Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism Maintenance and Postbiotic Production
2.2. Total Antioxidant Activity of Postbiotics
2.2.1. DPPH Radical Scavenging Assay
2.2.2. ABTS+ Radical Scavenging Assay
2.3. Feeding Trial and Sample Collection
2.4. Serum and Ruminal Fluid Antioxidant Enzymes Activity
2.5. Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Antioxidant Activity of Postbiotics
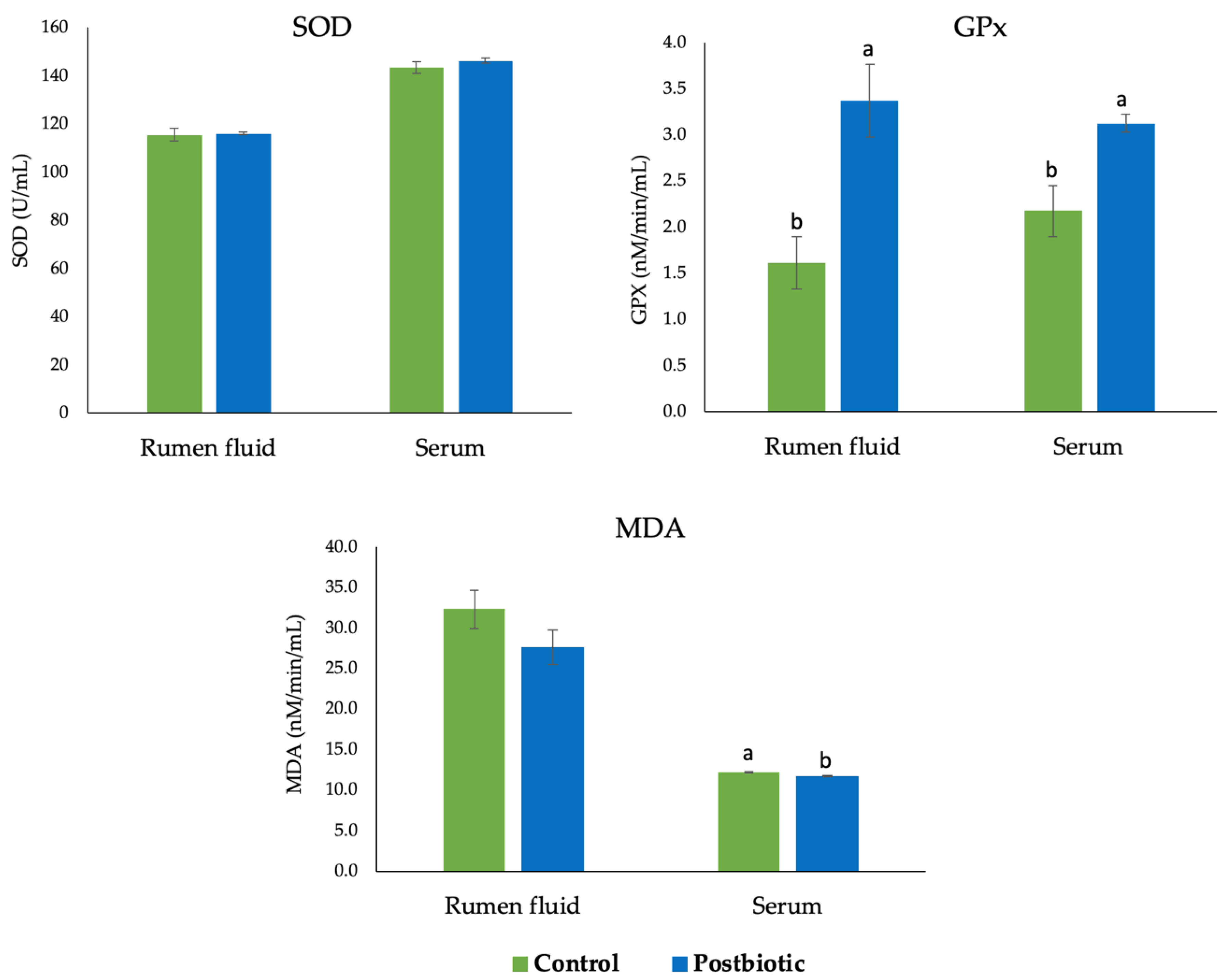
3.2. Antioxidant Enzyme Activity
3.3. Gene expression of Hepatic Antioxidant Enzymes
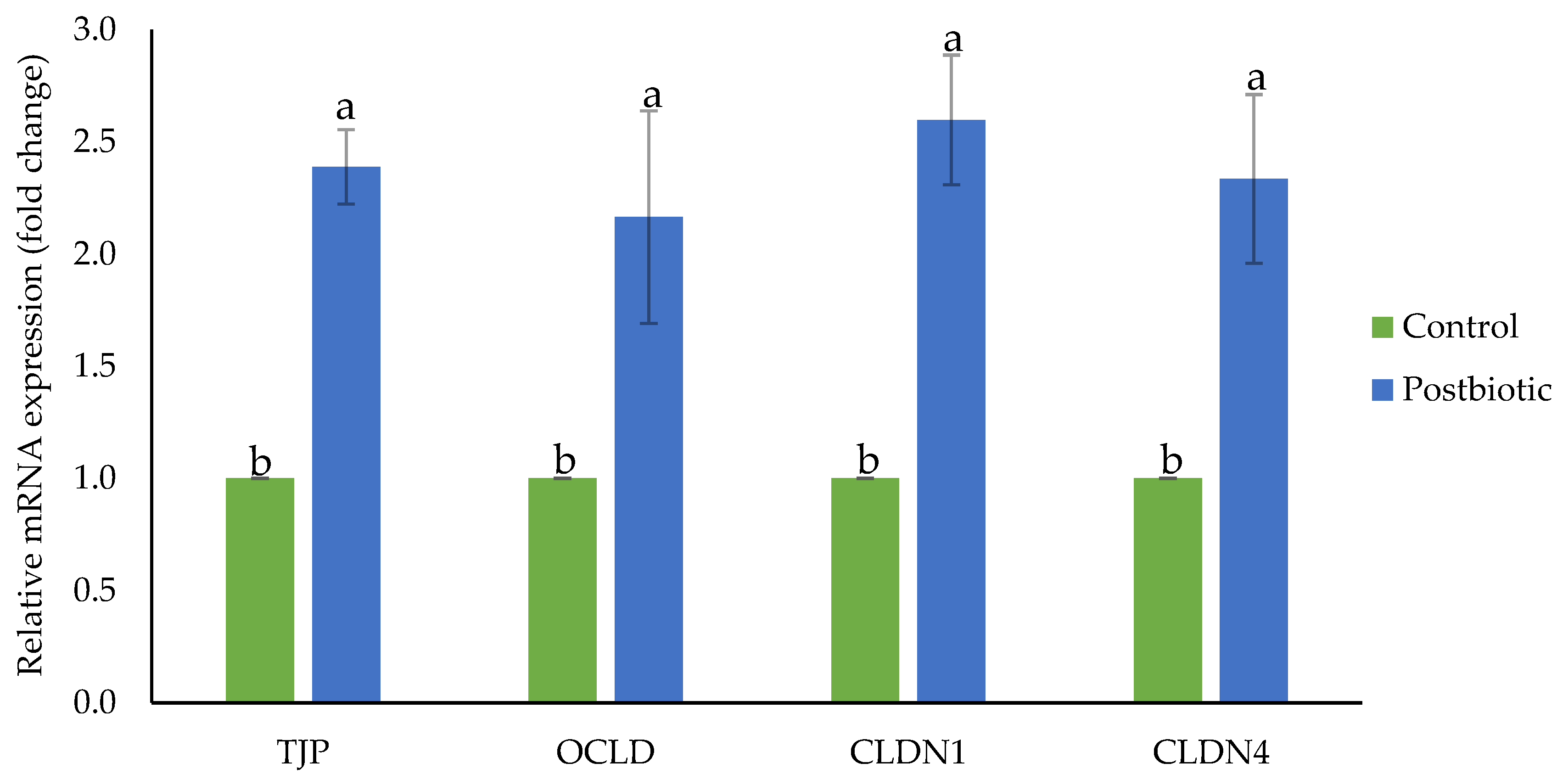
3.4. Gene Expression of Ruminal Barrier Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thanh, N.T.; Loh, T.C.; Foo, H.L.; Hair-Bejo, M.; Kasim, A. Inhibitory activity of metabolites produced by strains of Lactobacillus plantarum isolated from Malaysian fermented food. Int. J. Probiotics Prebiotics 2010, 5, 37. [Google Scholar]
- Choe, D.; Foo, H.; Loh, T.; Bejo, M.; Sazili, A. Inhibitory property of metabolite combinations produced from Lactobacillus plantarum strains. Pertanika J. Trop. Agric. Sci. 2013, 36, 79–88. [Google Scholar]
- Loh, T.C.; Thanh, N.T.; Foo, H.L.; Hair-Bejo, M.; Azhar, B.K. Feeding of different levels of metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microflora, volatile fatty acids and villi height in broilers. Anim. Sci. J. 2010, 81, 205–214. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Samsudin, A.A. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Asmara, S.A.; Akit, H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 2016, 96, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.C.; Choe, D.W.; Foo, H.L.; Sazili, A.Q.; Bejo, M.H. Effects of feeding different postbiotic metabolite combinations produced by Lactobacillus plantarum strains on egg quality and production performance, faecal parameters and plasma cholesterol in laying hens. BMC. Vet. Res. 2014, 10, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, T.C.; Thu, T.V.; Foo, H.L.; Bejo, M.H. Effects of different levels of metabolite combination produced by Lactobacillus plantarum on growth performance, diarrhoea, gut environment and digestibility of postweaning piglets. J. Appl. Anim. Res. 2013, 41, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Thu, T.V.; Loh, T.C.; Foo, H.L.; Yaakub, H.; Bejo, M.H. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets. Trop. Anim. Health Prod. 2011, 43, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef] [Green Version]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L. In vitro study of postbiotics from Lactobacillus plantarum RG14 on rumen fermentation and microbial population. R. Bras. Zootec. 2018, 47, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M. Postbiotic L. plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC. Vet. Res. 2019, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Schogor, A.L.B.; Palin, M.-F.; dos Santos, G.T.; Benchaar, C.; Lacasse, P.; Petit, H.V. Mammary gene expression and activity of antioxidant enzymes and oxidative indicators in the blood, milk, mammary tissue and ruminal fluid of dairy cows fed flax meal. Br. J. Nutr. 2013, 110, 1743–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.; Brzezinska-Slebodzinska, E.; Madsen, F. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Tang, W.; Li, C.; He, Z.; Pan, F.; Pan, S.; Wang, Y. Probiotic properties and cellular antioxidant activity of Lactobacillus plantarum MA2 isolated from Tibetan kefir grains. Probiotics Antimicro. 2018, 10, 523–533. [Google Scholar] [CrossRef]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Shimamura, S.; Abe, F.; Ishibashi, N.; Miyakawa, H.; Yaeshima, T.; Araya, T.; Tomita, M. Relationship between oxygen sensitivity and oxygen metabolism of Bifidobacterium species. J. Dairy Sci. 1992, 75, 3296–3306. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb. Cell Fact. 2019, 18, 112. [Google Scholar] [CrossRef] [Green Version]
- Sabry Mousa, A.E.; Marghani, B.; Ateya, A. Effects of supplementation of Bacillus spp. on blood metabolites, antioxidant status, and gene expression pattern of selective cytokines in growing Barki lambs. J. Adv. Vet. Anim. Res. 2019, 6, 333. [Google Scholar] [CrossRef]
- Jia, P.; Cui, K.; Ma, T.; Wan, F.; Wang, W.; Yang, D.; Wang, Y.; Guo, B.; Zhao, L.; Diao, Q. Influence of dietary supplementation with Bacillus licheniformis and Saccharomyces cerevisiae as alternatives to monensin on growth performance, antioxidant, immunity, ruminal fermentation and microbial diversity of fattening lambs. Sci. Rep. 2018, 8, 16712. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, M.; Xu, M.; Song, L.; Gao, M.; Hu, H. The effects of subacute ruminal acidosis on rumen epithelium barrier function in dairy goats. Small. Rumin. Res. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Shen, H.; Xu, Z.; Shen, Z.; Lu, Z. The regulation of ruminal short-chain fatty acids on the functions of rumen barriers. Front. Physiol. 2019, 10, 1305. [Google Scholar] [CrossRef]
- Hu, F.; Xue, Y.; Guo, C.; Liu, J.; Mao, S. The response of ruminal fermentation, epithelium-associated microbiota, and epithelial barrier function to severe feed restriction in pregnant ewes. J. Animal Sci. 2018, 96, 4293–4305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Meng, M.; Gao, L.; Tu, Y.; Bai, Y. Sodium butyrate improves high-concentrate-diet-induced impairment of ruminal epithelium barrier function in goats. J. Agric. Food Chem. 2018, 66, 8729–8736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tu, Y.; Gao, L.; Meng, M.; Bai, Y. Replacement of grains with soybean hulls ameliorates SARA-induced impairment of the colonic epithelium barrier function of goats. BMC. Vet. Res. 2018, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.; Krause, D.; Gozho, G.; McBride, B. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Gäbel, P.; Bestmann, M.; Martens, H. Influences of diet, short-chain fatty acids, lactate and chloride on bicarbonate movement across the reticulo-rumen wall of sheep. J. Vet. Med. A. 1991, 38, 523–529. [Google Scholar] [CrossRef]
- Keunen, J.; Plaizier, J.; Kyriazakis, L.; Duffield, T.; Widowski, T.; Lindinger, M.; McBride, B. Effects of a subacute ruminal acidosis model on the diet selection of dairy cows. J. Dairy Sci. 2002, 85, 3304–3313. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Zebeli, Q.; Patra, A.K.; Greco, G.; Amasheh, S.; Penner, G.B. Symposium review: The importance of the ruminal epithelial barrier for a healthy and productive cow. J. Dairy Sci. 2019, 102, 1866–1882. [Google Scholar] [CrossRef] [Green Version]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. Claudin clusters as determinants of epithelial barrier function. IUBMB Life 2015, 67, 29–35. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food. Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Goyal, A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT-Food Sci. Technol. 2015, 61, 263–268. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Yang, Y.; Xiong, Z.; Lv, F.; Zhou, W.; Ai, L. Lactic acid bacteria with antioxidant activities alleviating oxidized oil induced hepatic injury in mice. Front. Microbiol. 2018, 9, 2684. [Google Scholar] [CrossRef]
- Moghadam, M.S.; Foo, H.L.; Leow, T.C.; Rahim, R.A.; Loh, T.C. Novel bacteriocinogenic Lactobacillus plantarum strains and their differentiation by sequence analysis of 16 S rDNA, 16 S-23 S and 23 S-5 S intergenic spacer regions and randomly amplified polymorphic DNA analysis. Food Technol. Biotechnol. 2010, 48, 476–483. [Google Scholar]
- Foo, H.; Loh, T.; Lai, P.; Lim, Y.; Kufli, C.; Rusul, G. Effects of adding Lactobacillus plantarum I-UL4 metabolites in drinking water of rats. Pakistan J. Nutr. 2003, 2, 283–288. [Google Scholar]
- Loh, T.C.; Chong, S.W.; Foo, H.L.; Law, F.L. Effects on growth performance, faecal microflora and plasma cholesterol after supplementation of spray-dried metabolite to postweaning rats. Czech J. Animal Sci. 2009, 54, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.W.C.; Tan, Y.P.; Chin, S.J.; Gan, L.Y.; Kang, K.X.; Fong, C.H.; Chang, H.Q.; How, Y.C. Antioxidant properties of selected fresh and processed herbs and vegetables. Free Radic. Antiox. 2014, 4, 39. [Google Scholar] [CrossRef]
- Malaysian Standard, M. Halal food—Production, Preparation, Handling and Storage—General Guideline; Department of Standards Malaysia: Cyberjaya, Malaysia, 2009. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saeed, O.A.; Kee, L.T.; Sazili, A.Q.; Akit, H.; Jahromi, M.F.; Alimon, A.R.; Samsudin, A.A. Effects of corn supplementation on the antioxidant activity, selected minerals, and gene expression of selenoprotein and metallothionein in serum, liver, and kidney of sheep-fed palm kernel cake: Urea-treated rice straw diets. 3 Biotech 2019, 9, 146. [Google Scholar] [CrossRef]
- Thibessard, A.; Borges, F.; Fernandez, A.; Gintz, B.; Decaris, B.; Leblond-Bourget, N. Identification of Streptococcus thermophilus CNRZ368 genes involved in defense against superoxide stress. Appl. Environ. Microbiol. 2004, 70, 2220–2229. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, P.; Musco, N.; Cutrignelli, M.; Mollica, M.; Trinchese, G.; Calabrò, S.; Tudisco, R.; Grossi, M.; Mastellone, V.; Vassalotti, G. The association of aloe and β-carotene supplementation improves oxidative stress and inflammatory state in pregnant buffalo cows. Buffalo Bull. 2017, 36, 497–503. [Google Scholar]
- Halliwell, B.; Gutteridge, J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1. [Google Scholar] [CrossRef]
- Pan, D.; Mei, X. Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Yi, Z.-J.; Fu, Y.-R.; Li, M.; Gao, K.-S.; Zhang, X.-G. Effect of LTA isolated from bifidobacteria on D-galactose-induced aging. Exp. Gerontol. 2009, 44, 760–765. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Zhang, D.; Liu, H.; Shan, D.; Wang, Y. Lactobacillus plantarum ZLP001: In vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian Australas. J. Anim. Sci. 2012, 25, 1153. [Google Scholar] [CrossRef] [Green Version]
- Kareem, K.Y.; Ling, F.H.; Chwen, L.T.; Foong, O.M.; Asmara, S.A. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Yen, C.-L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef]
- Bhatia, S.; Shukla, R.; Madhu, S.V.; Gambhir, J.K.; Prabhu, K.M. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003, 36, 557–562. [Google Scholar] [CrossRef]
- Wang, A.; Yi, X.; Yu, H.; Dong, B.; Qiao, S. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing–finishing pigs. J. Appl. Microbiol. 2009, 107, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2016, 96, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K.; Samak, G. Protection and restitution of gut barrier by probiotics: Nutritional and clinical implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [PubMed] [Green Version]
- Lebeer, S.; Vanderleyden, J.; de Keersmaecker, S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Sun, Q.; Qi, R.; Wang, J.; Qiu, X.; Liu, Z.; Huang, J. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Rokana, N.; Singh, R.; Mallappa, R.H.; Batish, V.K.; Grover, S. Modulation of intestinal barrier function to ameliorate Salmonella infection in mice by oral administration of fermented milks produced with Lactobacillus plantarum MTCC 5690–a probiotic strain of Indian gut origin. J. Med. Microbiol. 2016, 65, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef] [Green Version]

| Control | Postbiotic | |
|---|---|---|
| Feed composition (%) | ||
| Grass | 30.00 | 30.00 |
| Corn | 40.00 | 40.00 |
| Soybean meal | 23.80 | 23.80 |
| Wheat pollard | 3.40 | 3.40 |
| Palm oil | 0.90 | 0.90 |
| Calcium carbonate | 1.70 | 1.70 |
| Sodium chloride | 0.40 | 0.40 |
| Mineral premix 1 | 0.90 | 0.90 |
| Vitamin premix 2 | 0.90 | 0.90 |
| Postbiotic RG14 | - | 0.90 |
| Nutrient composition (% DM) | ||
| ME (MJ/kg) | 8.09 | 8.09 |
| Crude protein | 16.90 | 16.90 |
| Crude fat | 2.70 | 2.67 |
| NDF | 59.60 | 59.60 |
| ADF | 16.90 | 16.60 |
| Gene | Primer Sequence (5′-3′) | Product Size (bp) | NCBI Accession Number |
|---|---|---|---|
| Cu/Zn SOD | F-GAC TTG GGC AGA GGT GGA AA R-CAG GGA ATG TTT ACG GGG CA | 100 | NM_000454.4 |
| GPX1 | F-CCT GGT CGT ACT CGG CTT C R-CCT TCT CGC CAT TCA CCT C | 154 | NM_000581.3 |
| GPX4 | F-GGG AGT AAT GCG GAG ATC AA R-CAT ACC GCT TCA CCA CAC AG | 210 | NM_001039847.2 |
| TJP1 | F-CGACCAGATCCTCAGGGTAA R-AATCACCCACATCGGATTCT | 161 | XM_015101949.1 |
| OCLD | F-GTTCGACCAATGCTCTCTCAG R-CAGCTCCCATTAAGGTTCCA | 196 | XM_015101256.1 |
| CLDN1 | F-CACCCTTGGCATGAAGTGTA R-AGCCAATGAAGAGAGCCTGA | 212 | NM_001185016.1 |
| CLDN4 | F-AAGGTGTACGACTCGCTGCT R-GACGTTGTTAGCCGTCCAG | 237 | NM_001185017.1 |
| GAPDH | F-ACCACTTTGGCATCGTGGAG R-GGGCCATCCACAGTCTTCTG | 76 | NM_001190390.1 |
| Postbiotics | TL1 | RG11 | RG14 | SEM | p-Value |
|---|---|---|---|---|---|
| DPPH (µg AAEAC/mL) | 67.04 | 72.295 | 74.284 | 1.8102 | 0.266 |
| ABTS (µg AAEAC/mL) | 151.822 b | 150.617 b | 202.831 a | 8.7876 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izuddin, W.I.; Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A. Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs. Antioxidants 2020, 9, 250. https://doi.org/10.3390/antiox9030250
Izuddin WI, Humam AM, Loh TC, Foo HL, Samsudin AA. Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs. Antioxidants. 2020; 9(3):250. https://doi.org/10.3390/antiox9030250
Chicago/Turabian StyleIzuddin, Wan Ibrahim, Ali Merzza Humam, Teck Chwen Loh, Hooi Ling Foo, and Anjas Asmara Samsudin. 2020. "Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs" Antioxidants 9, no. 3: 250. https://doi.org/10.3390/antiox9030250
APA StyleIzuddin, W. I., Humam, A. M., Loh, T. C., Foo, H. L., & Samsudin, A. A. (2020). Dietary Postbiotic Lactobacillus plantarum Improves Serum and Ruminal Antioxidant Activity and Upregulates Hepatic Antioxidant Enzymes and Ruminal Barrier Function in Post-Weaning Lambs. Antioxidants, 9(3), 250. https://doi.org/10.3390/antiox9030250





