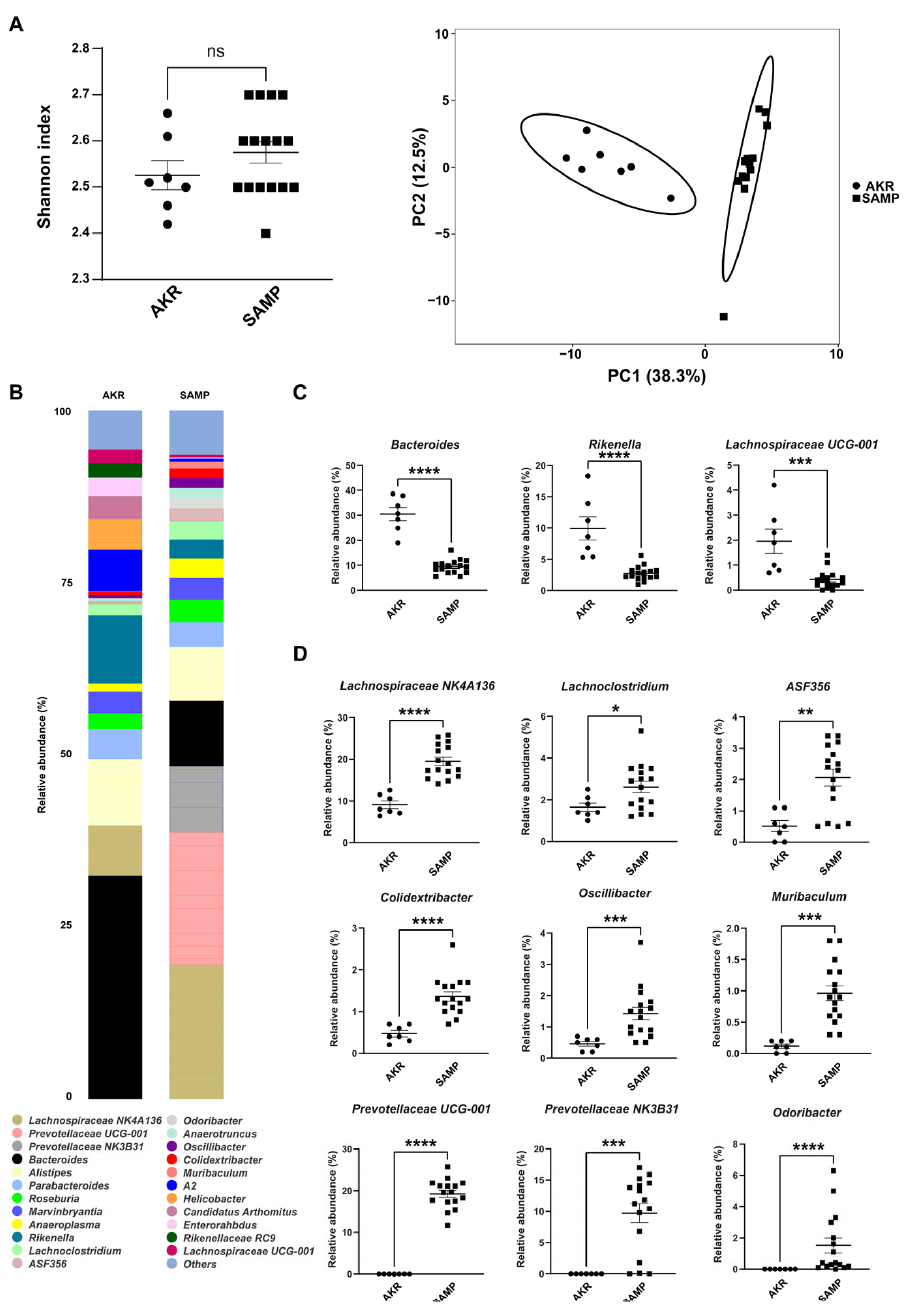
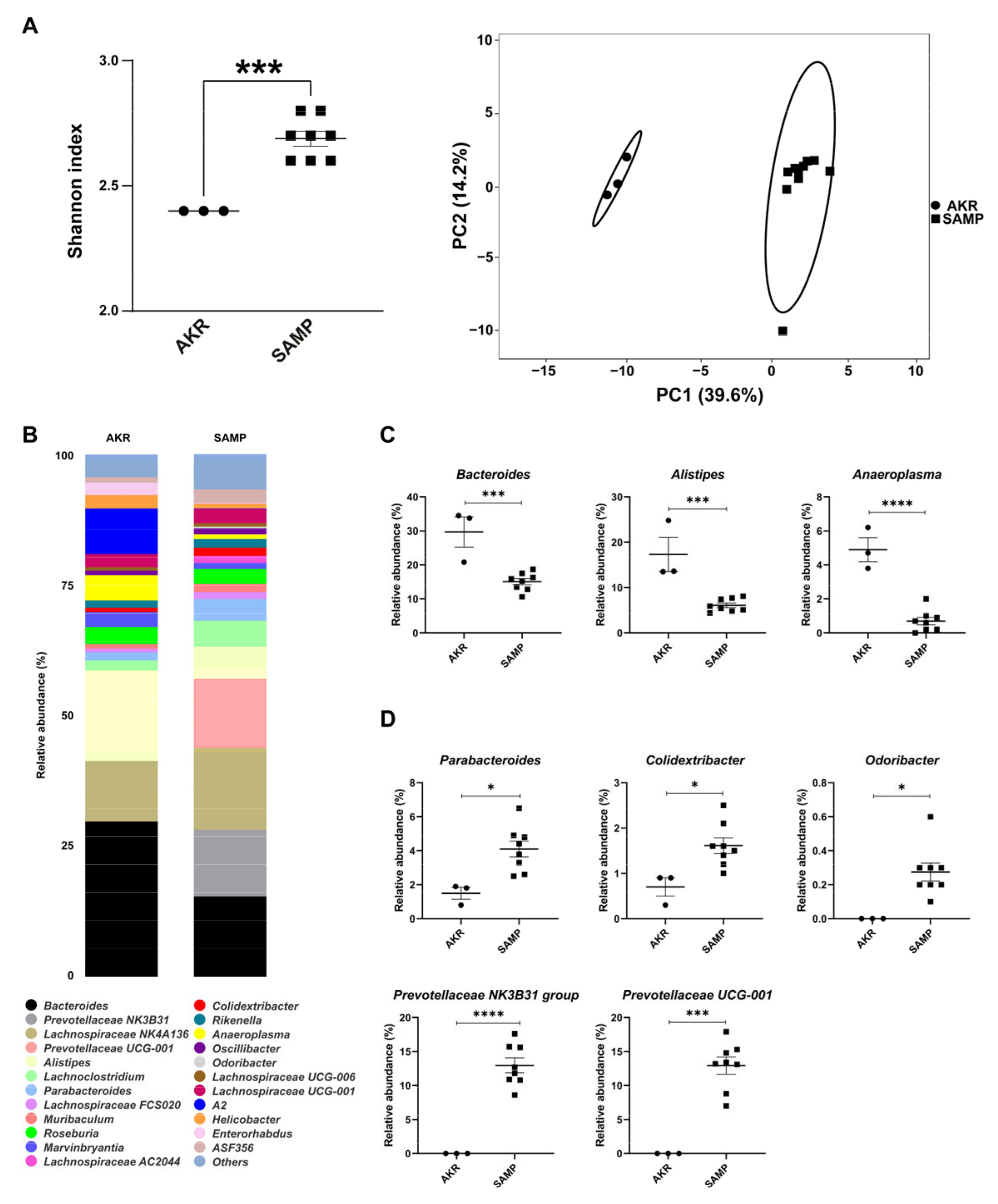
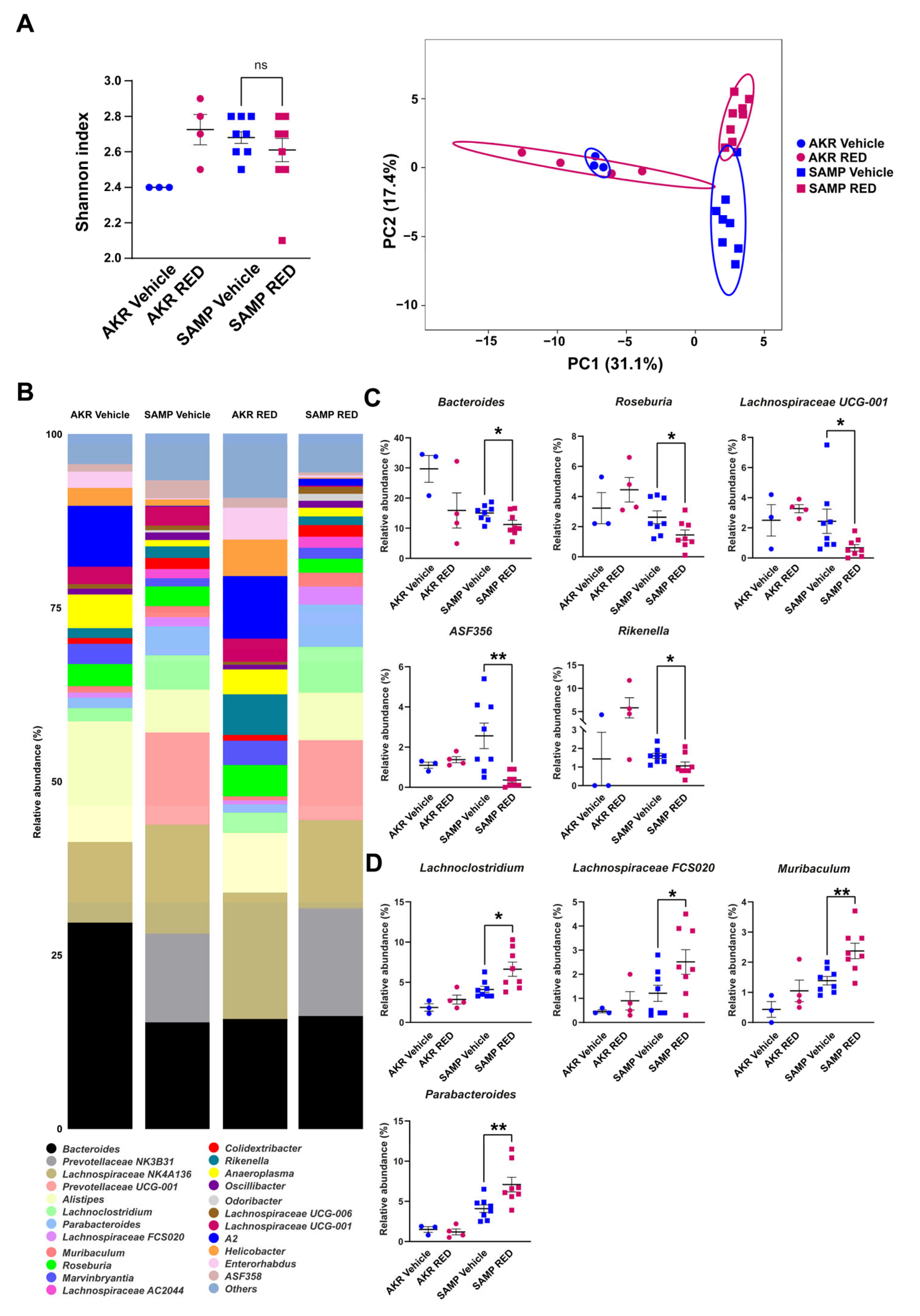
3.1. Fecal Mice Microbiota in 5- and 15-Week-Old SAMP Mice
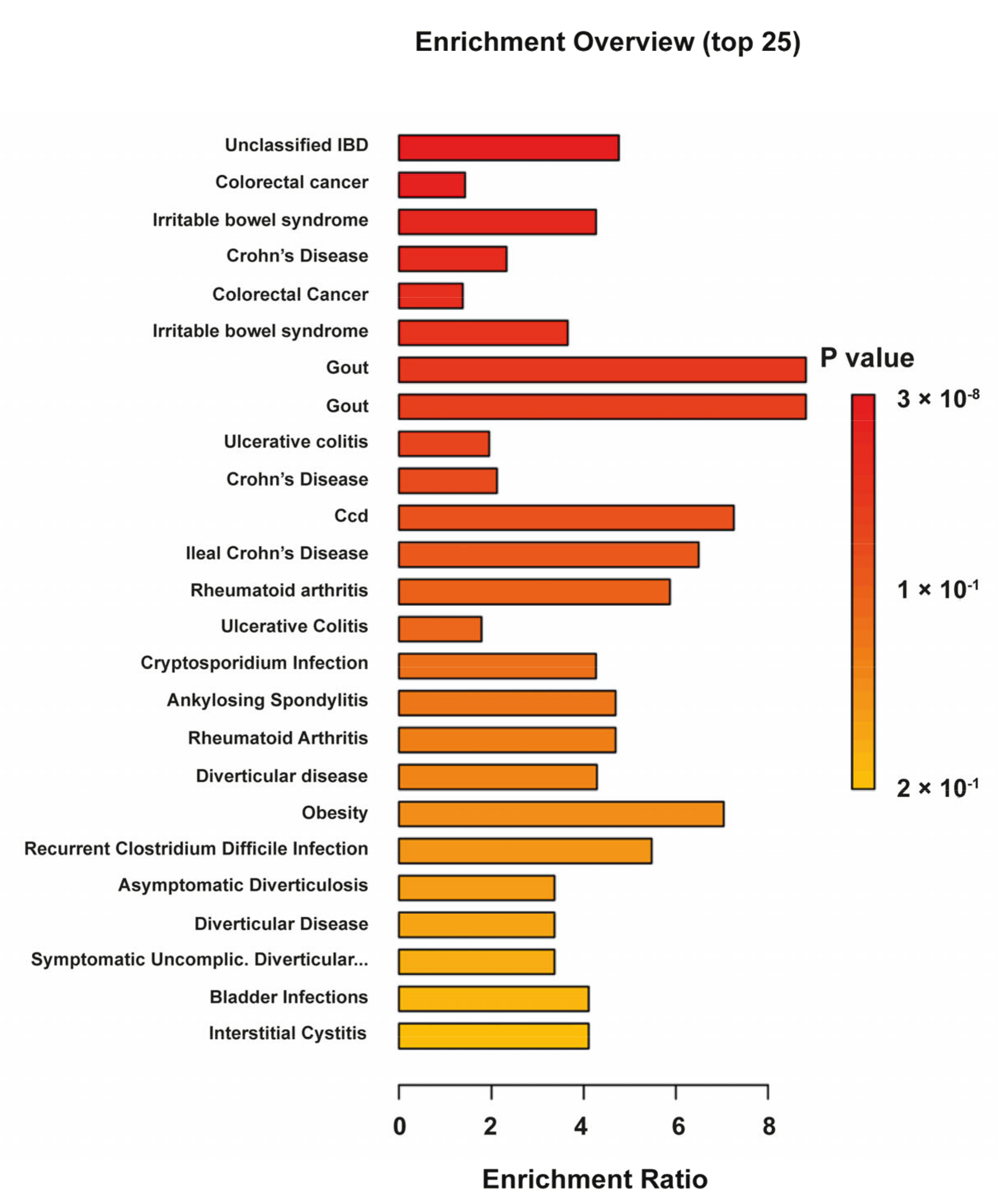
3.2. Fecal Mice Metabolome and Lipidome in 5-Week-Old AKR and SAMP Mice
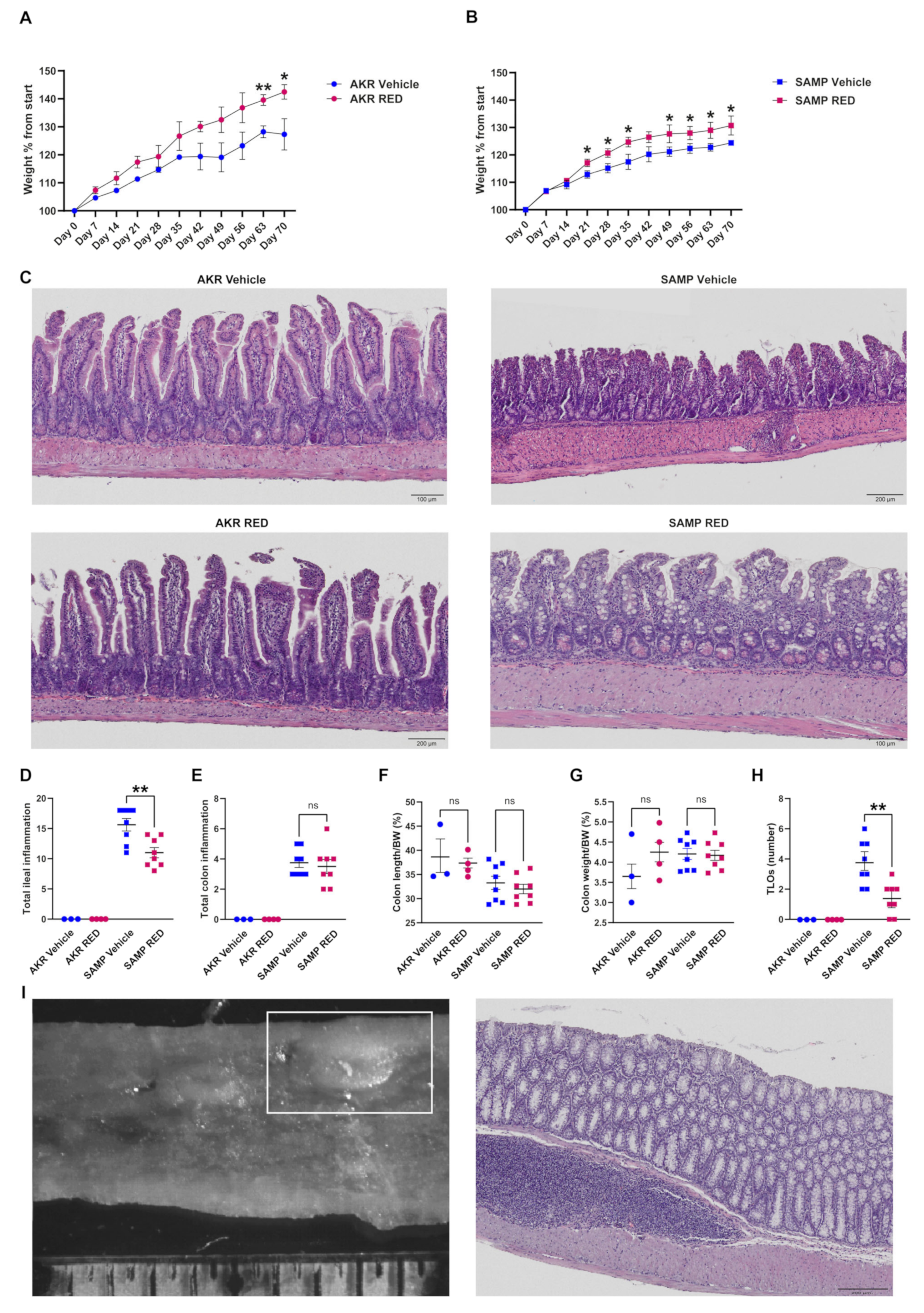
3.3. In Vivo Effects of RED Administration in SAMP Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RED | Anthocyanin-rich extract |
| CD | Crohn’s disease |
| SAMP | Senescence-prone mice |
| IBD | Inflammatory bowel disease |
| TNF | Tumor necrosis factor |
| IFX | Infliximab |
| TH1 | T-helper cell 1 |
| TH2 | T-helper cell 2 |
| IL-33 | Interleukin 33 |
| DCs | Dendritic cells |
| UC | Ulcerative colitis |
| RFT | Red-fruit tea |
| IACUC | Institutional Animal Care and Use Committee |
| SPF | Specific pathogen-free |
| ARC | Animal research center |
| CWRU | Case Western Reserve University |
| FDR | False Discovery Rate |
| EPO | External Parameter Orthogonalization |
| CCSWA | Common Component and Specific Weight Analysis |
| PCA | Principal component analysis |
| DSS | Dextran-sodium sulfate |
| LCFA | Long-chain fatty acids |
| SCFA | Short-chain fatty acids |
| TNBS | 2,4,6-Trinitrobenzenesulfonic acid |
| CERs | Ceramides |
| SM | Sphingomyelin |
| PE | Phosphatidylethanolamine |
| PGs | Phosphatidylglycerols |
| PI | Phosphatidylinositol |
| LPCs | Lysophosphatidylcholines |
| HDMB | Human metabolome database |
| BW | Body weight |
| TLOs | Tertiary lymphoid organs |
References
- Corridoni, D.; Arseneau, K.O.; Cominelli, F. Inflammatory Bowel Disease. Immunol. Lett. 2014, 161, 231–235. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, S.; Xue, X.; Al Naggar, Y.; Wu, L.; Wang, K. Understanding the Gastrointestinal Protective Effects of Polyphenols Using Foodomics-Based Approaches. Front. Immunol. 2021, 12, 671150. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Jiang, X.; Peerani, F.; Narula, N.; Chaudrey, K.; Whitehead, D.; Hudesman, D.; Lukin, D.; Swaminath, A.; et al. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am. J. Gastroenterol. 2016, 111, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Stidham, R.W.; Lee, T.C.H.; Higgins, P.D.R.; Deshpande, A.R.; Sussman, D.A.; Singal, A.G.; Elmunzer, B.J.; Saini, S.D.; Vijan, S.; Waljee, A.K. Systematic Review with Network Meta-Analysis: The Efficacy of Anti-TNF Agents for the Treatment of Crohn’s Disease. Aliment. Pharmacol. Ther. 2014, 39, 1349–1362. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F.; Siegmund, B. Personalizing Treatment in IBD: Hype or Reality in 2020? Can We Predict Response to Anti-TNF? Front. Med. 2020, 7, 517. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s Disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef]
- Serrano-Gómez, G.; Mayorga, L.; Oyarzun, I.; Roca, J.; Borruel, N.; Casellas, F.; Varela, E.; Pozuelo, M.; Machiels, K.; Guarner, F.; et al. Dysbiosis and Relapse-Related Microbiome in Inflammatory Bowel Disease: A Shotgun Metagenomic Approach. Comput. Struct. Biotechnol. J. 2021, 19, 6481–6489. [Google Scholar] [CrossRef]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory Bowel Disease: A Review of Pre-Clinical Murine Models of Human Disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Pizarro, T.T.; Pastorelli, L.; Bamias, G.; Garg, R.R.; Reuter, B.K.; Mercado, J.R.; Chieppa, M.; Arseneau, K.O.; Ley, K.; Cominelli, F. SAMP1/YitFc Mouse Strain: A Spontaneous Model of Crohn’s Disease-like Ileitis. Inflamm. Bowel Dis. 2010, 17, 2566–2584. [Google Scholar] [CrossRef] [PubMed]
- Chieppa, M.; De Santis, S.; Verna, G. Winnie Mice: A Chronic and Progressive Model of Ulcerative Colitis. Inflamm. Bowel Dis. 2025, izaf006. [Google Scholar] [CrossRef]
- De Salvo, C.; Wang, X.-M.; Pastorelli, L.; Mattioli, B.; Omenetti, S.; Buela, K.A.; Chowdhry, S.; Garg, R.R.; Goodman, W.A.; Rodriguez-Palacios, A.; et al. IL-33 Drives Eosinophil Infiltration and Pathogenic Type 2 Helper T-Cell Immune Responses Leading to Chronic Experimental Ileitis. Am. J. Pathol. 2016, 186, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Verna, G.; Liso, M.; Cavalcanti, E.; Bianco, G.; Di Sarno, V.; Santino, A.; Campiglia, P.; Chieppa, M. Quercetin Administration Suppresses the Cytokine Storm in Myeloid and Plasmacytoid Dendritic Cells. Int. J. Mol. Sci. 2021, 22, 8349. [Google Scholar] [CrossRef]
- Sommella, E.; Verna, G.; Liso, M.; Salviati, E.; Esposito, T.; Carbone, D.; Pecoraro, C.; Chieppa, M.; Campiglia, P. Hop-Derived Fraction Rich in Beta Acids and Prenylflavonoids Regulates the Inflammatory Response in Dendritic Cells Differently from Quercetin: Unveiling Metabolic Changes by Mass Spectrometry-Based Metabolomics. Food Funct. 2021, 12, 12800–12811. [Google Scholar] [CrossRef]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet Influences the Functions of the Human Intestinal Microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef] [PubMed]
- Liso, M.; De Santis, S.; Scarano, A.; Verna, G.; Dicarlo, M.; Galleggiante, V.; Campiglia, P.; Mastronardi, M.; Lippolis, A.; Vacca, M.; et al. A Bronze-Tomato Enriched Diet Affects the Intestinal Microbiome under Homeostatic and Inflammatory Conditions. Nutrients 2018, 10, 1862. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Plant Polyphenols-Biofortified Foods as a Novel Tool for the Prevention of Human Gut Diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef]
- Scarano, A.; Laddomada, B.; Blando, F.; De Santis, S.; Verna, G.; Chieppa, M.; Santino, A. The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota. Antioxidants 2023, 12, 630. [Google Scholar] [CrossRef]
- Liso, M.; Sila, A.; Verna, G.; Scarano, A.; Donghia, R.; Castellana, F.; Cavalcanti, E.; Pesole, P.L.; Sommella, E.M.; Lippolis, A.; et al. Nutritional Regimes Enriched with Antioxidants as an Efficient Adjuvant for IBD Patients under Infliximab Administration, a Pilot Study. Antioxidants 2022, 11, 138. [Google Scholar] [CrossRef]
- Vacca, M.; Sommella, E.M.; Liso, M.; Verna, G.; Scarano, A.; Sila, A.; Curlo, M.; Mastronardi, M.; Petroni, K.; Tonelli, C.; et al. Anthocyanins from Purple Corn Affect Gut Microbiota and Metabolome in Inflammatory Bowel Disease Patients under Infliximab Infusion: The SiCURA Pilot Study. Food Sci. Hum. Wellness 2024, 13, 3536–3543. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Merciai, F.; Musella, S.; Sommella, E.; Bertamino, A.; D’Ursi, A.M.; Campiglia, P. Development and Application of a Fast Ultra-High Performance Liquid Chromatography-Trapped Ion Mobility Mass Spectrometry Method for Untargeted Lipidomics. J. Chromatogr. A 2022, 1673, 463124. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Vestuto, V.; Ferraro, M.R.; Ciaglia, T.; Pecoraro, C.; Sommella, E.; Cascioferro, S.; Salviati, E.; Novi, S.; Tecce, M.F.; et al. Metabolomics-Assisted Discovery of a New Anticancer GLS-1 Inhibitor Chemotype from a Nortopsentin-Inspired Library: From Phenotype Screening to Target Identification. Eur. J. Med. Chem. 2022, 234, 114233. [Google Scholar] [CrossRef]
- Scisciola, L.; Chianese, U.; Caponigro, V.; Basilicata, M.G.; Salviati, E.; Altucci, L.; Campiglia, P.; Paolisso, G.; Barbieri, M.; Benedetti, R.; et al. Multi-Omics Analysis Reveals Attenuation of Cellular Stress by Empagliflozin in High Glucose-Treated Human Cardiomyocytes. J. Transl. Med. 2023, 21, 662. [Google Scholar] [CrossRef]
- Shannon, C.E. The Mathematical Theory of Communication. MD Comput. 1997, 14, 306–317. [Google Scholar]
- Chao, A.; Bunge, J. Estimating the Number of Species in a Stochastic Abundance Model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef]
- Sommella, E.; Carrizzo, A.; Merciai, F.; Di Sarno, V.; Carbone, D.; De Lucia, M.; Musella, S.; Vecchione, C.; Campiglia, P. Analysis of the Metabolic Switch Induced by the Spirulina Peptide SP6 in High Fat Diet ApoE-/- Mice Model: A Direct Infusion FT-ICR-MS Based Approach. J. Pharm. Biomed. Anal. 2021, 195, 113865. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Merciai, F.; Carrizzo, A.; Sommella, E.; Di Pietro, P.; Caponigro, V.; Salviati, E.; Musella, S.; di Sarno, V.; Rusciano, M.; et al. Untargeted Lipidomics Reveals Specific Lipid Profiles in COVID-19 Patients with Different Severity from Campania Region (Italy). J. Pharm. Biomed. Anal. 2022, 217, 114827. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 2018, 57, 289–300. [Google Scholar] [CrossRef]
- Roger, J.-M.; Chauchard, F.; Bellon-Maurel, V. EPO–PLS External Parameter Orthogonalisation of PLS Application to Temperature-Independent Measurement of Sugar Content of Intact Fruits. Chemom. Intellig. Lab. Syst. 2003, 66, 191–204. [Google Scholar] [CrossRef]
- Qannari, E.M.; Wakeling, I.; Courcoux, P.; MacFie, H.J.H. Defining the Underlying Sensory Dimensions. Food Qual. Prefer. 2000, 11, 151–154. [Google Scholar] [CrossRef]
- Cariou, V.; Jouan-Rimbaud Bouveresse, D.; Qannari, E.M.; Rutledge, D.N. Chapter 7—ComDim Methods for the Analysis of Multiblock Data in a Data Fusion Perspective. In Data Handling in Science and Technology; Cocchi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 31, pp. 179–204. ISBN 0922-3487. [Google Scholar]
- El Ghaziri, A.; Cariou, V.; Rutledge, D.N.; Qannari, E.M. Analysis of Multiblock Datasets Using ComDim: Overview and Extension to the Analysis of (K + 1) Datasets. J. Chemom. 2016, 30, 420–429. [Google Scholar] [CrossRef]
- Petroni, K.; Trinei, M.; Fornari, M.; Calvenzani, V.; Marinelli, A.; Micheli, L.A.; Pilu, R.; Matros, A.; Mock, H.P.; Tonelli, C.; et al. Dietary Cyanidin 3-Glucoside from Purple Corn Ameliorates Doxorubicin-Induced Cardiotoxicity in Mice. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 462–469. [Google Scholar] [CrossRef]
- Magni, G.; Marinelli, A.; Riccio, D.; Lecca, D.; Tonelli, C.; Abbracchio, M.P.; Petroni, K.; Ceruti, S. Purple Corn Extract as Anti-Allodynic Treatment for Trigeminal Pain: Role of Microglia. Front. Cell. Neurosci. 2018, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Neto, J.C.; Kittana, H.; Mantz, S.; Segura Munoz, R.R.; Schmaltz, R.J.; Bindels, L.B.; Clarke, J.; Hostetter, J.M.; Benson, A.K.; Walter, J.; et al. A Gut Pathobiont Synergizes with the Microbiota to Instigate Inflammatory Disease Marked by Immunoreactivity against Other Symbionts but Not Itself. Sci. Rep. 2017, 7, 17707. [Google Scholar] [CrossRef]
- Brand, S. Crohn’s Disease: Th1, Th17 or Both? The Change of a Paradigm: New Immunological and Genetic Insights Implicate Th17 Cells in the Pathogenesis of Crohn’s Disease. Gut 2009, 58, 1152–1167. [Google Scholar] [CrossRef]
- Baumgartner, M.; Lang, M.; Holley, H.; Crepaz, D.; Hausmann, B.; Pjevac, P.; Moser, D.; Haller, F.; Hof, F.; Beer, A.; et al. Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis. Gastroenterology 2021, 161, 1245–1256.e20. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, L.; Han, T.; Huang, H.; Chen, J. Dynamic Changes in Gut Microbiome of Ulcerative Colitis: Initial Study from Animal Model. J. Inflamm. Res. 2022, 15, 2631–2647. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.-H.; Xue, M.; Raygoza Garay, J.A.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated With Gut Barrier Dysfunction in Healthy Relatives of Patients With Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e10. [Google Scholar] [CrossRef]
- Frau, A.; Ijaz, U.Z.; Slater, R.; Jonkers, D.; Penders, J.; Campbell, B.J.; Kenny, J.G.; Hall, N.; Lenzi, L.; Burkitt, M.D.; et al. Inter-Kingdom Relationships in Crohn’s Disease Explored Using a Multi-Omics Approach. Gut Microbes 2021, 13, 1930871. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Tian, Z.; Li, N.; Mao, R.; Li, X.; Zhao, M.; Xiong, S.; Zeng, Z.; Feng, R.; Chen, M. Gut Microbiota Profiles and Microbial-Based Therapies in Post-Operative Crohn’s Disease: A Systematic Review. Front. Med. 2021, 7, 615858. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, H.; Kang, C.-S.; Shin, T.-S.; Kim, J.W.; Park, J.-M.; Kim, J.G.; Kim, Y.-K. Dysbiotic Change in Gastric Microbiome and Its Functional Implication in Gastric Carcinogenesis. Sci. Rep. 2022, 12, 4285. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Fan, L.; Zheng, N.; Blecker, C.; Delcenserie, V.; Li, H.; Wang, J. 2’-Fucosyllactose Ameliorates Inflammatory Bowel Disease by Modulating Gut Microbiota and Promoting MUC2 Expression. Front. Nutr. 2022, 9, 822020. [Google Scholar] [CrossRef]
- Thipart, K.; Gruneck, L.; Phunikhom, K.; Sharpton, T.J.; Sattayasai, J.; Popluechai, S. Dark-Purple Rice Extract Modulates Gut Microbiota Composition in Acetic Acid- and Indomethacin-Induced Inflammatory Bowel Disease in Rats. Int. Microbiol. 2022, 26, 423–434. [Google Scholar] [CrossRef]
- Dobranowski, P.A.; Tang, C.; Sauvé, J.P.; Menzies, S.C.; Sly, L.M. Compositional Changes to the Ileal Microbiome Precede the Onset of Spontaneous Ileitis in SHIP Deficient Mice. Gut Microbes 2019, 10, 578–598. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.; Dunkel, A.; Waldschmitt, N.; Raj, A.C.D.; Lagkouvardos, I.; Corraliza, A.M.; Mayorgas, A.; Martinez-Medina, M.; Reiter, S.; Schloter, M.; et al. Integrated Microbiota and Metabolite Profiles Link Crohn’s Disease to Sulfur Metabolism. Nat. Commun. 2020, 11, 4322. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Harding, A.; Menghini, P.; Himmelman, C.; Retuerto, M.; Nickerson, K.P.; Lam, M.; Croniger, C.M.; McLean, M.H.; Durum, S.K.; et al. The Artificial Sweetener Splenda Promotes Gut Proteobacteria, Dysbiosis, and Myeloperoxidase Reactivity in Crohn’s Disease-Like Ileitis. Inflamm. Bowel Dis. 2018, 24, 1005–1020. [Google Scholar] [CrossRef]
- Butera, A.; Di Paola, M.; Pavarini, L.; Strati, F.; Pindo, M.; Sanchez, M.; Cavalieri, D.; Boirivant, M.; De Filippo, C. Nod2 Deficiency in Mice Is Associated with Microbiota Variation Favouring the Expansion of Mucosal CD4+ LAP+ Regulatory Cells. Sci. Rep. 2018, 8, 14241. [Google Scholar] [CrossRef]
- Gryaznova, M.; Dvoretskaya, Y.; Burakova, I.; Syromyatnikov, M.; Popov, E.; Kokina, A.; Mikhaylov, E.; Popov, V. Dynamics of Changes in the Gut Microbiota of Healthy Mice Fed with Lactic Acid Bacteria and Bifidobacteria. Microorganisms 2022, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Naes, S.M.; Ab-Rahim, S.; Mazlan, M.; Amir Hashim, N.A.; Abdul Rahman, A. Increased ENT2 Expression and Its Association with Altered Purine Metabolism in Cell Lines Derived from Different Stages of Colorectal Cancer. Exp. Ther. Med. 2023, 25, 212. [Google Scholar] [CrossRef]
- Calzadilla, N.; Qazi, A.; Sharma, A.; Mongan, K.; Comiskey, S.; Manne, J.; Youkhana, A.G.; Khanna, S.; Saksena, S.; Dudeja, P.K.; et al. Mucosal Metabolomic Signatures in Chronic Colitis: Novel Insights into the Pathophysiology of Inflammatory Bowel Disease. Metabolites 2023, 13, 873. [Google Scholar] [CrossRef]
- Chiaro, T.R.; Soto, R.; Zac Stephens, W.; Kubinak, J.L.; Petersen, C.; Gogokhia, L.; Bell, R.; Delgado, J.C.; Cox, J.; Voth, W.; et al. A Member of the Gut Mycobiota Modulates Host Purine Metabolism Exacerbating Colitis in Mice. Sci. Transl. Med. 2017, 9, eaaf9044. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ye, S.; He, Y.; Wang, S.; Xiao, Y.; Xiang, X.; Deng, M.; Luo, W.; Chen, X.; Wang, X. Fatty Acids and Lipid Mediators in Inflammatory Bowel Disease: From Mechanism to Treatment. Front. Immunol. 2023, 14, 1286667. [Google Scholar] [CrossRef]
- Nieto, N.; Giron, M.D.; Suarez, M.D.; Gil, A. Changes in Plasma and Colonic Mucosa Fatty Acid Profiles in Rats with Ulcerative Colitis Induced by Trinitrobenzene Sulfonic Acid. Dig. Dis. Sci. 1998, 43, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Lu, L.; Yang, W.; Huang, T.; Lin, Z.; Lin, C.; Kwan, H.; Wong, H.L.X.; Chen, Y.; et al. Saturated Long-Chain Fatty Acid-Producing Bacteria Contribute to Enhanced Colonic Motility in Rats. Microbiome 2018, 6, 107. [Google Scholar] [CrossRef]
- Grosheva, I.; Zheng, D.; Levy, M.; Polansky, O.; Lichtenstein, A.; Golani, O.; Dori-Bachash, M.; Moresi, C.; Shapiro, H.; Del Mare-Roumani, S.; et al. High-Throughput Screen Identifies Host and Microbiota Regulators of Intestinal Barrier Function. Gastroenterology 2020, 159, 1807–1823. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L. Elevated IL-23R Expression and Foxp3+Rorgt+ Cells in Intestinal Mucosa During Acute and Chronic Colitis. Med. Sci. Monit. 2016, 22, 2785–2792. [Google Scholar] [CrossRef]
- Huang, C.; Wang, J.; Liu, H.; Huang, R.; Yan, X.; Song, M.; Tan, G.; Zhi, F. Ketone Body β-Hydroxybutyrate Ameliorates Colitis by Promoting M2 Macrophage Polarization through the STAT6-Dependent Signaling Pathway. BMC Med. 2022, 20, 148. [Google Scholar] [CrossRef]
- Oertel, S.; Scholich, K.; Weigert, A.; Thomas, D.; Schmetzer, J.; Trautmann, S.; Wegner, M.S.; Radeke, H.H.; Filmann, N.; Brüne, B.; et al. Ceramide Synthase 2 Deficiency Aggravates AOM-DSS-Induced Colitis in Mice: Role of Colon Barrier Integrity. Cell Mol. Life Sci. 2017, 74, 3039–3055. [Google Scholar] [CrossRef]
- Li, Z.; Kabir, I.; Tietelman, G.; Huan, C.; Fan, J.; Worgall, T.; Jiang, X.-C. Sphingolipid de Novo Biosynthesis Is Essential for Intestine Cell Survival and Barrier Function. Cell Death Dis. 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Kayser, B.D.; Lhomme, M.; Prifti, E.; Da Cunha, C.; Marquet, F.; Chain, F.; Naas, I.; Pelloux, V.; Dao, M.C.; Kontush, A.; et al. Phosphatidylglycerols Are Induced by Gut Dysbiosis and Inflammation, and Favorably Modulate Adipose Tissue Remodeling in Obesity. FASEB J. 2019, 33, 4741–4754. [Google Scholar] [CrossRef]
- Beller, A.; Kruglov, A.; Durek, P.; von Goetze, V.; Werner, K.; Heinz, G.A.; Ninnemann, J.; Lehmann, K.; Maier, R.; Hoffmann, U.; et al. Specific Microbiota Enhances Intestinal IgA Levels by Inducing TGF-β in T Follicular Helper Cells of Peyer’s Patches in Mice. Eur. J. Immunol. 2020, 50, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, S.; Goepp, M.; Pollock, J.; Robb, C.T.; Smyth, D.J.; Zhou, Y.; Andrews, R.; Tyrrell, V.; Gkikas, K.; Adima, A.; et al. Prostaglandin E2 Promotes Intestinal Inflammation via Inhibiting Microbiota-Dependent Regulatory T Cells. Sci. Adv. 2021, 7, eabd7954. [Google Scholar] [CrossRef] [PubMed]
- Bery, A.I.; Shepherd, H.M.; Li, W.; Krupnick, A.S.; Gelman, A.E.; Kreisel, D. Role of Tertiary Lymphoid Organs in the Regulation of Immune Responses in the Periphery. Cell Mol. Life Sci. 2022, 79, 359. [Google Scholar] [CrossRef]
- McNamee, E.N.; Rivera-Nieves, J. Ectopic Tertiary Lymphoid Tissue in Inflammatory Bowel Disease: Protective or Provocateur? Front. Immunol. 2016, 7, 308. [Google Scholar] [CrossRef]
- Gomez-Nguyen, A.; Gupta, N.; Sanaka, H.; Gruszka, D.; Pizarro, A.; DiMartino, L.; Basson, A.; Menghini, P.; Osme, A.; DeSalvo, C.; et al. Chronic Stress Induces Colonic Tertiary Lymphoid Organ Formation and Protection against Secondary Injury through IL-23/IL-22 Signaling. PLoS ONE 2022, 119, e2208160119. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides Produces Acetate to Alleviate Heparanase-Exacerbated Acute Pancreatitis through Reducing Neutrophil Infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Brockman, J.A.; Jewell, D.E. A Diet Supplemented with Polyphenols, Prebiotics and Omega-3 Fatty Acids Modulates the Intestinal Microbiota and Improves the Profile of Metabolites Linked with Anxiety in Dogs. Biology 2022, 11, 976. [Google Scholar] [CrossRef]
- Cladis, D.P.; Simpson, A.M.R.; Cooper, K.J.; Nakatsu, C.H.; Ferruzzi, M.G.; Weaver, C.M. Blueberry Polyphenols Alter Gut Microbiota & Phenolic Metabolism in Rats. Food Funct. 2021, 12, 2442–2456. [Google Scholar] [CrossRef]
- Di Martino, L.; Osme, A.; Ghannoum, M.; Cominelli, F. A Novel Probiotic Combination Ameliorates Crohn’s Disease–Like Ileitis by Increasing Short-Chain Fatty Acid Production and Modulating Essential Adaptive Immune Pathways. Inflamm. Bowel Dis. 2023, 29, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Hou, S.; Sui, Y.; Chang, N.; Zhou, Y.; Sun, C. Sonchus arvensis L. Water Extract Attenuates Dextran Sulfate Sodium-Induced Colitis by Adjusting Gut Microbiota. Heliyon 2023, 9, e14168. [Google Scholar] [CrossRef]
- Komatsu, Y.; Shimizu, Y.; Yamano, M.; Kikuchi, M.; Nakamura, K.; Ayabe, T.; Aizawa, T. Disease Progression-Associated Alterations in Fecal Metabolites in SAMP1/YitFc Mice, a Crohn’s Disease Model. Metabolomics 2020, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Herring, C.A.; Chen, B.; Kim, H.; Simmons, A.J.; Southard-Smith, A.N.; Allaman, M.M.; White, J.R.; Macedonia, M.C.; McKinley, E.T.; et al. Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology 2020, 159, 2101–2115.e5. [Google Scholar] [CrossRef]
- Liu, H. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Gao, H.; Wang, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W. Bifidobacterium Breve CCFM683 Could Ameliorate DSS-Induced Colitis in Mice Primarily via Conjugated Linoleic Acid Production and Gut Microbiota Modulation. J. Funct. Foods 2018, 49, 61–72. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Viladomiu, M.; Pedragosa, M.; De Simone, C.; Carbo, A.; Shaykhutdinov, R.; Jobin, C.; Arthur, J.C.; Corl, B.A.; Vogel, H.; et al. Probiotic Bacteria Produce Conjugated Linoleic Acid Locally in the Gut That Targets Macrophage PPAR γ to Suppress Colitis. PLoS ONE 2012, 7, e31238. [Google Scholar] [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of Linoleic Acid by Human Gut Bacteria: Different Routes for Biosynthesis of Conjugated Linoleic Acid. J. Bacteriol. 2007, 189, 2566–2570. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Yu, H.; Lu, Y.; Yu, W.; Miao, M.; Shi, H. Anti-Aging Effects of a Functional Food via the Action of Gut Microbiota and Metabolites in Aging Mice. Aging 2021, 13, 17880–17900. [Google Scholar] [CrossRef] [PubMed]
- Medina-Larqué, A.-S.; Rodríguez-Daza, M.-C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, É.; Marette, A.; Roy, D.; Jacques, H.; Desjardins, Y. Cranberry Polyphenols and Agave Agavins Impact Gut Immune Response and Microbiota Composition While Improving Gut Barrier Function, Inflammation, and Glucose Metabolism in Mice Fed an Obesogenic Diet. Front. Immunol. 2022, 13, 871080. [Google Scholar] [CrossRef]
- Wan, M.; Li, Q.; Lei, Q.; Zhou, D.; Wang, S. Polyphenols and Polysaccharides from Morus Alba L. Fruit Attenuate High-Fat Diet-Induced Metabolic Syndrome Modifying the Gut Microbiota and Metabolite Profile. Foods 2022, 11, 1818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X. Fermentation in Vitro of EGCG, GCG and EGCG3”Me Isolated from Oolong Tea by Human Intestinal Microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Langhi, C.; Vallier, M.; Bron, A.; Otero, Y.F.; Maura, M.; Le Joubioux, F.; Blomberg, N.; Giera, M.; Guigas, B.; Maugard, T.; et al. A Polyphenol-Rich Plant Extract Prevents Hypercholesterolemia and Modulates Gut Microbiota in Western Diet-Fed Mice. Front. Cardiovasc. Med. 2024, 11, 1342388. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Li, R.; Liu, Y.; Wang, X.; Zhang, X.; Xu, C.; Li, Y.; Guo, Y.; Yao, Q. Berberine Regulates Fecal Metabolites to Ameliorate 5-Fluorouracil Induced Intestinal Mucositis through Modulating Gut Microbiota. Biomed. Pharmacother. 2020, 124, 109829. [Google Scholar] [CrossRef]
- Anderson, B.D.; Sepulveda, D.E.; Nachnani, R.; Cortez-Resendiz, A.; Coates, M.D.; Beckett, A.; Bisanz, J.E.; Kellogg, J.J.; Raup-Konsavage, W.M. High Cannabigerol Hemp Extract Moderates Colitis and Modulates the Microbiome in an Inflammatory Bowel Disease Model. J. Pharmacol. Exp. Ther. 2024, 390, 331–341. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, F.; He, X.; Fu, S.; Fan, B. Anthocyanin Extract from Black Rice Attenuates Chronic Inflammation in DSS-Induced Colitis Mouse Model by Modulating the Gut Microbiota. Open Chem. 2023, 21, 20220288. [Google Scholar] [CrossRef]
- Yang, S.; Huang, J.; Tan, W.; Xia, X.; Gan, D.; Ren, Y.; Su, H.; Xiang, M. Xiaoyankangjun Tablet Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Regulating Gut Microbiota and JAK2/STAT3 Pathway. Nat. Prod. Bioprospect. 2024, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and Plant Therapy in Patients with Inflammatory Bowel Disease. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 210–220. [Google Scholar]
- Caban, M.; Owczarek, K.; Lewandowska, U. Effects of Polyphenol-Rich Extracts on Inflammatory Bowel Diseases. Food Rev. Int. 2024, 40, 2448–2485. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verna, G.; Caponigro, V.; Santis, S.D.; Salviati, E.; Merciai, F.; Celio, F.D.A.; Campiglia, P.; Petroni, K.; Tonelli, C.; Scarano, A.; et al. A Diet Fortified with Anthocyanin-Rich Extract (RED) Reduces Ileal Inflammation in a Senescence-Prone Mice Model of Crohn’s-Disease-like Ileitis. Antioxidants 2025, 14, 473. https://doi.org/10.3390/antiox14040473
Verna G, Caponigro V, Santis SD, Salviati E, Merciai F, Celio FDA, Campiglia P, Petroni K, Tonelli C, Scarano A, et al. A Diet Fortified with Anthocyanin-Rich Extract (RED) Reduces Ileal Inflammation in a Senescence-Prone Mice Model of Crohn’s-Disease-like Ileitis. Antioxidants. 2025; 14(4):473. https://doi.org/10.3390/antiox14040473
Chicago/Turabian StyleVerna, Giulio, Vicky Caponigro, Stefania De Santis, Emanuela Salviati, Fabrizio Merciai, Fabiano De Almeida Celio, Pietro Campiglia, Katia Petroni, Chiara Tonelli, Aurelia Scarano, and et al. 2025. "A Diet Fortified with Anthocyanin-Rich Extract (RED) Reduces Ileal Inflammation in a Senescence-Prone Mice Model of Crohn’s-Disease-like Ileitis" Antioxidants 14, no. 4: 473. https://doi.org/10.3390/antiox14040473
APA StyleVerna, G., Caponigro, V., Santis, S. D., Salviati, E., Merciai, F., Celio, F. D. A., Campiglia, P., Petroni, K., Tonelli, C., Scarano, A., Santino, A., Basilicata, M. G., Chieppa, M., & Cominelli, F. (2025). A Diet Fortified with Anthocyanin-Rich Extract (RED) Reduces Ileal Inflammation in a Senescence-Prone Mice Model of Crohn’s-Disease-like Ileitis. Antioxidants, 14(4), 473. https://doi.org/10.3390/antiox14040473












