Impact of Melatonin Application in Winemaking on Phenolic Content, Tryptophan Metabolites, and Bioactivity of Red Wine †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Wine Sampling
2.3. Total Phenolic Content
2.4. Total Flavonoid Content
2.5. Anti-DPPH Radical Activity
2.6. FRAP Assay
2.7. UPLC Analysis
2.7.1. Qualitative Analysis of Wine Samples by UPLC with Tandem Mass Spectrometry Detector
2.7.2. Solid-Phase Extraction Procedure
2.7.3. Quantitative Determination of Tryptophan Metabolites Using UPLC with Fluorescent Detector
2.8. Cytotoxic Activity
2.9. Statistical Analysis
3. Results and Discussion
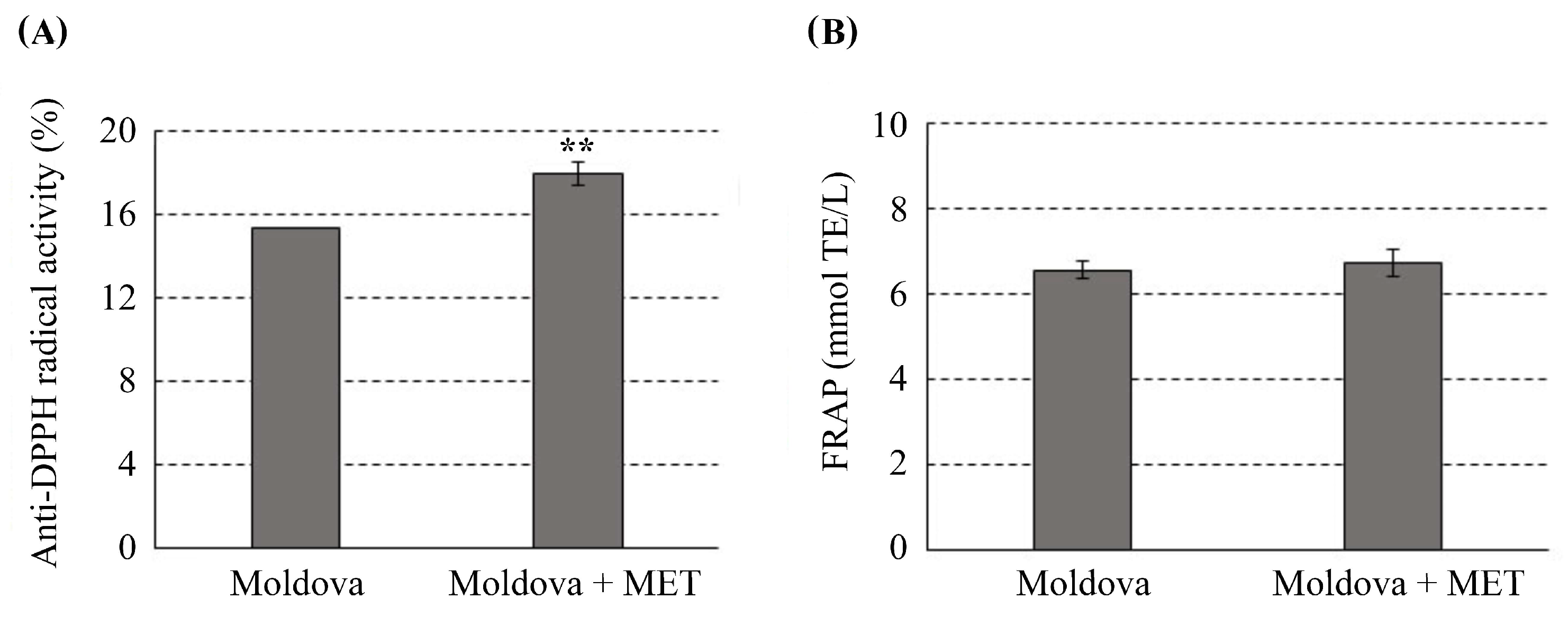
3.1. Total Phenolic/Flavonoid Content and Antioxidant Activity in Moldova Wine
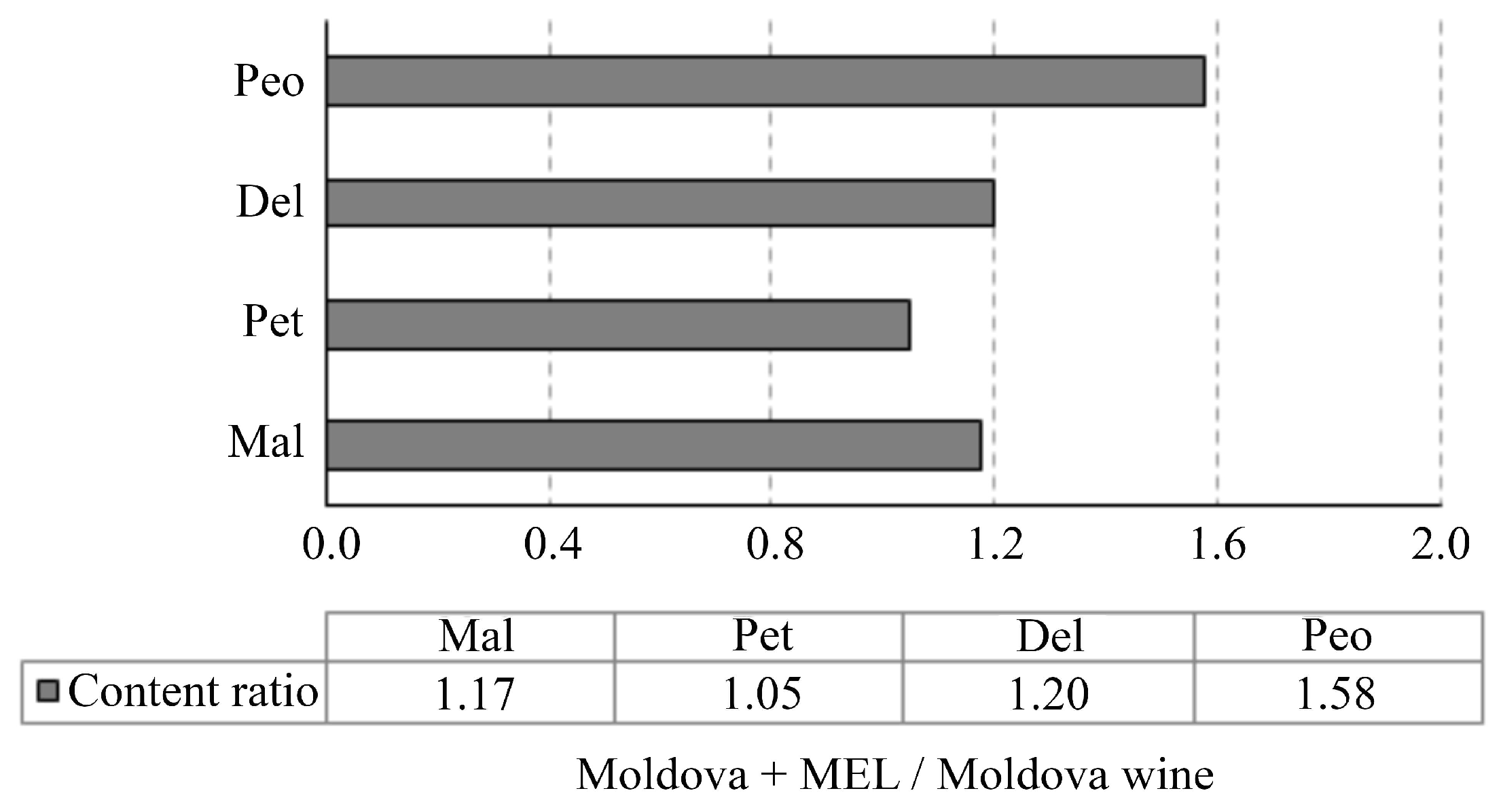
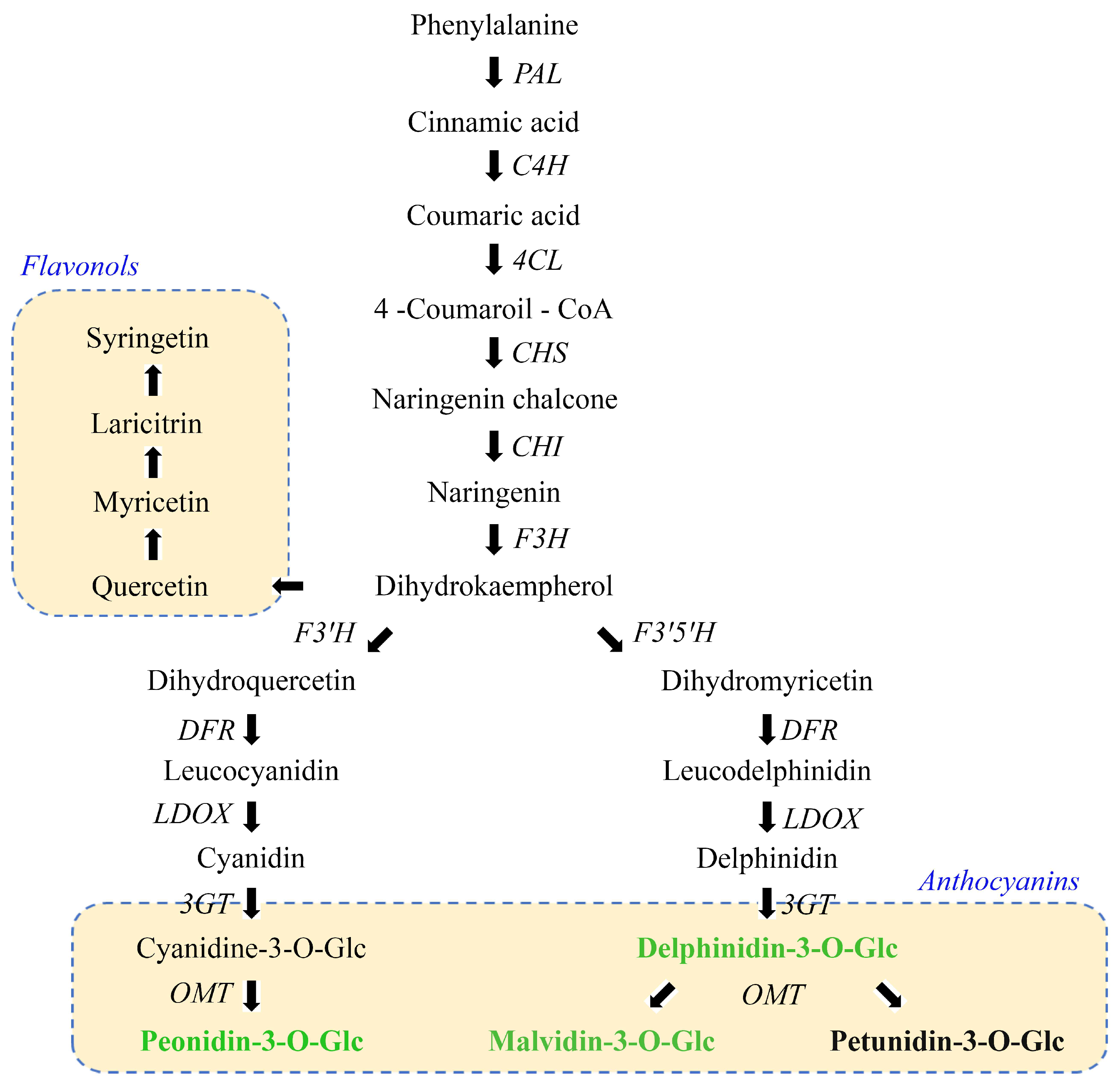
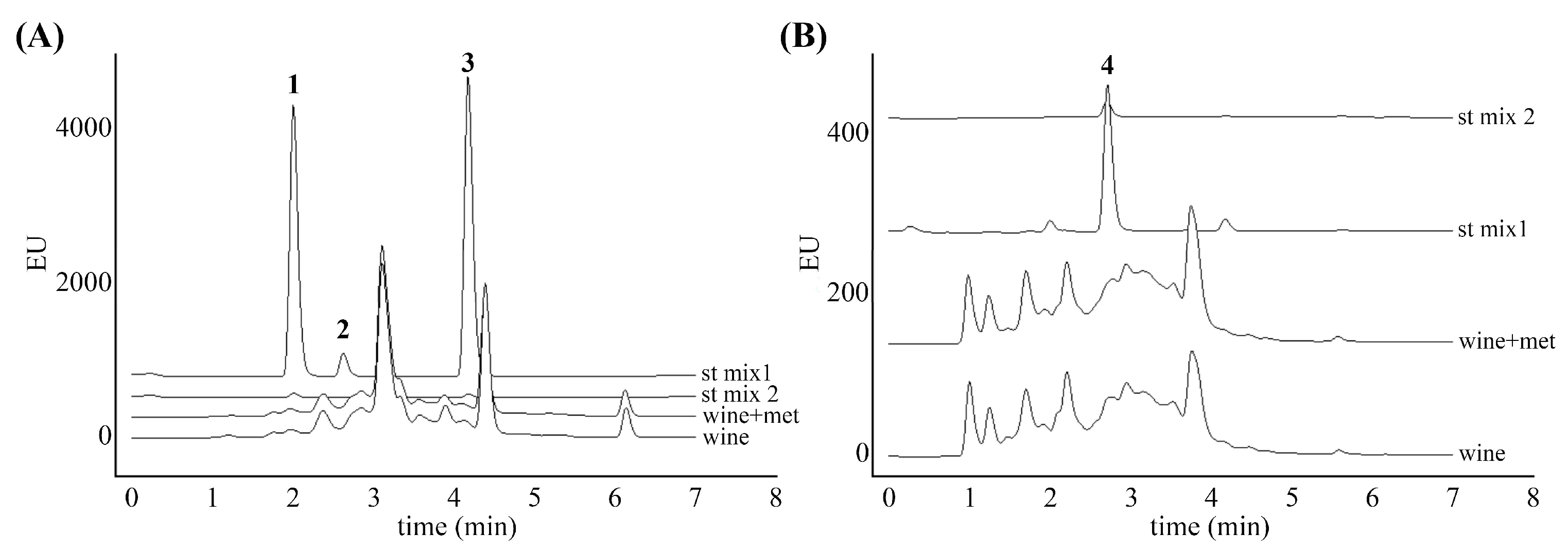
3.2. Anthocyanins Profile Analysis
3.3. Tryptophan Metabolites Analysis
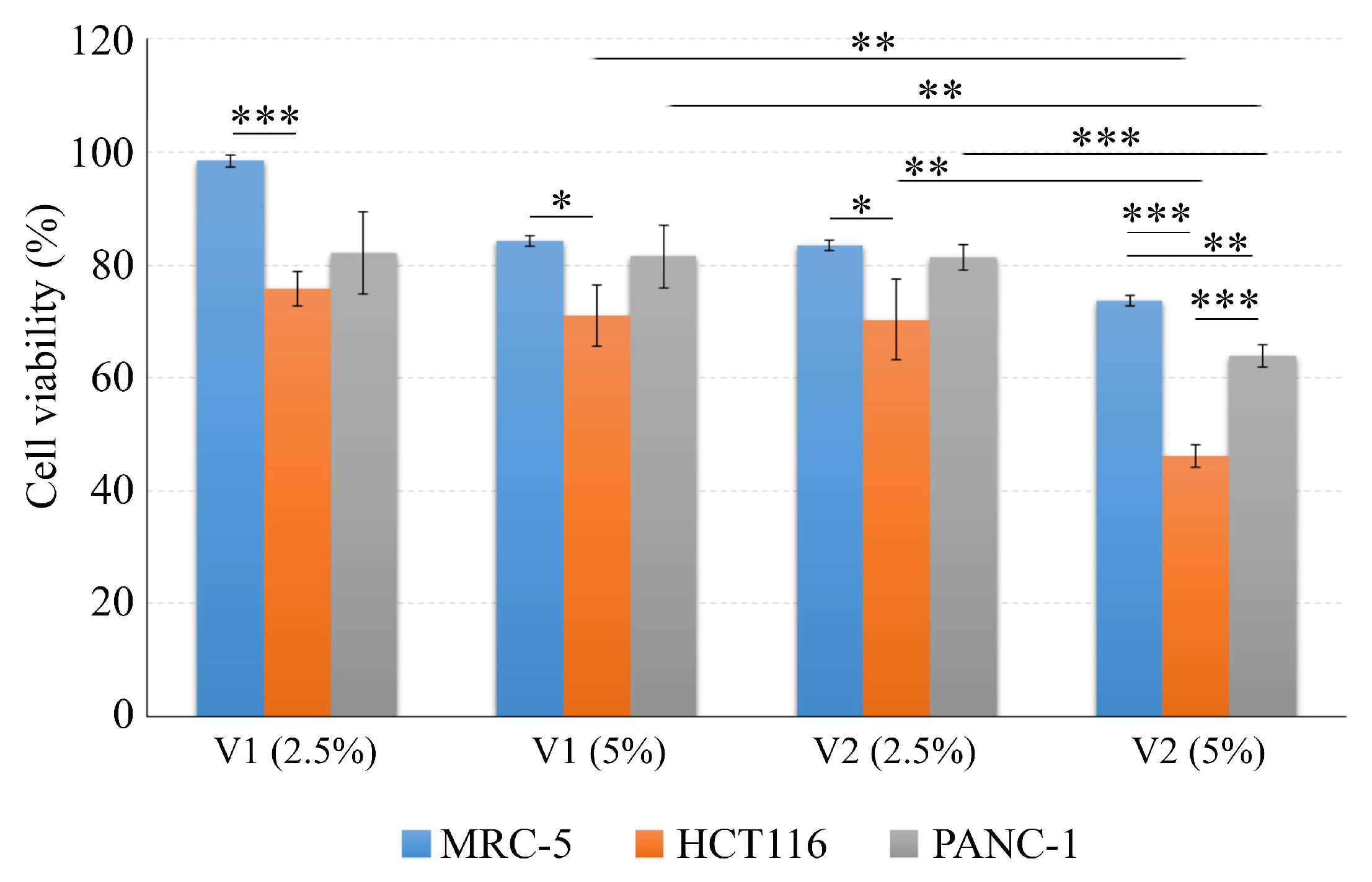
3.4. Cytotoxic Effects of Moldova Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsoupras, A.; Ni, V.L.J.; O’Mahony, É.; Karali, M. Winemaking: “With One Stone, Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products. Fermentation 2023, 9, 838. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Tzachristas, A.; Pasvanka, K.; Calokerinos, A.; Proestos, C. Polyphenols: Natural Antioxidants to Be Used as a Quality Tool in Wine Authenticity. Appl. Sci. 2020, 10, 5908. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Sabadashka, M.; Hertsyk, D.; Strugała-Danak, P.; Dudek, A.; Kanyuka, O.; Kucharska, A.Z.; Kaprelyants, L.; Sybirna, N. Anti-Diabetic and Antioxidant Activities of Red Wine Concentrate Enriched with Polyphenol Compounds under Experimental Diabetes in Rats. Antioxidants 2021, 10, 1399. [Google Scholar] [CrossRef]
- Rocha-Parra, D.; Chirife, J.; Zamora, C.; De Pascual-Teresa, S. Chemical Characterization of an Encapsulated Red Wine Powder and Its Effects on Neuronal Cells. Molecules 2018, 23, 842. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, M.D.; Kimura, S.; Ferro, M.; Foerster, B.; Abufaraj, M.; Briganti, A.; Karakiewicz, P.I.; Shariat, S.F. The Impact of Moderate Wine Consumption on the Risk of Developing Prostate Cancer. Clin. Epidemiol. 2018, 10, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, N.O.; Stanisavljević, N.; Todorović Vukotić, N.; Novović, K.; Žakula, J.J.; Stanković, D.; Pajović, S.B. Antioxidant and Cytotoxic Activity of Red Wine after in Vitro Simulated Digestion in the Presence of Complex Food Matrix. Nat. Prod. Res. 2022, 37, 990–995. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.L.; Alecu, A.; Brinduse, E. The Influence of Melatonin Treatment in the Vinification of Feteasca Neeagra and Cabernet Sauvignon Wines on the Profile of Polyphenolic Compounds and Antioxidant Activity. Antioxidants 2023, 12, 1214. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Niță, R.G.; Vlase, L.; Zamfir, C.I.; Cioroiu, B.I.; Colibaba, L.C.; Muntean, D.; Luchian, C.E.; Vlase, A.M.; Cotea, V. Maximizing Wine Antioxidants: Yeast’s Contribution to Melatonin Formation. Antioxidants 2024, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Xia, Y.; Yang, M.; Zheng, J.; Liu, Y.; Cao, Z. Differences in Grape-Surface Yeast Populations Significantly Influence the Melatonin Level of Wine in Spontaneous Fermentation. LWT 2022, 163, 113568. [Google Scholar] [CrossRef]
- Albu, C.; Radu, L.E.; Radu, G.L. Assessment of Melatonin and Its Precursors Content by a HPLC-MS/MS Method from Different Romanian Wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin Is Synthesised by Yeast during Alcoholic Fermentation in Wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Muñoz-Jurado, A.; Escribano, B.M. Presence of Melatonin in Foods of Daily Consumption: The Benefit of This Hormone for Health. Food Chem. 2024, 458, 140172. [Google Scholar] [CrossRef]
- Woldańska-Okońska, M.; Koszela, K. The Physiological Impact of Melatonin, Its Effect on the Course of Diseases and Their Therapy and the Effect of Magnetic Fields on Melatonin Secretion—Potential Common Pathways of Influence. Biomolecules 2024, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Kim, S.; Lee, S. Melatonin’s Impact on Wound Healing. Antioxidants 2024, 13, 1197. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in Wine and Beer: Beneficial Effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhou, Y.; Liu, Z.; Wei, B.; Feng, X. Melatonin in Different Food Samples: Recent Update on Distribution, Bioactivities, Pretreatment and Analysis Techniques. Food Res. Int. 2023, 163, 112272. [Google Scholar] [CrossRef]
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting Immune System against Cancer by Melatonin: A Mechanistic Viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef]
- Ma, H.; Yan, J.; Sun, W.; Jiang, M.; Zhang, Y. Melatonin Treatment for Sleep Disorders in Parkinson’s Disease: A Meta-Analysis and Systematic Review. Front. Aging Neurosci. 2022, 14, 784314. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jurado, A.; Escribano, B.M.; Caballero-Villarraso, J.; Galván, A.; Agüera, E.; Santamaría, A.; Túnez, I. Melatonin and Multiple Sclerosis: Antioxidant, Anti-Inflammatory and Immunomodulator Mechanism of Action. Inflammopharmacology 2022, 30, 1569–1596. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Song, C.Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z.W.; Xi, Z.M. Melatonin Treatment of Pre-Veraison Grape Berries to Increase Size and Synchronicity of Berries and Modify Wine Aroma Components. Food Chem. 2015, 185, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-Viguera, C.; Domínguez-Perles, R.; Medina, S. Winery By-Products as Sources of Bioactive Tryptophan, Serotonin, and Melatonin: Contributions to the Antioxidant Power. Foods 2023, 12, 1571. [Google Scholar] [CrossRef]
- Leclercq, S.; Schwarz, M.; Delzenne, N.M.; Stärkel, P.; de Timary, P. Alterations of Kynurenine Pathway in Alcohol Use Disorder and Abstinence: A Link with Gut Microbiota, Peripheral Inflammation and Psychological Symptoms. Transl. Psychiatry 2021, 11, 503. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Formation of Amino Acid Derivatives in White and Red Wines during Fermentation: Effects of Non-Saccharomyces Yeasts and Oenococcus Oeni. Food Chem. 2021, 343, 128415. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Szűgyi-Reiczigel, Z.; Ladányi, M.; Bisztray, G.D.; Varga, Z.; Bodor-Pesti, P. Morphological Traits Evaluated with Random Forest Method Explains Natural Classification of Grapevine (Vitis vinifera L.) Cultivars. Plants 2022, 11, 3428. [Google Scholar] [CrossRef]
- Volynkin, V.; Vasylyk, I.; Volodin, V.; Grigoreva, E.; Karzhaev, D.; Lushchay, E.; Ulianich, P.; Volkov, V.; Risovannaya, V.; Blinova, S.; et al. The Assessment of Agrobiological and Disease Resistance Traits of Grapevine Hybrid Populations (Vitis vinifera L. × Muscadinia rotundifolia Michx.) in the Climatic Conditions of Crimea. Plants 2021, 10, 1215. [Google Scholar] [CrossRef]
- Wagner, M.; Stanbury, P.; Dietrich, T.; Döring, J.; Ewert, J.; Foerster, C.; Freund, M.; Friedel, M.; Kammann, C.; Koch, M.; et al. Developing a Sustainability Vision for the Global Wine Industry. Sustainability 2023, 15, 10487. [Google Scholar] [CrossRef]
- Vitis International Variety Cataloque VIVC. Available online: https://www.vivc.de/index.php?r=passport%2Fview&id=7896 (accessed on 19 April 2025).
- Sunyer-Figueres, M.; Mas, A.; Beltran, G.; Torija, M.-J.J. Protective Effects of Melatonin on Saccharomyces Cerevisiae under Ethanol Stress. Antioxidants 2021, 10, 1735. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Đorđević, N.; Novaković, M.; Pejin, B.; Živković, M.; Savić, A.; Mutić, J.; Tešević, V. An Insight into Chemical Composition and Biological Activity of Montenegrin Vranac Red Wine. Sci. Hortic. 2018, 230, 142–148. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing (Antioxidant) Power as a Measure of Antioxidant Capacity: The FRAP Assay. Anal. Biochem. 1999, 299, 15–36. [Google Scholar] [CrossRef]
- Stój, A.; Kapusta, I.; Domagała, D. Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules 2020, 25, 1342. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Jyoti, A.; Mishra, M.K.; Louissaint, N.; Romero, R.; Chugani, D.C.; Kannan, S.; Kannan, R.M. Concurrent Quantification of Tryptophan and Its Major Metabolites. Anal. Biochem. 2013, 443, 222–231. [Google Scholar] [CrossRef]
- Fracassetti, D.; Vigentini, I.; Lo Faro, A.F.F.; De Nisi, P.; Foschino, R.; Tirelli, A.; Orioli, M.; Iriti, M. Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods. Foods 2019, 8, 99. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yue, Q.; Bian, F.; Zhai, H.; Yao, Y. Melatonin Treatment Enhances the Polyphenol Content and Antioxidant Capacity of Red Wine. Hortic. Plant J. 2018, 4, 144–150. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous Melatonin Treatment Induces Disease Resistance against Botrytis Cinerea on Post-Harvest Grapes by Activating Defence Responses. Foods 2022, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, L.; Belwal, T.; Zhang, X.; Lu, H.; Chen, C.; Li, L. Exogenous Melatonin and Abscisic Acid Expedite the Flavonoids Biosynthesis in Grape Berry of Vitis vinifera Cv. Kyoho. Molecules 2020, 25, 12. [Google Scholar] [CrossRef]
- Meng, J.F.; Yu, Y.; Shi, T.C.; Fu, Y.S.; Zhao, T.; Zhang, Z.W. Melatonin Treatment of Pre-Veraison Grape Berries Modifies Phenolic Components and Antioxidant Activity of Grapes and Wine. Food Sci. Technol. 2019, 39, 35–42. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Liang, D.; Hu, R.; Wang, Z.; et al. Melatonin Application Improves Berry Coloration, Sucrose Synthesis, and Nutrient Absorption in ‘Summer Black’ Grape. Food Chem. 2021, 356, 129713. [Google Scholar] [CrossRef]
- Đorđević, N.O.; Novaković, M.M.; Pejin, B.; Mutić, J.J.; Vajs, V.E.; Pajović, S.B.; Tešević, V.V. Comparative Analytical Study of the Selected Wine Varieties Grown in Montenegro. Nat. Prod. Res. 2017, 31, 1825–1830. [Google Scholar] [CrossRef]
- Mitrevska, K.; Grigorakis, S.; Loupassaki, S.; Calokerinos, A.C. Antioxidant Activity and Polyphenolic Content of North Macedonian Wines. Appl. Sci. 2020, 10, 2010. [Google Scholar] [CrossRef]
- Đorđević, N.; Todorović Vukotić, N.; Nastasijević, B.; Perić, I.; Živković, M.B.; Pejić, S.; Pajović, S.B. Effect of Melatonin Treatment during Vinification on Total Phenolic Content and Antioxidant Activity of Moldova Wine. In Proceedings of the 8th Workshop: Food and Drug Safety and Quality, Belgrade, Serbia, 26 September 2024; pp. 190–193. [Google Scholar]
- Gulcin, I.; Buyukokuroglu, M.E.; Kufrevioglu, O.I. Metal Chelating and Hydrogen Peroxide Scavenging Effects of Melatonin. J. Pineal Res. 2003, 34, 278–281. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.; Osuna, C.; Gitto, E. Actions of melatonin in the Reduction of Oxidative Stress. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Effects of Melatonin Treatment on the Enzymatic Browning and Nutritional Quality of Fresh-Cut Pear Fruit. Food Chem. 2019, 299, 125116. [Google Scholar] [CrossRef] [PubMed]
- Charoenphun, N.; Lekjing, S.; Venkatachalam, K. Effect of Exogenous Melatonin Application on Maintaining Physicochemical Properties, Phytochemicals, and Enzymatic Activities of Mango Fruits During Cold Storage. Horticulturae 2025, 11, 222. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Singh, Z.; Hasan, M.U.; Kaur, J.; Afrifa-Yamoah, E.; Woodward, A. Melatonin Application Suppresses Oxidative Stress and Maintains Fruit Quality of Cold Stored ‘Esperanza’ Raspberries by Regulating Antioxidant System. Postharvest Biol. Technol. 2024, 207, 112597. [Google Scholar] [CrossRef]
- Đorđević, N.O.; Todorović, N.; Novaković, I.T.; Pezo, L.L.; Pejin, B.; Maraš, V.; Tešević, V.V.; Pajović, S.B. Antioxidant Activity of Selected Polyphenolics in Yeast Cells: The Case Study of Montenegrin Merlot Wine. Molecules 2018, 23, 1971. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yue, Q.; Bian, F.; Sun, H.; Zhai, H.; Yao, Y. Melatonin Enhances Phenolics Accumulation Partially via Ethylene Signaling and Resulted in High Antioxidant Capacity in Grape Berries. Front. Plant Sci. 2017, 8, 1426. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation Mechanisms Occurring in Wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In Vivo Antioxidant Activity of Grape, Pomace and Wine from Three Red Varieties Grown in Argentina: Its Relationship to Phenolic Profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Mas, A.; Guillamon, J.M.; Torija, M.J.; Beltran, G.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Bioactive Compounds Derived from the Yeast Metabolism of Aromatic Amino Acids during Alcoholic Fermentation. Biomed. Res. Int. 2014, 2014, 898045. [Google Scholar] [CrossRef]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B.; Han, S.-Z. Melatonin and Other 5-Methoxylated Indoles in Yeast: Presence in High Concentrations and Dependence on Tryptophan Availability. Cytologia 1999, 64, 209–213. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of Melatonin Treatment on the Postharvest Quality of Strawberry Fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Gardana, C.; Iriti, M.; Stuknyte, M.; De Noni, I.; Simonetti, P. “Melatonin Isomer” in Wine Is Not an Isomer of the Melatonin but Tryptophan-Ethylester. J. Pineal Res. 2014, 57, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vigentini, I. Tryptophan-Ethylester, the False (Unveiled) Melatonin Isomer in Red Wine. Int. J. Tryptophan Res. 2015, 8, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Vigentini, I.; Gardana, C.; Fracassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast Contribution to Melatonin, Melatonin Isomers and Tryptophan Ethyl Ester during Alcoholic Fermentation of Grape Musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef]
- Dei Cas, M.; Vigentini, I.; Vitalini, S.; Laganaro, A.; Iriti, M.; Paroni, R.; Foschino, R. Tryptophan Derivatives by Saccharomyces Cerevisiae Ec1118: Evaluation, Optimization, and Production in a Soybean-based Medium. Int. J. Mol. Sci. 2021, 22, 472. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Determination of Tryptophan Derivatives in Kynurenine Pathway in Fermented Foods Using Liquid Chromatography Tandem Mass Spectrometry. Food Chem. 2018, 243, 420–427. [Google Scholar] [CrossRef]
- Li, Y.; Hu, N.; Yang, D.; Oxenkrug, G.; Yang, Q. Regulating the Balance between the Kynurenine and Serotonin Pathways of Tryptophan Metabolism. FEBS J. 2017, 284, 948–966. [Google Scholar] [CrossRef]
- Ji, G.; Zhou, W.; Li, X.; Du, J.; Li, X.; Hao, H. Melatonin Inhibits Proliferation and Viability and Promotes Apoptosis in Colorectal Cancer Cells via Upregulation of the MicroRNA-34a/449a Cluster. Mol. Med. Rep. 2021, 23, 187. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Z.; Du, L.; Xu, C.; Wang, Y.; Yang, B.; He, N.; Wang, J.; Ji, K.; Liu, Y.; et al. Melatonin Sensitizes Human Colorectal Cancer Cells to γ-Ray Ionizing Radiation in Vitro and in Vivo. Int. J. Mol. Sci. 2018, 19, 3974. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, X.; Huang, J.; Li, J.; Chen, Q.; Sun, Y.; Wu, J. Melatonin Attenuates Endoplasmic Reticulum Stress in Acute Pancreatitis. Pancreas 2018, 47, 884–891. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Mirhosseini, N.; Reiter, R.J.; Behnamfar, M.; Asemi, Z. Melatonin and Pancreatic Cancer: Current Knowledge and Future Perspectives. J. Cell. Physiol. 2019, 234, 5372–5378. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef]

| Wine Sample | Total Phenolic Content (mg GAE L−1) | Total Flavonoid Content (mg RTE L−1) |
|---|---|---|
| Moldova | 1045.0 ± 12.50 | 135.5 ± 3.36 |
| Moldova + MEL | 1132.5 ± 16.54 ** | 149.2 ± 4.30 ** |
| Peak | Rt (min) | Ion Species | MRM m/z Transition | Cone Voltage (V)/Collision E (eV) | Putative Constituent |
|---|---|---|---|---|---|
| 1 | 2.01 | [M + H]+ | 465 > 303 | 40/20 | Delphinidin-3-O-Glc |
| 2 | 3.22 | [M + H]+ | 479 > 317 | 40/20 | Petunidin-3-O-Glc |
| 3 | 3.70 | [M + H]+ | 463 > 301 | 40/20 | Peonidin-3-O-Glc |
| 4 | 3.86 | [M + H]+ | 493 > 331 | 40/20 | Malvidine-3-O-Glc |
| Sample | Serotonin | Tryptophan | Kynurenic Acid | Melatonin |
|---|---|---|---|---|
| Moldova | 0.103 ± 0.005 | <LOD | 0.362 ± 0.012 | 0.035 ± 0.002 |
| Moldova + melatonin | 0.098 ± 0.013 | <LOD | 0.417 ± 0.013 ** | 0.104 ± 0.002 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đorđević, N.; Todorović Vukotić, N.; Perić, I.; Keta, O.; Petković, V.; Pajović, S.B.; Nastasijević, B. Impact of Melatonin Application in Winemaking on Phenolic Content, Tryptophan Metabolites, and Bioactivity of Red Wine. Antioxidants 2025, 14, 504. https://doi.org/10.3390/antiox14050504
Đorđević N, Todorović Vukotić N, Perić I, Keta O, Petković V, Pajović SB, Nastasijević B. Impact of Melatonin Application in Winemaking on Phenolic Content, Tryptophan Metabolites, and Bioactivity of Red Wine. Antioxidants. 2025; 14(5):504. https://doi.org/10.3390/antiox14050504
Chicago/Turabian StyleĐorđević, Neda, Nevena Todorović Vukotić, Ivana Perić, Otilija Keta, Vladana Petković, Snežana B. Pajović, and Branislav Nastasijević. 2025. "Impact of Melatonin Application in Winemaking on Phenolic Content, Tryptophan Metabolites, and Bioactivity of Red Wine" Antioxidants 14, no. 5: 504. https://doi.org/10.3390/antiox14050504
APA StyleĐorđević, N., Todorović Vukotić, N., Perić, I., Keta, O., Petković, V., Pajović, S. B., & Nastasijević, B. (2025). Impact of Melatonin Application in Winemaking on Phenolic Content, Tryptophan Metabolites, and Bioactivity of Red Wine. Antioxidants, 14(5), 504. https://doi.org/10.3390/antiox14050504







