COVID-19 Vaccination in Kidney Transplant Candidates and Recipients
Abstract
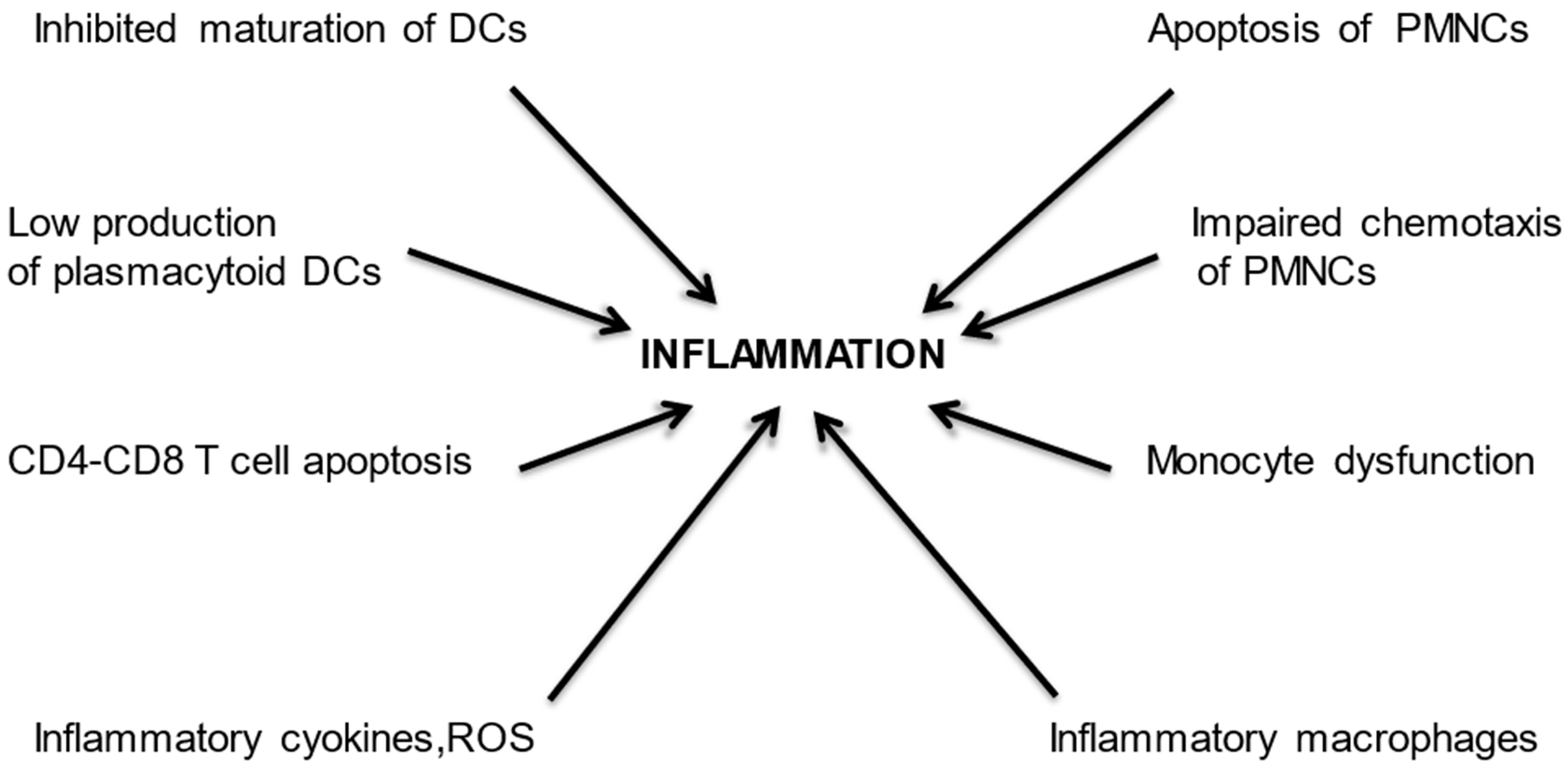
:1. Introduction
2. Methods
3. Pretransplant Vaccination
4. Post-Transplant Vaccination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bossini, N.; Alberici, F.; Delbarba, E.; Valerio, F.; Manenti, C.; Possenti, S.; Econimo, L.; Maffei, C.; Pola, A.; Terlizzi, V.; et al. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am. J. Transplant. 2020, 20, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Pievani, D.; Randoux, C.; Louis, K.; Denis, B.; DeLion, A.; Le Goff, O.; Antoine, C.; Greze, C.; Pillebout, E.; et al. COVID-19 Infection in Kidney Transplant Recipients: Disease Incidence and Clinical Outcomes. J. Am. Soc. Nephrol. 2020, 31, 2413–2423. [Google Scholar] [CrossRef]
- Favà, A.; Cucchiari, D.; Montero, N.; Toapanta, N.; Centellas, F.J.; Vila-Santandreu, A.; Coloma, A.; Meneghini, M.; Manonelles, A.; Sellarés, J.; et al. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: A multicentric cohort study. Am. J. Transplant. 2020, 20, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. COVID-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Mahalingasivam, V.; Craik, A.; Tomlinson, L.A. A Systematic Review of COVID-19 and Kidney Transplantation. Kidney Int. Rep. 2020, 6, 24–45. [Google Scholar] [CrossRef]
- Savina, K.; Sreekumar, R.; Soonu, V.K.; Variyar, E.J. Various vaccine platforms in the field of COVID-19. Beni. Suef. Univ. J. Basic Appl. Sci. 2022, 11, 35. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Das, S.; Kar, S.S.; Samanta, S.; Banerjee, J.; Giri, B.; Dash, S.K. Immunogenic and reactogenic efficacy of Covaxin and Covishield: A comparative review. Immunol. Res. 2022, 70, 289–315. [Google Scholar] [CrossRef]
- Janssen. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of Its Phase 3 ENSEMBLE Trial. Available online: https://www.janssen.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints (accessed on 13 October 2022).
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- COVAXIN® (BBV152) Booster Dose Study Shows Promising Results. Available online: https://www.bharatbiotech.com/covaxin.html (accessed on 13 October 2022).
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Vanholder, R. Special Issue: Immune Dysfunction in Uremia. Toxins 2021, 13, 70. [Google Scholar] [CrossRef]
- Agrawal, S.; Gollapudi, P.; Elahimehr, R.; Pahl, M.V.; Vaziri, N.D. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol. Dial Transplant. 2010, 25, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesselink, D.A.; Betjes, M.G.H.; Verkade, M.A.; Athanassopoulos, P.; Baan, C.C.; Weimar, W. The effects of chronic kidney disease and renal replacement therapy on circulating dendritic cells. Nephrol. Dial Transplant. 2005, 20, 1868–1873. [Google Scholar] [CrossRef]
- Kim, J.U.; Kim, M.; Kim, S. Dendritic Cell Dysfunction in Patients with End-stage Renal Disease. Immune Netw. 2017, 17, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, S.; Matos, C.; Caioni, M. Indoxyl 3-sulfate inhibits maturation and activation of human monocyte-derived dendritic cells. Immunobiology 2018, 223, 239–245. [Google Scholar] [CrossRef]
- Cendoroglo, M.; Jaber, B.L.; Balakrishnan, V.S.; Perianayagam, M.; King, A.J.; Pereira, B.J.G. Neutrophil Apoptosis and Dysfunction in Uremia. J. Am. Soc. Nephrol. 1999, 10, 93–100. [Google Scholar] [CrossRef]
- Mahajan, S.; Kalra, O.P.; Asit, K.T.; Ahuja, G.; Kalra, V. Phagocytic polymorphonuclear function in patients with progressive uremia and the effect of acute hemodialysis. Ren Fail. 2005, 27, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Rudnicki, M.; Walter, F.; Niwa, T.; Hörl, W.H. Glucose-Modified Proteins Modulate Essential Functions and Apoptosis of Polymorphonuclear Leukocytes. J. Am. Soc. Nephrol. 2001, 12, 1264–1271. [Google Scholar] [CrossRef]
- Ottonello, L.; Gnerre, P.; Bertolotto, M. Leptin as a Uremic Toxin Interferes with Neutrophil Chemotaxis. J. Am. Soc. Nephrol. 2004, 15, 2366–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho, J.T.G.; Dalboni, M.A.; Watanabe, R.; Peres, A.T.; Goes, M.A.; Manfredi, S.R.; Canziani, M.E.; Cendoroglo, G.S.; Guimarães-Souza, N.; Batista, M.C.; et al. Effects of spermidine and p-cresol on polymorphonuclear cell apoptosis and function. Artif Organs. 2011, 35, E27–E32. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Hörl, W.H. Free Immunoglobulin Light Chains as a Risk Factor in Renal and Extrarenal Complications. Semin. Dial. 2009, 22, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Wallquist, C.; Mansouri, L.; Norrbäck, M.; Hylander, B.; Jacobson, S.H.; Lundahl, J. Early Changes in Monocyte Adhesion Molecule Expression and Tumor Necrosis Factor-α Levels in Chronic Kidney Disease—A 5-Year Prospective Study. Am. J. Nephrol. 2016, 44, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Girndt, M.; Trojanowicz, B.; Ulrich, C. Monocytes in Uremia. Toxins 2020, 12, 340. [Google Scholar] [CrossRef]
- Barisione, C.; Garibaldi, S.; Furfaro, A.L.; Nitti, M.; Palmieri, D.; Passalacqua, M.; Garuti, A.; Verzola, D.; Parodi, A.; Ameri, P.; et al. Moderate Increase of Indoxyl Sulfate Promotes Monocyte Transition into Profibrotic Macrophages. PLoS ONE 2016, 11, e0149276. [Google Scholar] [CrossRef] [Green Version]
- Bonan, N.B.; Schepers, E.; Pecoits-Filho, R. Contribution of the uremic milieu to an increased pro-inflammatory monocytic phenotype in chronic kidney disease. Sci. Rep. 2019, 9, 10236. [Google Scholar] [CrossRef] [Green Version]
- Hartzell, S.; Bin, S.; Cantarelli, C.; Haverly, M.; Manrique, J.; Angeletti, A.; La Manna, G.; Murphy, B.; Zhang, W.; Levitsky, J.; et al. Kidney Failure Associates with T Cell Exhaustion and Imbalanced Follicular Helper T Cells. Front. Immunol. 2020, 11, 583702. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Gollapudi, S.; Pahl, M.; Vaziri, N. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006, 70, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Betjes, M.G.H. Uremia-Associated Ageing of the Thymus and Adaptive Immune Responses. Toxins 2020, 12, 224. [Google Scholar] [CrossRef]
- Betjes, M.G.H.; Meijers, R.W.J.; Litjens, N.H.R. Loss of renal function causes premature aging of the immune system. Blood Purif. 2013, 36, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Graboski, A.L.; Redinbo, M.R. Gut-Derived Protein-Bound Uremic Toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Gryp, T.; Perna, A. Gut-Derived Metabolites and Their Role in Immune Dysfunction in Chronic Kidney Disease. Toxins 2020, 12, 245. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, M.T.; Cosola, C.; Ranieri, E.; Gesualdo, L. Protein-Bound Uremic Toxins and Immunity. Methods Mol. Biol. 2021, 2325, 215–227. [Google Scholar] [CrossRef]
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L.; Henson, J.D.; Hall, S. The intestinal microbiota and improving the efficacy of COVID-19 vaccinations. J. Funct. Foods 2021, 87, 104850. [Google Scholar] [CrossRef]
- Caggiano, G.; Cosola, C.; Di Leo, V.; Gesualdo, M.; Gesualdo, L. Microbiome modulation to correct uremic toxins and to preserve kidney functions. Curr. Opin. Nephrol. Hypertens. 2020, 29, 49–56. [Google Scholar] [CrossRef]
- Heijnen, B.F.J.; Nelissen, J.; Van Essen, H. Irreversible Renal Damage after Transient Renin-Angiotensin System Stimulation: Involvement of an AT1-Receptor Mediated Immune Response. PLoS ONE 2013, 8, e57815. [Google Scholar] [CrossRef]
- Frost, J.N.; Tan, T.K.; Abbas, M.; Wideman, S.K.; Bonadonna, M.; Stoffel, N.U.; Wray, K.; Kronsteiner, B.; Smits, G.; Campagna, D.R.; et al. Hepcidin-Mediated Hypoferremia Disrupts Immune Responses to Vaccination and Infection. Medicine 2021, 2, 164–179.e12. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [Green Version]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Bacchetta, J.; Salusky, I.B.; Hewison, M. Beyond mineral metabolism, is there an interplay between FGF23 and vitamin D in innate immunity? Pediatr. Nephrol. 2013, 28, 577–582. [Google Scholar] [CrossRef]
- Czaya, B.; Faul, C. FGF23 and inflammation—A vicious coalition in CKD. Kidney Int. 2019, 96, 813–815. [Google Scholar] [CrossRef]
- Pinti, M.; Appay, V.; Campisi, J. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef] [Green Version]
- Mihai, S.; Codrici, E.; Popescu, I.D. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [Green Version]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Ponticelli, C.; Podestà, M.A.; Moroni, G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 2020, 98, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Song, J.; Singer, K. Age and Sex: Impact on adipose tissue metabolism and inflammation. Mech. Ageing Dev. 2021, 199, 111563. [Google Scholar] [CrossRef] [PubMed]
- Raupachova, J.; Kopecky, C.; Cohen, G. High-Density Lipoprotein from Chronic Kidney Disease Patients Modulates Polymorphonuclear Leukocytes. Toxins 2019, 11, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Disturbances of acquired immunity in hemodialysis patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, K.A.; Pindel, M.; Pietruczuk, K. The influence of a single hemodialysis procedure on human T lymphocytes. Sci. Rep. 2019, 9, 5041. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, J.; Ramírez, R.; Madueño, J.A. Cell apoptosis and hemodialysis-induced inflammation. Kidney Int Suppl. 2002, 61, S89–S93. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Liu, Z.H.; Chen, Z.H.; Gong, D.H.; Ji, D.X.; Li, L.S.H. Improvement of monocyte function and immune homeostasis by high volume continuous venovenous hemofiltration in patients with severe acute pancreatitis. Int. J. Artif. Organs 2008, 31, 882–890. [Google Scholar] [CrossRef]
- Nongnuch, A.; Ngampongpan, W.; Srichatrapimuk, S.; Wongsa, A.; Thongpraphai, S.; Boonarkart, C.; Sanmeema, N.; Chittaganpitch, M.; Auewarakul, P.; Tassaneetrithep, B.; et al. Immune response to influenza vaccination in ESRD patients undergoing hemodialysis vs. hemodiafiltration. PLoS ONE 2020, 15, e0227719. [Google Scholar] [CrossRef]
- Xiaoyan, J.; Rongyi, C.; Xuesen, C. The difference of T cell phenotypes in end stage renal disease patients under different dialysis modality. BMC Nephrol. 2019, 20, 301. [Google Scholar] [CrossRef] [Green Version]
- Caprara, C.; Corradi, V.; Scalzotto, E. Differential effects of peritoneal and hemodialysis on circulating regulatory T cells one month post initiation of renal replacement therapy. Clin. Nephrol. 2021, 95, 37–44. [Google Scholar] [CrossRef]
- Ducloux, D.; Legendre, M.; Bamoulid, J. ESRD-associated immune phenotype depends on dialysis modality and iron status: Clinical implications. Immun. Ageing 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Rubey, H.; Treipl, A. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol. Dial. Transplant. 2021, 36, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Anand, S.; Han, J. COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J. Am. Soc. Nephrol. 2022, 33, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Blazquez-Navarro, A.; Safi, L.; Kaliszczyk, S.; Paniskaki, K.; Neumann, I.E.; Schmidt, K.; Stockhausen, M.; Hörstrup, J.; Cinkilic, O.; et al. Impaired Humoral but Substantial Cellular Immune Response to Variants of Concern B1.1.7 and B.1.351 in Hemodialysis Patients after Vaccination with BNT162b2. J. Am. Soc. Nephrol. 2021, 32, 2725–2727. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, S.M.; Sibbel, S.; Karpinski, S.; Marlowe, G.; Walker, A.G.; Giullian, J.; Van Wyck, D.; Kelley, T.; Lazar, R.; Zywno, M.L.; et al. Comparative Effectiveness of mRNA-based BNT162b2 Vaccine versus Adenovirus Vector–Based Ad26.COV2. S Vaccine for the Prevention of COVID-19 among Dialysis Patients. J. Am. Soc. Nephrol. 2022, 33, 688–697. [Google Scholar] [CrossRef]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chen, J.; et al. Severity of COVID-19 after Vaccination among Hemodialysis Patients: An Observational Cohort Study. Clin. J. Am. Soc. Nephrol. 2022, 17, 843–850. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Coates, P.T.; Rovin, B.H.; Ronco, P. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int. 2021, 99, 1275–1279. [Google Scholar] [CrossRef]
- Oliver, M.J.; Thomas, D.; Balamchi, S.; Ip, J.; Naylor, K.; Dixon, S.N.; McArthur, E.; Kwong, J.; Perl, J.; Atiquzzaman, M.; et al. Vaccine Effectiveness Against SARS-CoV-2 Infection and Severe Outcomes in the Maintenance Dialysis Population in Ontario, Canada. J. Am. Soc. Nephrol. 2022, 33, 839–849. [Google Scholar] [CrossRef]
- Zitt, E.; Davidovic, T.; Schimpf, J. The Safety and Immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 Vaccine in Hemodialysis Patients. Front. Immunol. 2021, 12, 704773. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Ma, B.M.; Tam, A.R.; Chan, K.W. Immunogenicity and Safety of COVID-19 Vaccines in Patients Receiving Renal Replacement Therapy: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 827859. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Lu, K.-C.; Kuo, K.-L. The Efficacy of COVID-19 Vaccines in Chronic Kidney Disease and Kidney Transplantation Patients: A Narrative Review. Vaccines 2021, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Bensouna, I.; Caudwell, V.; Kubab, S. SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e1. [Google Scholar] [CrossRef] [PubMed]
- Dekervel, M.; Henry, N.; Torreggiani, M.; Pouteau, L.-M.; Imiela, J.-P.; Mellaza, C.; Garnier, A.-S.; Dujardin, A.; Asfar, M.; Ducancelle, A.; et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021, 14, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Biedunkiewicz, B.; Tylicki, L.; Ślizień, W.; Lichodziejewska-Niemierko, M.; Dąbrowska, M.; Kubanek, A.; Rodak, S.; Polewska, K.; Tylicki, P.; Renke, M.; et al. Waning Humoral Response after COVID-19 mRNA Vaccination in Maintenance Dialysis Patients and Recovery after a Complementary Third Dose. Vaccines 2022, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Rahamimov, R.; Mashraki, T.; Ben-Dor, N.; Steinmetz, T.; Agur, T.; Zingerman, B.; Herman-Edelstein, M.; Lichtenberg, S.; Ben-Zvi, H.; et al. Immune Response to Third Dose BNT162b2 COVID-19 Vaccine Among Kidney Transplant Recipients—A Prospective Study. Transpl. Int. 2022, 35, 10204. [Google Scholar] [CrossRef]
- Housset, P.; Kubab, S.; Hanafi, L. Humoral response after a fourth “booster” dose of a Coronavirus disease 2019 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int. 2022, 101, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.E.; Krawczyk, A.; Brochhagen, L.; van de Sand, L.; Sorge-Hädicke, B.; Tyczynski, B.; Witzke, O.; et al. Decline of Humoral Responses 6 Months after Vaccination with BNT162b2 (Pfizer–BioNTech) in Patients on Hemodialysis. Vaccines 2022, 10, 327. [Google Scholar] [CrossRef]
- Clarke, C.L.; Prendecki, M.; Dhutia, A.; Gan, J.; Edwards, C.; Prout, V.; Lightstone, L.; Parker, E.; Marchesin, F.; Griffith, M.; et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021, 99, 1470–1477. [Google Scholar] [CrossRef]
- Agur, T.; Ben-Dor, N.; Herman-Edelstein, M. Longevity of Humoral Response Six Months Following BNT162b2 Vaccine in Dialysis Patients. Front. Med. 2022, 9, 781888. [Google Scholar] [CrossRef]
- Dulovic, A.; Strengert, M.; Ramos, G.M.; Becker, M.; Griesbaum, J.; Junker, D.; Lürken, K.; Beigel, A.; Wrenger, E.; Lonnemann, G.; et al. Diminishing Immune Responses against Variants of Concern in Dialysis Patients 4 Months after SARS-CoV-2 mRNA Vaccination. Emerg. Infect. Dis. 2022, 28, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2013, 58, e44–e100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Boyle, S.; Malat, G.; Sharma, A.; Bias, T.; Doyle, A. Low rates of vaccination in listed kidney transplant candidates. Transpl. Infect. Dis. 2016, 18, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9, S1–S155. [Google Scholar] [CrossRef]
- Duchini, A.; Goss, J.A.; Karpen, S.; Pockros, P.J. Vaccinations for Adult Solid-Organ Transplant Recipients: Current Recommendations and Protocols. Clin. Microbiol. Rev. 2003, 16, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Katerinis, I.; Hadaya, K.; Duquesnoy, R.; Ferrari-Lacraz, S.; Meier, S.; Van Delden, C.; Martin, P.-Y.; Siegrist, C.-A.; Villard, J. De Novo Anti-HLA Antibody After Pandemic H1N1 and Seasonal Influenza Immunization in Kidney Transplant Recipients. Am. J. Transplant. 2011, 11, 1727–1733. [Google Scholar] [CrossRef]
- Fairhead, T.; Hendren, E.; Tinckam, K.; Rose, C.; Sherlock, C.; Shi, L.; Crowcroft, N.; Gubbay, J.; Landsberg, D.; Knoll, G.; et al. Poor seroprotection but allosensitization after adjuvanted pandemic influenza H1N1 vaccine in kidney transplant recipients. Transpl. Infect. Dis. 2012, 14, 575–583. [Google Scholar] [CrossRef]
- Blumberg, E.A.; Fitzpatrick, J.; Stutman, P.C. Safety of influenza vaccine in heart transplant recipients. J. Heart Lung Transplant. 1998, 17, 1075–1080. Available online: https://pubmed.ncbi.nlm.nih.gov/9855446/ (accessed on 12 October 2022).
- Salles, M.J.C.; Sens, Y.A.S.; Malafronte, P.; Souza, J.F.; Vilas Boas, L.S.; Machado, C.M. Antibody response to the non-adjuvanted and adjuvanted influenza A H1N1/09 monovalent vaccines in renal transplant recipients. Transpl. Infect. Dis. 2012, 14, 564–574. [Google Scholar] [CrossRef]
- Bosaeed, M.; Kumar, D. Seasonal influenza vaccine in immunocompromised persons. Hum. Vaccin. Immunother. 2018, 14, 1311–1322. [Google Scholar] [CrossRef]
- Zipeto, D.; da Fonseca Palmeira, J.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Banerjee, P.; Bhagavatula, S.; Kushwaha, P.P.; Kumar, S. Contributions of human ACE2 and TMPRSS2 in determining host–pathogen interaction of COVID-19. J. Genet. 2021, 100, 12. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Benigni, A.; Remuzzi, G. Angiotensin-converting enzyme 2: From a vasoactive peptide to the gatekeeper of a global pandemic. Curr. Opin. Nephrol. Hypertens. 2021, 30, 252–263. [Google Scholar] [CrossRef]
- Murgolo, N.; Therien, A.G.; Howell, B.; Klein, D.; Koeplinger, K.; Lieberman, L.A.; Adam, G.C.; Flynn, J.; McKenna, P.; Swaminathan, G.; et al. SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development. PLoS Pathog. 2021, 17, e1009225. [Google Scholar] [CrossRef] [PubMed]
- Tharmaraj, D.; Dendle, C.; Polkinghorne, K.R.; Mulley, W.R. Kidney transplant recipients’ attitudes toward COVID-19 vaccination and barriers and enablers to vaccine acceptance. Transpl. Infect. Dis. 2022, 24, e13749. [Google Scholar] [CrossRef]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Proß, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef]
- Mazzola, A.; Todesco, E.; Drouin, S.; Hazan, F.; Marot, S.; Thabut, D.; Varnous, S.; Soulié, C.; Barrou, B.; Marcelin, A.-G.; et al. Poor Antibody Response After Two Doses of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in Transplant Recipients. Clin. Infect. Dis. 2022, 74, 1093–1096. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Arab. Archaeol. Epigr. 2021, 21, 2719–2726. [Google Scholar] [CrossRef]
- Magicova, M.; Zahradka, I.; Fialova, M.; Neskudla, T.; Gurka, J.; Modos, I.; Hojny, M.; Raska, P.; Smejkal, P.; Striz, I.; et al. Determinants of Immune Response to Anti–SARS-CoV-2 mRNA Vaccines in Kidney Transplant Recipients: A Prospective Cohort Study. Transplantation 2022, 106, 842–852. [Google Scholar] [CrossRef]
- Azzi, Y.; Raees, H.; Wang, T.; Cleare, L.; Liriano-Ward, L.; Loarte-Campos, P.; Pynadath, C.; Ajaimy, M.; Alani, O.; Bao, Y.; et al. Risk factors associated with poor response to COVID-19 vaccination in kidney transplant recipients. Kidney Int. 2021, 100, 1127–1128. [Google Scholar] [CrossRef]
- Russo, G.; Lai, Q.; Poli, L.; Perrone, M.P.; Gaeta, A.; Rossi, M.; Mastroianni, C.M.; Garofalo, M.; Pretagostini, R. SARS-CoV-2 vaccination with BNT162B2 in renal transplant patients: Risk factors for impaired response and immunological implications. Clin. Transplant. 2022, 36, e14495. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Chiang, T.P.-Y.; Ou, M.T. Antibody Response to the Janssen COVID-19 Vaccine in Solid Organ Transplant Recipients. Transplantation 2021, 105, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Zhang, R.; Shin, B.; Gadsden, T.M.; Petrosyan, A.; Vo, A.; Ammerman, N.; Sethi, S.; Huang, E.; Peng, A.; Najjar, R.; et al. Assessment of humoral and cellular immune responses to SARS-CoV-2 vaccination (BNT162b2) in immunocompromised renal allograft recipients. Transpl. Infect. Dis. 2022, 24, e13813. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Charmetant, X.; Espi, M.; Barba, T. Predictive factors of a viral neutralizing humoral response after a third dose of COVID-19 mRNA vaccine. Am. J. Transplant. 2022, 22, 1442–1450. [Google Scholar] [CrossRef]
- Werbel, W.A.; Boyarsky, B.J.; Ou, M.T. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021, 174, 1330–1332. [Google Scholar] [CrossRef]
- Reindl-Schwaighofer, R.; Heinzel, A.; Mayrdorfer, M.; Jabbour, R.; Hofbauer, T.M.; Merrelaar, A.; Eder, M.; Regele, F.; Doberer, K.; Spechtl, P.; et al. Comparison of SARS-CoV-2 Antibody Response 4 Weeks After Homologous vs. Heterologous Third Vaccine Dose in Kidney Transplant Recipients: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 165–171. [Google Scholar] [CrossRef]
- Marlet, J.; Gatault, P.; Maakaroun, Z. Antibody Responses after a Third Dose of COVID-19 Vaccine in Kidney Transplant Recipients and Patients Treated for Chronic Lymphocytic Leukemia. Vaccines 2021, 9, 1055. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier, G.; Perrin, P. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients with Minimal Serologic Response to 2 Doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef]
- Anichini, G.; Terrosi, C.; Gandolfo, C. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021, 385, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Tylicki, L.; Dębska-Ślizień, A.; Muchlado, M. Boosting Humoral Immunity from mRNA COVID-19 Vaccines in Kidney Transplant Recipients. Vaccines 2021, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Cremoni, M.; Gérard, A.; Grabsi, H.; Rogier, L.; Blois, M.; Couzin, C.; Ben Hassen, N.; Rouleau, M.; Barbosa, S.; et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. eBioMedicine 2021, 73, 103679. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Jens, A.; Stefanski, A.-L.; Hammett, C.; Osmanodja, B.; Koch, N.; Zukunft, B.; Beck, J.; Oellerich, M.; et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination–specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022, 7, e157836. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, J.; Tonnus, W.; Paliege, A.; Rettig, R.; Steglich, A.; Gembardt, F.; Kessel, F.; Kröger, H.; Arndt, P.; Sradnick, J.; et al. Cellular and Humoral Immune Responses After 3 Doses of BNT162b2 mRNA SARS-CoV-2 Vaccine in Kidney Transplant. Transplantation 2021, 105, E267–E269. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Kumar, D.; Hu, Q.; Samson, R. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am. J. Transplant. 2022, 22, 2089–2093. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O.; Benotmane, I.; Masset, C.; Blancho, G. Antibody Response to a Fourth Messenger RNA COVID-19 Vaccine Dose in Kidney Transplant Recipients: A Case Series. Ann. Intern. Med. 2022, 175, 455–456. [Google Scholar] [CrossRef]
- Karaba, A.H.; Johnston, T.S.; Aytenfisu, T.Y.; Akinde, O.; Eby, Y.; Ruff, J.E.; Abedon, A.T.; Alejo, J.L.; Blankson, J.N.; Cox, A.L.; et al. A Fourth Dose of COVID-19 Vaccine Does Not Induce Neutralization of the Omicron Variant Among Solid Organ Transplant Recipients with Suboptimal Vaccine Response. Transplantation 2022, 106, 1440–1444. [Google Scholar] [CrossRef]
- Alejo, J.L.; Mitchell, J.; Chiang, T.P.-Y.; Abedon, A.T.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.; Levan, M.L.; Massie, A.B.; Garonzik-Wang, J.M.; et al. Antibody Response to a Fourth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2021, 105, E280–E281. [Google Scholar] [CrossRef]
- Masset, C.; Benotmane, I.; Dantal, J. A fourth SARS-CoV-2 mRNA vaccine in strictly seronegative kidney transplant recipients. Kidney Int. 2022, 101, 825–826. [Google Scholar] [CrossRef]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021, 21, 2727–2739. [Google Scholar] [CrossRef]
- Chukwu, C.A.; Mahmood, K.; Elmakki, S. Evaluating the antibody response to SARS-CoV-2 vaccination amongst kidney transplant recipients at a single nephrology centre. PLoS ONE 2022, 17, e0265130. [Google Scholar] [CrossRef]
- Kantauskaite, M.; Müller, L.; Kolb, T.; Fischer, S.; Hillebrandt, J.; Ivens, K.; Andree, M.; Luedde, T.; Orth, H.M.; Adams, O.; et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2022, 22, 634–639. [Google Scholar] [CrossRef]
- Altheaby, A.; Alloqmani, D.; AlShammari, R. Safety and Efficacy of the COVID-19 Vaccine in Kidney Transplant Recipients. Cureus 2022, 14, e24753. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Boedecker-Lips, S.C.; Lautem, A.; Runkel, S.; Klimpke, P.; Kraus, D.; Keil, P.; Holtz, S.; Tomalla, V.; Marczynski, P.; Boedecker, C.B.; et al. Six-Month Follow-Up after Vaccination with BNT162b2: SARS-CoV-2 Antigen-Specific Cellular and Humoral Immune Responses in Hemodialysis Patients and Kidney Transplant Recipients. Pathogens 2022, 11, 67. [Google Scholar] [CrossRef]
- Lai, Q.; Spoletini, G.; Bianco, G. SARS-CoV-2 and immunosuppression: A double-edged sword. Transpl. Infect. Dis. 2020, 22, e13404. [Google Scholar] [CrossRef]
- Netti, G.S.; Infante, B.; Troise, D.; Mercuri, S.; Panico, M.; Spadaccino, F.; Catalano, V.; Gigante, M.; Simone, S.; Pontrelli, P.; et al. mTOR inhibitors improve both humoral and cellular response to SARS-CoV-2 messenger RNA BNT16b2 vaccine in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1475–1482. [Google Scholar] [CrossRef]
- Baker, D.; Roberts, C.A.K.; Pryce, G. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020, 202, 149–161. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.D.; Kyttaris, V.C. Rituximab-associated hypogammaglobulinemia in autoimmune rheumatic diseases: A single-center retrospective cohort study. Rheumatol. Int. 2021, 41, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.T.; Boyarsky, B.J.; Chiang, T.P. Immunogenicity and Reactogenicity After SARS-CoV-2 mRNA Vaccination in Kidney Transplant Recipients Taking Belatacept. Transplantation 2021, 105, 2119–2123. [Google Scholar] [CrossRef] [PubMed]
- Chavarot, N.; Ouedrani, A.; Marion, O.; Leruez-Ville, M.; Vilain, E.; Baaziz, M.; Del Bello, A.; Burger, C.; Sberro-Soussan, R.; Martinez, F.; et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Transplantation 2021, 105, E94–E95. [Google Scholar] [CrossRef]
- Osmanodja, B.; Ronicke, S.; Budde, K.; Jens, A.; Hammett, C.; Koch, N.; Seelow, E.; Waiser, J.; Zukunft, B.; Bachmann, F.; et al. Serological Response to Three, Four and Five Doses of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J. Clin. Med. 2022, 11, 2565. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Fernandes, G.; Devresse, A.; Scohy, A.; Yombi, J.C.; Belkhir, L.; De Greef, J.; De Meyer, M.; Mourad, M.; Darius, T.; Buemi, A.; et al. Rapid Decline in Vaccine-induced Anti-SARS-CoV-2 Antibody Titers 3 Months After Kidney Transplantation: A Case Series from Belgium. Transplantation 2022, 106, E98–E99. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Ou, M.T.; Greenberg, R.S. Safety of the First Dose of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation 2021, 105, e56–e57. [Google Scholar] [CrossRef]
- Phadke, V.K.; Scanlon, N.; Jordan, S.C.; Rouphael, N.G. Immune Responses to SARS-CoV-2 in Solid Organ Transplant Recipients. Curr. Transplant. Rep. 2021, 8, 127–139. [Google Scholar] [CrossRef]
- Jurdi, A.A.; Gassen, R.B.; Borges, T.J.; Solhjou, Z.; Hullekes, F.E.; Lape, I.T.; Efe, O.; Alghamdi, A.; Patel, P.; Choi, J.Y.; et al. Non-Invasive Monitoring for Rejection in Kidney Transplant Recipients After SARS-CoV-2 mRNA Vaccination. Front. Immunol. 2022, 13, 838985. [Google Scholar] [CrossRef]
- del Bello, A.; Marion, O.; Delas, A.; Congy-Jolivet, N.; Colombat, M.; Kamar, N. Acute rejection after anti–SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 2021, 100, 238–239. [Google Scholar] [CrossRef]
- Vnučák, M.; Graňák, K.; Beliančinová, M. Acute kidney rejection after anti-SARS-CoV-2 virus-vectored vaccine—Case report. NPJ Vaccines 2022, 7, 30. [Google Scholar] [CrossRef]
- Lim, J.-H.; Han, M.-H.; Kim, Y.-J. New-onset Nephrotic Syndrome after Janssen COVID-19 Vaccination: A Case Report and Literature Review. J. Korean Med. Sci. 2021, 36, 1011–8934. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.J.; Gianotten, S.; van der Meijden, W.A.G. An Additional Case of Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 312. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, N.; Guarnieri, A.; Tripodi, S.; Salvo, D.P.; Garosi, G. Minimal change disease following vaccination for SARS-CoV-2. J. Nephrol. 2021, 34, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Unver, S.; Haholu, A.; Yildirim, S. Nephrotic syndrome and acute kidney injury following CoronaVac anti-SARS-CoV-2 vaccine. Clin. Kidney J. 2021, 14, 2608–2611. [Google Scholar] [CrossRef]
- Wu, H.H.L.; Kalra, P.A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 1252. [Google Scholar] [CrossRef]
- Caza, T.N.; Cassol, C.A.; Messias, N.; Hannoudi, A.; Haun, R.S.; Walker, P.D.; May, R.M.; Seipp, R.M.; Betchick, E.J.; Amin, H.; et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney360 2021, 2, 1770–1780. [Google Scholar] [CrossRef]
- Rieckmann, S.; Seibert, F.S.; Hogeweg, M. Acute interstitial nephritis after vaccination with BNT162b2. J. Nephrol. 2022, 35, 779–782. [Google Scholar] [CrossRef]
- Jefferis, J.; Kassianos, A.J.; Grivei, A. SARS-CoV-2 vaccination–associated collapsing glomerulopathy in a kidney transplant recipient. Kidney Int. 2022, 101, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Xu, J.; Xia, H.; Wang, Y.; Zhang, C.; Chen, W.; Zhang, H.; Liu, Q.; Zhu, R.; et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021, 7, 99. [Google Scholar] [CrossRef]
- Chu, D.K.; Duda, S. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Vandeberg, P.; Cruz, M.; Diez, J.M. Production of anti-SARS-CoV-2 hyperimmune globulin from convalescent plasma. Transfusion 2021, 61, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef]
- Hassler, L.; Wysocki, J.; Gelarden, I.; Sharma, I.; Tomatsidou, A.; Ye, M.; Gula, H.; Nicoleascu, V.; Randall, G.; Pshenychnyi, S.; et al. A Novel Soluble ACE2 Protein Provides Lung and Kidney Protection in Mice Susceptible to Lethal SARS-CoV-2 Infection. J. Am. Soc. Nephrol. 2022, 33, 1293–1307. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Loo, Y.-M.; McTamney, P.M.; Arends, R.H.; Abram, M.E.; Aksyuk, A.A.; Diallo, S.; Flores, D.J.; Kelly, E.J.; Ren, K.; Roque, R.; et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci. Transl. Med. 2022, 14, eabl8124. [Google Scholar] [CrossRef]


| Vaccine Name | Brand Name | Manufacturer | Approach | Dose Regimens | Vaccine Efficacy% (95% CI) |
|---|---|---|---|---|---|
| BNT162b2 | Comirnaty | Pfizer-BioNTech | mRNA | 2 doses (21 days apart) | 94.6% [7] |
| mRNA-1273 | Spikevax | Moderna | mRNA | 2 doses (28 days apart) | 94.1% [8] |
| AZD1222 | Vaxzevria | AstraZeneca-Oxford | Viral vector | 2 doses (28 days apart) | 66.7% 55.1% (2 doses < 6 weeks apart) 81.3% (2 doses > 12 weeks apart) [9] |
| Covishield | Serum Institute of India | Viral vector | 2 doses (4–8 weeks apart) | ~90% [10] | |
| Ad26.COV2.S | Janssen COVID-19 Vaccine | Johnson & Johnson | Viral vector | 1 dose | 66% [11] |
| BBIBP-CorV | Covilo | Sinopharm | Inactivated virus | 2 doses (21 days apart) | 79% [12] |
| COVID-19 Vaccine | CoronaVac | Sinovac Biotech | Inactivated virus | 2 doses (14 days apart) | 50.4% (Brazil), 67% (Chile), 65% (Indonesia), 78% (Brazil), 84% (Turkey) |
| BBV152 | Covaxin | Bharat Biotech | Viral vector | 2 doses (28 days apart) | 81% [13] |
| NVX-CoV2373 | Nuvaxoid | Novavax | Protein subunit | 2 doses (21 days apart) | 89.7% [14] |
| Covovax | Serum Institute of India | Protein subunit | 2 doses (21 days apart) | 90.4% (USA) 89.7% (UK and Mexico) COVOVAX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponticelli, C.; Campise, M. COVID-19 Vaccination in Kidney Transplant Candidates and Recipients. Vaccines 2022, 10, 1808. https://doi.org/10.3390/vaccines10111808
Ponticelli C, Campise M. COVID-19 Vaccination in Kidney Transplant Candidates and Recipients. Vaccines. 2022; 10(11):1808. https://doi.org/10.3390/vaccines10111808
Chicago/Turabian StylePonticelli, Claudio, and Mariarosaria Campise. 2022. "COVID-19 Vaccination in Kidney Transplant Candidates and Recipients" Vaccines 10, no. 11: 1808. https://doi.org/10.3390/vaccines10111808
APA StylePonticelli, C., & Campise, M. (2022). COVID-19 Vaccination in Kidney Transplant Candidates and Recipients. Vaccines, 10(11), 1808. https://doi.org/10.3390/vaccines10111808





