Development of Foot-and-Mouth Disease Vaccines in Recent Years
Abstract
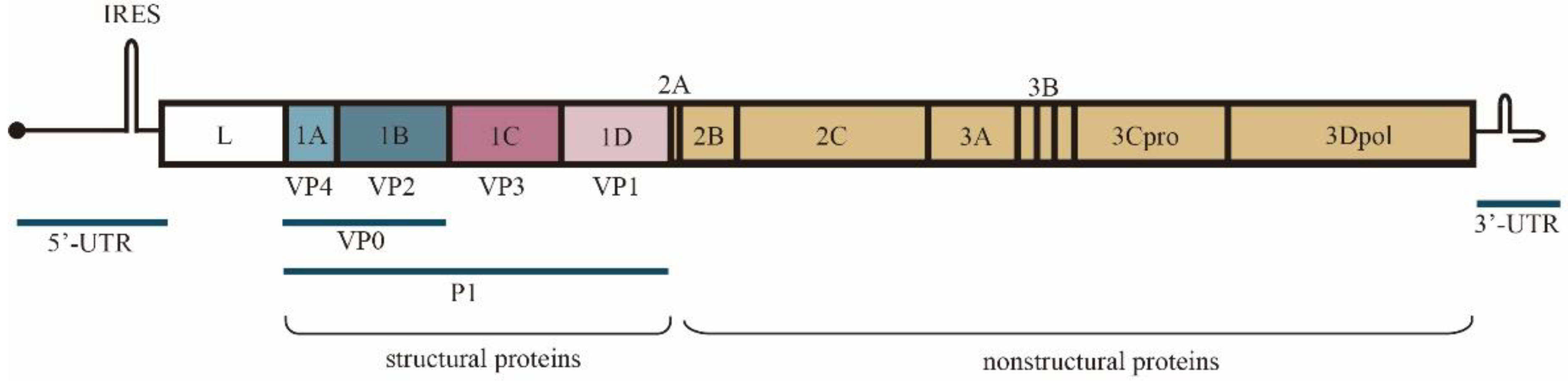
:1. Introduction
2. Inactivated Virus Vaccine
3. Virus-Like Particle Vaccine
3.1. Adenovirus Vector Vaccine
3.2. Phage Vaccine
3.3. Nucleic Acid Vaccine
3.4. E. coli Expression System
3.5. Mammalian Expression System
3.6. Chimeric Vaccine
4. Synthetic Peptide Vaccine
5. Adjuvant and Delivery System
| Type | Adjuvant or Delivery System | Mechanism | Applicable Vaccines |
|---|---|---|---|
| Saponin | The imine carbonyl group formed contributes to T-cell activation (inducing Th1/Th2 response) and permeabilizes cell membranes [78,79,80] | Adenovirus vector vaccine | |
| CAvant ® SOE (CA V AC, Daejeon, Korea) | Delivery of antigens to APCs or by direct stimulation of immune cells [112] | Inactivated viruses | |
| Agonists | Cationic liposomes and monophosphate liposome A | VLP is encapsulated in a cationic liposome and/or MPL based on DDA [86] | VLP vaccine |
| Agonists | Heparin-binding hemagglutinin (HBHA) | The multi-epitope immunogen HAO of serotype O and A FMDV was combined with HBHA, a novel TLR4 agonist [87] | VLP vaccine |
| Agonists | CVC1302 | Contains three PRR agonists that can increase B-cell numbers to increase antibody response [83,113] | Multi-epitope recombinant vaccine |
| Chinese herbal medicine | Panax ginseng stem and leaf saponins | The carbohydrate groups on the saponin molecule can interact with receptors on the APCs, and the acyl domain can facilitate the entry of antigens into the APCs [88] | Inactivated viruses |
| Chinese herbal medicine | Crude polysaccharides of Cistanche deserticola (CPCD) | DCs were activated by TLR-2 and TLR-4, and MAPKs and NF-κB pathway were induced [89] | Inactivated viruses |
| Chinese herbal medicine | Artemisia rupestris L., (AEAR) | Increase serum antibody titers, enhance cytokine secretion, and stimulate T-cell-mediated immune responses [90] | Inactivated viruses |
| Chinese herbal medicine | Achyranthes bidentata Polysaccharide (ABP) | The stable polysaccharide nanoemulsion delivery system can better deliver antigen and promote immune enhancement [91] | VLP vaccine |
| Noncoding synthetic RNAs | IRES, S and 3′NCR domains transcribed in vitro from plasmids induce a powerful antiviral response [92] | Inactivated viruses | |
| Nanoparticle polymers | Mesoporous silica | Unique center–radial hole structure for greater load capacity and control of FMDV release rate [97,98,99,103] | Inactivated viruses |
| Nanoparticle polymers | Chitosan (CP) | The flexible configuration and deformation of the vaccine particles can increase the contact area with cells [100,101,102] | VLP vaccine and inactivated vaccine |
| Nanoparticle polymers | Gold nanocages (AuNCs) | Proteins can bind to gold nanomaterials by electrostatic interaction, hydrophobicity and Au-S bond cooperation [104] | VLP vaccine |
| Nanoparticle polymers | Layered dihydroxide (LDH) | These particles, with interspace layers that can be loaded with antigens, provide improved and sustained delivery of antigen in vivo [107] | Inactivated viruses |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamal, S.M.; Belsham, G.J. Foot-and-mouth disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef] [Green Version]
- Alexandersen, S.; Zhang, Z.; Donaldson, A.; Garland, A. The Pathogenesis and Diagnosis of Foot-and-Mouth Disease. J. Comp. Pathol. 2003, 129, 1–36. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.; Rodriguez, L.L.; Arzt, J. Early events in the pathogenesis of foot-and-mouth disease in pigs; identification of oropharyngeal tonsils as sites of primary and sustained viral replication. PLoS ONE 2014, 9, e106859. [Google Scholar] [CrossRef]
- Arzt, J.; Pacheco, J.M.; Rodriguez, L.L. The early pathogenesis of foot-and-mouth disease in cattle after aerosol inoculation: Identification of the nasopharynx as the primary site of infection. Vet. Pathol. 2010, 47, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Arzt, J.; Baxt, B.; Grubman, M.J.; Jackson, T.; Juleff, N.; Rhyan, J.; Rieder, E.; Waters, R.; Rodriguez, L.L. The pathogenesis of foot-and-mouth disease II: Viral pathways in swine, small ruminants, and wildlife; myotropism, chronic syndromes, and molecular virus-host interactions. Transbound. Emerg. Dis. 2011, 58, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, S.; Quan, M.; Murphy, C.; Knight, J.; Zhang, Z. Studies of Quantitative Parameters of Virus Excretion and Transmission in Pigs and Cattle Experimentally Infected with Foot-and-Mouth Disease Virus. J. Comp. Pathol. 2003, 129, 268–282. [Google Scholar] [CrossRef]
- Donaldson, A.I.; Herniman, K.A.J.; Parker, J.; Sellers, R.F. Further investigations on the airborne excretion of foot-and-mouth disease virus. J. Hyg. 1970, 68, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenfeldt, C.; Arzt, J. The Carrier Conundrum; A Review of Recent Advances and Persistent Gaps Regarding the Carrier State of Foot-and-Mouth Disease Virus. Pathogens 2020, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Woodbury, E.L. A review of the possible mechanisms for the persistence of foot-and-mouth disease virus. Epidemiol. Infect. 1995, 114, 1–13. [Google Scholar] [CrossRef]
- Mason, P.W.; Grubman, M.J.; Baxt, B. Molecular basis of pathogenesis of FMDV. Virus Res. 2003, 91, 9–32. [Google Scholar] [CrossRef]
- Carrillo, C.; Tulman, E.R.; Delhon, G.; Lu, Z.; Carreno, A.; Vagnozzi, A.; Kutish, G.F.; Rock, D.L. Comparative genomics of foot-and-mouth disease virus. J. Virol. 2005, 79, 6487–6504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, A.R.; Knowles, N.J. Foot-and-mouth disease type O viruses exhibit genetically and geographically distinct evolutionary lineages. J. Gen. Virol. 2001, 82, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Valarcher, J.F.; Leforban, Y.; Rweyemamu, M.; Roeder, P.L.; Gerbier, G.; Mackay, D.K.J.; Sumption, K.J.; Paton, D.J.; Knowles, N.J. Incursions of foot-and-mouth disease virus into Europe between 1985 and 2006. Transbound. Emerg. Dis. 2008, 55, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Stenfeldt, C.; Diaz-San Segundo, F.; de Los Santos, T.; Rodriguez, L.L.; Earzt, J. The Pathogenesis of Foot-and-Mouth Disease in Pigs. Front. Vet. Sci. 2016, 3, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey-Bryars, M.; Reeve, R.; Bastola, U.; Knowles, N.J.; Auty, H.; Bachanek-Bankowska, K.; Fowler, V.L.; Fyumagwa, R.; Kazwala, R.; Kibona, T.; et al. Waves of endemic foot-and-mouth disease in eastern Africa suggest feasibility of proactive vaccination approaches. Nat. Ecol. Evol. 2018, 2, 1449–1457. [Google Scholar] [CrossRef] [Green Version]
- Leforban, Y.; Gerbier, G. Review of the status of foot and mouth disease and approach to control/eradication in Europe and Central Asia. Rev. Sci. Et Tech. De L’oie 2003, 21, 477–492. [Google Scholar] [CrossRef]
- Mansilla, F.C.; Turco, C.S.; Miraglia, M.C.; Bessone, F.A.; Franco, R.; Pérez-Filgueira, M.; Sala, J.M.; Capozzo, A.V. The role of viral particle integrity in the serological assessment of foot-and-mouth disease virus vaccine-induced immunity in swine. PLoS ONE 2020, 15, e0232782. [Google Scholar] [CrossRef]
- Nagendrakumar, S.B.; Srinivasan, V.A.; Madhanmohan, M.; Yuvaraj, S.; Parida, S.; Di Nardo, A.; Horsington, J.; Paton, D.J. Evaluation of cross-protection between O1 Manisa and O1 Campos in cattle vaccinated with foot-and-mouth disease virus vaccine incorporating different payloads of inactivated O1 Manisa antigen. Vaccine 2011, 29, 1906–1912. [Google Scholar] [CrossRef]
- Harmsen, M.M.; Seago, J.; Perez, E.; Charleston, B.; Eblé, P.L.; Dekker, A. Isolation of Single-Domain Antibody Fragments That Preferentially Detect Intact (146S) Particles of Foot-and-Mouth Disease Virus for Use in Vaccine Quality Control. Front. Immunol. 2017, 8, 960. [Google Scholar] [CrossRef] [Green Version]
- Doel, T.R.; Chong, W.K.T. Comparative Immunofenieity of 146S, 75S and 12S Particles of Foot-and~Mouth Disease Virus. Arch. Virol. 1982, 73, 185–191. [Google Scholar] [CrossRef]
- Maradei, E.; Malirat, V.; Beascoechea, C.P.; Espinoza, A.M.; Novo, S.G.; Smitsaart, E.; Salgado, G.; Mattion, N.; Toledo, J.R.; Bergmann, I.E. Emergence of antigenic variants of Foot-and-Mouth Disease Virus serotype O in Ecuador and preliminary evaluation of a field strain as a vaccine candidate. Vaccine 2014, 32, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; Di Nardo, A.; Knowles, N.J.; Wadsworth, J.; Pituco, E.M.; Cosivi, M.; Rivera, A.M.; Kassimi, L.B.; Brocchi, E.; de Clercq, K.; et al. The history of foot-and-mouth disease virus serotype C: The first known extinct serotype? Virus Evol. 2021, 7, veab009. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.P.; Rodriguez, L.L.; Hammond, J.M.; Pinto, J.; Perez, A.M. Review of the Global Distribution of Foot-and-Mouth Disease Virus from 2007 to 2014. Transbound. Emerg. Dis. 2017, 64, 316–332. [Google Scholar] [CrossRef]
- Dekker, A.; Sanz-Bernardo, B.; Singanallur, N.B.; Ludi, A.B.; Horsington, J.; Eblé, P.L.; King, D.P.; Vosloo, W. Cross-Protection Induced by a A/MAY/97 Emergency Vaccine Against Intra-Serotype Heterologous Challenge with a Foot-and-Mouth Disease Virus from the A/ASIA/G-VII Lineage. Vaccines 2020, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Hwang, J.-H.; Park, J.-H.; Lee, M.J.; Kim, B.; Kim, S.-M. Vaccine strain of O/ME-SA/Ind-2001e of foot-and-mouth disease virus provides high immunogenicity and broad antigenic coverage. Antivir. Res. 2020, 182, 104920. [Google Scholar] [CrossRef]
- Singanallur, N.B.; Dekker, A.; Eblé, P.L.; Van Hemert-Kluitenberg, F.; Weerdmeester, K.; Horsington, J.; Vosloo, W.W. Emergency Foot-and-Mouth Disease Vaccines A Malaysia 97 and A22 Iraq 64 Offer Good Protection against Heterologous Challenge with A Variant Serotype A ASIA/G-IX/SEA-97 Lineage Virus. Vaccines 2020, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Li, K.; Cao, Y.; Sun, Z.; Li, P.; Bao, H.; Wang, S.; Zhu, G.; Bai, X.; Sun, P.; et al. Structures of Foot-and-mouth Disease Virus with neutralizing antibodies derived from recovered natural host reveal a mechanism for cross-serotype neutralization. PLoS Pathog. 2021, 17, e1009507. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Ryu, K.-H.; Kim, A.-Y.; Kim, J.; Ko, Y.-J.; Lee, E. Production of a Foot-and-Mouth Disease Vaccine Antigen Using Suspension-Adapted BHK-21 Cells in a Bioreactor. Vaccines 2021, 9, 505. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Ren, S.; Meng, X.; Yin, X.; Zhang, J.; Tarasiuk, K.; Pejsak, Z.; Jiang, T.; Mao, R.; et al. Knockout of HDAC9 Gene Enhances Foot-and-Mouth Disease Virus Replication. Front. Microbiol. 2022, 13, 805606. [Google Scholar] [CrossRef]
- Harvey, Y.; Jackson, B.; Carr, B.V.; Childs, K.; Moffat, K.; Freimanis, G.; Tennakoon, C.; Juleff, N.; Seago, J. An Improved alphavbeta6-Receptor-Expressing Suspension Cell Line for Foot-and-Mouth Disease Vaccine Production. Viruses 2022, 14, 621. [Google Scholar] [CrossRef]
- Quattrocchi, V.; Bidart, J.; Mignaqui, A.C.; Ruiz, V.; Ferella, A.; Langellotti, C.; Gammella, M.; Ferraris, S.; Carrillo, J.; Wigdorovitz, A.; et al. Bovine Dendritic Cell Activation, T Cell Proliferation and Antibody Responses to Foot-And-Mouth Disease, Is Similar With Inactivated Virus and Virus Like Particles. Front. Vet. Sci. 2020, 7, 594. [Google Scholar] [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef] [PubMed]
- Grubman, M.J. Adenovirus serotype 5-vectored foot-and-mouth disease subunit vaccines: The first decade. Future Virol. 2010, 5, 51–64. [Google Scholar] [CrossRef]
- Grubman, M.J. Use of replication-defective adenoviruses to develop vaccines and biotherapeutics against foot-and-mouth disease. Future Virol. 2012, 7, 767–778. [Google Scholar] [CrossRef]
- Mayr, G.A. Development of Replication-Defective Adenovirus Serotype 5 Containing the Capsid and 3C Protease Coding Regions of Foot-and-Mouth Disease Virus as a Vaccine Candidate. Virology 1999, 263, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Barrera, J.; Brake, D.A.; Schutta, C.; Ettyreddy, D.; Kamicker, B.J.; Rasmussen, M.V.; de Rueda, C.B.; Zurita, M.; Pisano, M.; Hurtle, W.; et al. Versatility of the adenovirus-vectored foot-and-mouth disease vaccine platform across multiple foot-and-mouth disease virus serotypes and topotypes using a vaccine dose representative of the AdtA24 conditionally licensed vaccine. Vaccine 2018, 36, 7345–7352. [Google Scholar] [CrossRef]
- Moraes, M.P.; Mayr, G.; Mason, P.; Grubman, M. Early protection against homologous challenge after a single dose of replication-defective human adenovirus type 5 expressing capsid proteins of foot-and-mouth disease virus (FMDV) strain A24. Vaccine 2002, 20, 1631–1639. [Google Scholar] [CrossRef]
- Pena, L.; Moraes, M.P.; Koster, M.; Burrage, T.; Pacheco, J.M.; Segundo, F.D.-S.; Grubman, M.J. Delivery of a foot-and-mouth disease virus empty capsid subunit antigen with nonstructural protein 2B improves protection of swine. Vaccine 2008, 26, 5689–5699. [Google Scholar] [CrossRef]
- Fernandez-Sainz, I.; Medina, G.N.; Ramirez-Medina, E.; Koster, M.J.; Grubman, M.J.; de Los Santos, T. Adenovirus-vectored foot-and-mouth disease vaccine confers early and full protection against FMDV O1 Manisa in swine. Virology 2017, 502, 123–132. [Google Scholar] [CrossRef]
- Rweyemamu, M. Stability and Immnnogenicity of Empty Particles of Foot-and-Mouth Disease Virus. Arch. Virol. 1979, 59, 69–79. [Google Scholar] [CrossRef]
- Caron, L. Granulocyte-macrophage colony-stimulating factor does not increase the potency or efficacy of a foot-and-mouth disease virus subunit vaccine. Pesqui. Vet. Bras. 2005, 25, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Ziraldo, M.; Bidart, J.E.; Prato, C.A.; Tribulatti, M.V.; Zamorano, P.; Mattion, N.; D’Antuono, A.L. Optimized Adenoviral Vector That Enhances the Assembly of FMDV O1 Virus-Like Particles in situ Increases Its Potential as Vaccine for Serotype O Viruses. Front. Microbiol. 2020, 11, 591019. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Li, Y.; Yang, M.; Mao, C. T7 Phage as an Emerging Nanobiomaterial with Genetically Tunable Target Specificity. Adv. Sci. 2022, 9, e2103645. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Yong, C.Y.; Lee, K.W. Phage T7 as a Potential Platform for Vaccine Development. Methods Mol. Biol. 2022, 2412, 75–93. [Google Scholar]
- Wu, P.; Yang, N.; Wang, Y.; Xu, M.; Zhang, Y.; Chen, C. A meta-analysis and experiment assessing phage-based FMDV vaccine. biorXiv 2020. [Google Scholar] [CrossRef]
- Fowler, V.; Robinson, L.; Bankowski, B.; Cox, S.; Parida, S.; Lawlor, C.; Gibson, D.; O’Brien, F.; Ellefsen, B.; Hannaman, D.; et al. A DNA vaccination regime including protein boost and electroporation protects cattle against foot-and-mouth disease. Antivir. Res. 2012, 94, 25–34. [Google Scholar] [CrossRef]
- Dory, D.; Rémond, M.; Béven, V.; Cariolet, R.; Zientara, S.; Jestin, A. Foot-and-Mouth Disease Virus neutralizing antibodies production induced by pcDNA3 and Sindbis virus based plasmid encoding FMDV P1-2A3C3D in swine. Antivir. Res. 2009, 83, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Aggarwal, N.; Takamatsu, H.-H.; Sterling, C.M.; Voyce, C.; Barnett, P.V. Enhancing immune responses against a plasmid DNA vaccine encoding a FMDV empty capsid from serotype O. Vaccine 2006, 24, 4602–4606. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, S.; Yan, H.; Geng, X.; Wang, Y.; Xu, X.; Wang, M.; Zhang, H.; Huang, B.; Pang, W.; et al. The High Immunity Induced by the Virus-Like Particles of Foot-and-Mouth Disease Virus Serotype O. Front. Vet. Sci. 2021, 8, 633706. [Google Scholar] [CrossRef]
- Li, L.; Dong, H.; Lu, Y.; Wang, M.; Sun, S.; Guo, H. Effect of amino acid site modification on stability of foot-and-mouth disease virus-like particles. Sheng Wu Gong Cheng Xue Bao 2021, 37, 2435–2442. [Google Scholar]
- Zhi, Y.; Ji, H.; Guo, H.; Lim, J.; Byun, E.-B.; Kim, W.; Seo, H. Salmonella Vaccine Vector System for Foot-and-Mouth Disease Virus and Evaluation of Its Efficacy with Virus-Like Particles. Vaccines 2021, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, L.; Lv, J.; Zhang, Z.; Zhou, P.; Ma, Z.; Wang, Y.; Zhang, Y.; Pan, L. The immune response to a recombinant Lactococcus lactis oral vaccine against foot-and-mouth disease virus in mice. Biotechnol. Lett. 2020, 42, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Mignaqui, A.C.; Ferella, A.; Cass, B.; Mukankurayija, L.; L’Abbé, D.; Bisson, L.; Sánchez, C.; Scian, R.; Cardillo, S.B.; Durocher, Y.; et al. Foot-and-Mouth Disease: Optimization, Reproducibility, and Scalability of High-Yield Production of Virus-Like Particles for a Next-Generation Vaccine. Front. Vet. Sci. 2020, 7, 601. [Google Scholar] [CrossRef] [PubMed]
- Puckette, M.; Primavera, V.; Martel, E.; Barrera, J.; Hurtle, W.; Clark, B.; Kamicker, B.; Zurita, M.; Brake, D.; Neilan, J. Transiently Transfected Mammalian Cell Cultures: An Adaptable and Effective Platform for Virus-like Particle-Based Vaccines against Foot-and-Mouth Disease Virus. Viruses 2022, 14, 989. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.; Fragkoudis, R.; Hawes, P.; Newman, J.; Burman, A.; Panjwani, A.; Stonehouse, N.; Tuthill, T. Generation of Antibodies against Foot-and-Mouth-Disease Virus Capsid Protein VP4 Using Hepatitis B Core VLPs as a Scaffold. Life 2021, 11, 338. [Google Scholar] [CrossRef]
- Rangel, G.; Bárcena, J.; Moreno, N.; Mata, C.; Castón, J.; Alejo, A.; Blanco, E. Chimeric RHDV Virus-Like Particles Displaying Foot-and-Mouth Disease Virus Epitopes Elicit Neutralizing Antibodies and Confer Partial Protection in Pigs. Vaccines 2021, 9, 470. [Google Scholar] [CrossRef]
- Rangel, G.; Martín, V.; Bárcena, J.; Blanco, E.; Alejo, A. An Adenovirus Vector Expressing FMDV RNA Polymerase Combined with a Chimeric VLP Harboring a Neutralizing Epitope as a Prime Boost Strategy to Induce FMDV-Specific Humoral and Cellular Responses. Pharmaceuticals 2021, 14, 675. [Google Scholar] [CrossRef]
- Fontana, D.; Garay, E.; Cervera, L.; Kratje, R.; Prieto, C.; Gòdia, F. Chimeric VLPs Based on HIV-1 Gag and a Fusion Rabies Glycoprotein Induce Specific Antibodies against Rabies and Foot-and-Mouth Disease Virus. Vaccines 2021, 9, 251. [Google Scholar] [CrossRef]
- Crisci, E.; Almanza, H.; Mena, I.; Córdoba, L.; Gómez-Casado, E.; Castón, J.; Fraile, L.; Bárcena, J.; Montoya, M. Chimeric calicivirus-like particles elicit protective anti-viral cytotoxic responses without adjuvant. Virology 2009, 387, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Crisci, E.; Fraile, L.; Novellas, R.; Espada, Y.; Cabezón, R.; Martínez, J.; Cordoba, L.; Bárcena, J.; Benitez-Ribas, D.; Montoya, M. In vivo tracking and immunological properties of pulsed porcine monocyte-derived dendritic cells. Mol. Immunol. 2015, 63, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Peers-Adams, A.; Win, S.J.; Scullion, S.; Wilson, M.; Young, V.L.; Jennings, P.; Ward, V.; Baird, M.A.; Young, S.L. Antigen Incorporated In Virus-like Particles Is Delivered to Specific Dendritic Cell Subsets That Induce An Effective Antitumor Immune Response In Vivo. J. Immunother. 2013, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Avendano, C.; Giraldo, C.T.C.; Ordoñez, D.; Diaz-Arevalo, D.; Rodríguez-Habibe, I.; Oviedo, J.; Curtidor, H.; García-Castiblanco, S.; Martínez-Panqueva, F.; Camargo-Castañeda, A.; et al. Evaluating the immunogenicity of chemically-synthesised peptides derived from foot-and-mouth disease VP1, VP2 and VP3 proteins as vaccine candidates. Vaccine 2020, 38, 3942–3951. [Google Scholar] [CrossRef] [PubMed]
- Mansuroğlu, B.; Derman, S.; Kızılbey, K.; Ateş, S.C.; Akdeste, Z.M. Synthetic peptide vaccine for Foot-and-Mouth Disease: Synthesis, characterization and immunogenicity. Turk. J. Biochem. 2020, 45, 859–868. [Google Scholar] [CrossRef]
- van Lierop, M.-J.C.; Van Noort, J.M.; Wagenaar, J.P.A.; Rutten, V.P.M.G.; Langeveld, J.; Meloen, R.H.; Hensen, E.J. T cell-stimulatory fragments of foot-and-mouth disease virus released by mild treatment with cathepsin D. J. Gen. Virol. 1994, 75, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; McCullough, K.; Summerfield, A.; Fiorini, J.; Andreu, D.; Chiva, C.; Borràs, E.; Barnett, P.; Sobrino, F. Interspecies Major Histocompatibility Complex-Restricted Th Cell Epitope on Foot-and-Mouth Disease Virus Capsid Protein VP4. J. Virol. 2000, 74, 4902–4907. [Google Scholar] [CrossRef] [Green Version]
- Cubillos, C.; de la Torre, B.G.; Jakab, A.; Clementi, G.; Borrás, E.; Bárcena, J.; Andreu, D.; Sobrino, F.; Blanco, E. Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J. Virol. 2008, 82, 7223–7230. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Guerra, B.; de la Torre, B.G.; Defaus, S.; Dekker, A.; Andreu, D.; Sobrino, F. Full protection of swine against foot-and-mouth disease by a bivalent B-cell epitope dendrimer peptide. Antivir. Res. 2016, 129, 74–80. [Google Scholar] [CrossRef]
- Canas-Arranz, R.; Forner, M.; Defaus, S.; de León, P.; Bustos, M.J.; Torres, E.; Sobrino, F.; Andreu, D.; Blanco, E. A Single Dose of Dendrimer B2T Peptide Vaccine Partially Protects Pigs against Foot-and-Mouth Disease Virus Infection. Vaccines 2020, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Collen, T.; Baron, J.; Childerstone, A.; Corteyn, A.; Doel, T.; Flint, M.; Garcia-Valcarcel, M.; Parkhouse, R.; Ryan, M. Heterotypic recognition of recombinant FMDV proteins by bovine T-cells: The polymerase (P3Dpol) as an immunodominant T-cell immunogen. Virus Res. 1998, 56, 125–133. [Google Scholar] [CrossRef]
- Foster, M.; Cook, A.; Cedillo, L.; Parkhouse, R.M. Serological and cellular immune responses to non-structural proteins in animals infected with FMDV. Vet. Q. 1998, 20 (Suppl. S2), S28–S30. [Google Scholar] [CrossRef]
- Canas-Arranz, R.; De León, P.; Forner, M.; Defaus, S.; Bustos, M.J.; Torres, E.; Andreu, D.; Blanco, E.; Sobrino, F. Immunogenicity of a Dendrimer B2T Peptide Harboring a T-Cell Epitope From FMDV Non-structural Protein 3D. Front. Vet. Sci. 2020, 7, 498. [Google Scholar] [CrossRef]
- Defaus, S.; Forner, M.; Cañas-Arranz, R.; De León, P.; Bustos, M.J.; Rodríguez-Pulido, M.; Blanco, E.; Sobrino, F.; Andreu, D. Designing Functionally Versatile, Highly Immunogenic Peptide-Based Multiepitopic Vaccines against Foot-and-Mouth Disease Virus. Vaccines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- De León, P.; Cañas-Arranz, R.; Saez, Y.; Forner, M.; Defaus, S.; Cuadra, D.; Bustos, M.J.; Torres, E.; Andreu, D.; Blanco, E.; et al. Association of Porcine Swine Leukocyte Antigen (SLA) Haplotypes with B- and T-Cell Immune Response to Foot-and-Mouth Disease Virus (FMDV) Peptides. Vaccines 2020, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.E.; Ho, C.-S.; Ando, A.; Rogel-Gaillard, C.; Charles, M.; Tector, M.; Tector, A.J.; Lunney, J.K. Importance of the Major Histocompatibility Complex (Swine Leukocyte Antigen) in Swine Health and Biomedical Research. Annu. Rev. Anim. Biosci. 2020, 8, 171–198. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.S.; Lunney, J.K.; Lee, J.-H.; Franzo-Romain, M.H.; Martens, G.W.; Rowland, R.R.R.; Smith, D.M. Molecular characterization of swine leucocyte antigen class II genes in outbred pig populations. Anim. Genet. 2010, 41, 428–432. [Google Scholar] [CrossRef]
- Harmsen, M.M.; Fijten, H.; Westra, D.; Dekker, A. Stabilizing effects of excipients on dissociation of intact (146S) foot-and-mouth disease virions into 12S particles during storage as oil-emulsion vaccine. Vaccine 2015, 33, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Q.; Li, L.; Zeng, Q.; Li, H.; Gong, T.; Zhang, Z.; Sun, X. Turning the Old Adjuvant from Gel to Nanoparticles to Amplify CD8(+) T Cell Responses. Adv. Sci. 2018, 5, 1700426. [Google Scholar] [CrossRef]
- Marciani, D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [Google Scholar] [CrossRef]
- Rajput, Z.I.; Hu, S.-H.; Xiao, C.-W.; Arijo, A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Bazid, A.I.; El-Alfy, H.A.; El-Didamony, G.; Elfeil, W.K.; El-Sayed, M.M.; Fawzy, M. Adjuvant effect of saponin in an oil-based monovalent (serotype O) foot-and-mouth disease virus vaccine on the antibody response in guinea pigs and cattle. Arch. Virol. 2021, 166, 1977–1984. [Google Scholar] [CrossRef]
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barteling, S.J.; Vreeswijk, J. Developments in foot-and-mouth disease vaccines. Vaccine 1991, 9, 75–88. [Google Scholar] [CrossRef]
- Du, L.; Hou, L.; Yu, X.; Cheng, H.; Chen, J.; Zheng, Q.; Hou, J. Pattern-Recognition Receptor Agonist-Containing Immunopotentiator CVC1302 Boosts High-Affinity Long-Lasting Humoral Immunity. Front. Immunol. 2021, 12, 697292. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.V.; Lefevre, E.A.; Windsor, M.A.; Inghese, C.; Gubbins, S.; Prentice, H.; Juleff, N.D.; Charleston, B. CD4+ T-cell responses to foot-and-mouth disease virus in vaccinated cattle. J. Gen. Virol. 2013, 94 Pt. 1, 97–107. [Google Scholar] [CrossRef]
- Guzman, E.; Taylor, G.; Charleston, B.; Ellis, S.A. Induction of a cross-reactive CD8(+) T cell response following foot-and-mouth disease virus vaccination. J. Virol. 2010, 84, 12375–12384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.S.; Zhi, Y.; Guo, H.; Byun, E.-B.; Lim, J.H.; Seo, H.S. Promotion of Cellular and Humoral Immunity against Foot-and-Mouth Disease Virus by Immunization with Virus-Like Particles Encapsulated in Monophosphoryl Lipid A and Liposomes. Vaccines 2020, 8, 633. [Google Scholar] [CrossRef]
- Lei, Y.; Shao, J.; Ma, F.; Lei, C.; Chang, H.; Zhang, Y. Enhanced efficacy of a multi-epitope vaccine for type A and O footand-mouth disease virus by fusing multiple epitopes with Mycobacterium tuberculosis heparin-binding hemagglutinin (HBHA), a novel TLR4 agonist. Mol. Immunol. 2020, 121, 118–126. [Google Scholar] [CrossRef]
- Xu, H.; Niu, Y.; Hong, W.; Liu, W.; Zuo, X.; Bao, X.; Guo, C.; Lu, Y.; Deng, B. Development of a water-in-oil-in-water adjuvant for foot-and-mouth disease vaccine based on ginseng stem-leaf saponins as an immune booster. Comp. Immunol. Microbiol. Infect. Dis. 2020, 71, 101499. [Google Scholar] [CrossRef]
- Li, Q.; Ba, X.; Cao, H.; Weng, X.; Yang, Y.; Wang, B.; Zhang, A. Crude polysaccharides from Cistanche deserticola Y.C. Ma as an immunoregulator and an adjuvant for foot-and-mouth disease vaccine. J. Funct. Foods 2021, 87, 104800. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Li, J.; Wang, B.; Zhang, A. Enhancing immune responses to inactivated foot-and-mouth virus vaccine by a polysaccharide adjuvant of aqueous extracts from Artemisia rupestris L. J. Vet. Sci. 2021, 22, e30. [Google Scholar] [CrossRef]
- Yang, K.; Song, H.; Shi, X.; Ru, J.; Tan, S.; Teng, Z.; Dong, H.; Guo, H.; Wei, F.; Sun, S. Preparation of a polysaccharide adjuvant and its application in the production of a foot-and-mouth disease virus-like particles vaccine. Biochem. Eng. J. 2022, 184, 108479. [Google Scholar] [CrossRef]
- Rodriguez-Pulido, M.; Polo, M.; Borrego, B.; Sáiz, M. Use of Foot-and-Mouth Disease Virus Non-coding Synthetic RNAs as Vaccine Adjuvants. Methods Mol. Biol. 2022, 2465, 125–135. [Google Scholar] [PubMed]
- Cañas-Arranz, R.; Forner, M.; Defaus, S.; Rodríguez-Pulido, M.; de León, P.; Torres, E.; Bustos, M.J.; Borrego, B.; Sáiz, M.; Blanco, E.; et al. A bivalent B-cell epitope dendrimer peptide can confer long-lasting immunity in swine against foot-and-mouth disease. Transbound. Emerg. Dis. 2020, 67, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, F.; Ru, J.; Liu, L.; Wang, Z.; Wang, N.; Shu, X.; Wei, Z.; Guo, H. Evaluation of the Effect of Inactivated Transmissible Gastroenteritis Virus Vaccine with Nano Silicon on the Phenotype and Function of Porcine Dendritic Cells. Viruses 2021, 13, 2158. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, Y.; Wan, X.; Chen, Q.; Wang, H.; Li, J.; Chen, J.; Gao, R. Enhancement of Immune Response and Anti-Infection of Mice by Porcine Antimicrobial Peptides and Interleukin-4/6 Fusion Gene Encapsulated in Chitosan Nanoparticles. Vaccines 2020, 8, 552. [Google Scholar] [CrossRef]
- Ma, W.; Chen, M.; Kaushal, S.; McElroy, M.; Zhang, Y.; Ozkan, C.; Bouvet, M.; Minev, B.; Kruse, C.; Grotjahn, D.; et al. PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses. Int. J. Nanomed. 2012, 7, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- An, W.; Defaus, S.; Andreu, D.; Rivera-Gil, P. In Vivo Sustained Release of Peptide Vaccine Mediated by Dendritic Mesoporous Silica Nanocarriers. Front. Immunol. 2021, 12, 684612. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Lin, H.; Du, W.; Chen, H.; Shi, J. Ultrasmall mesoporous organosilica nanoparticles: Morphology modulations and redox-responsive biodegradability for tumor-specific drug delivery. Biomaterials 2018, 161, 292–305. [Google Scholar] [CrossRef]
- Yin, W.; Xuan, D.; Wang, H.; Zhou, M.; Deng, B.; Ma, F.; Lu, Y.; Zhang, J. Biodegradable Imiquimod-Loaded Mesoporous Organosilica as a Nanocarrier and Adjuvant for Enhanced and Prolonged Immunity against Foot-and-Mouth Disease Virus in Mice. ACS Appl. Bio Mater. 2022, 5, 3095–3106. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Lin, X.; Li, Z.; Ma, G.; Su, Z.; Zhang, S. A Novel Particulate Delivery System Based on Antigen-Zn(2+) Coordination Interactions Enhances Stability and Cellular Immune Response of Inactivated Foot and Mouth Disease Virus. Mol. Pharm. 2020, 17, 2952–2963. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, N.; Miao, C.; Yue, H.; Wu, J.; Ma, G. A novel multiple emulsion enhanced immunity via its biomimetic delivery approach. J. Mater. Chem. B 2020, 8, 7365–7374. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Teng, Z.; Lu, Y.; Luo, X.; Mu, S.; Ru, J.; Zhao, X.; Guo, H.; Ran, X.; Wen, X.; et al. Enhanced immunogenicity of foot and mouth disease DNA vaccine delivered by PLGA nanoparticles combined with cytokine adjuvants. Res. Vet. Sci. 2021, 136, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Pan, L.; Lv, J.; Zhang, Z.; Wang, Y.; Hu, W.; Liu, X.; Zhou, P.; Wang, Y.; Zhang, Y. Comparison of immune responses in guinea pigs by intranasal delivery with different nanoparticles-loaded FMDV DNA vaccine. Microb. Pathog. 2020, 142, 104061. [Google Scholar] [CrossRef]
- Teng, Z.; Sun, S.; Luo, X.; Zhang, Z.; Seo, H.; Xu, X.; Huang, J.; Dong, H.; Mu, S.; Du, P.; et al. Bi-functional gold nanocages enhance specific immunological responses of foot-and-mouth disease virus-like particles vaccine as a carrier and adjuvant. Nanomedicine 2021, 33, 102358. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Chen, W.; Zuo, H.; Li, B.; Duan, C.; Rolfe, B.; Zhang, B.; Mahony, T.J.; Xu, Z.P. Clay Nanoparticles Elicit Long-Term Immune Responses by Forming Biodegradable Depots for Sustained Antigen Stimulation. Small 2018, 14, e1704465. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, Y.; Yin, X.; He, Y.; Zhang, Q.; Chen, C. Layered double hydroxide nanoparticles as an adjuvant for inactivated foot-and-mouth disease vaccine in pigs. BMC Vet. Res. 2020, 16, 474. [Google Scholar] [CrossRef]
- Bidart, J.; Mignaqui, A.; Kornuta, C.; Lupi, G.; Gammella, M.; Soria, I.; Galarza, R.; Ferella, A.; Cardillo, S.; Langellotti, C.; et al. FMD empty capsids combined with the Immunostant Particle Adjuvant -ISPA or ISA206 induce protective immunity against foot and mouth disease virus. Virus Res. 2021, 297, 198339. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Chen, H.; Li, X.; Qian, P. A ferritin nanoparticle vaccine for foot-and-mouth disease virus elicited partial protection in mice. Vaccine 2020, 38, 5647–5652. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Lin, X.; Li, Z.; Ma, G.; Su, Z.; Zhang, S. Biocompatible cationic solid lipid nanoparticles as adjuvants effectively improve humoral and T cell immune response of foot and mouth disease vaccines. Vaccine 2020, 38, 2478–2486. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Teng, Z.; Ren, M.; Dong, H.; Zhang, Y.; Ru, J.; Du, P.; Sun, S.; Guo, H. Four Simple Biomimetic Mineralization Methods to Improve the Thermostability and Immunogenicity of Virus-like Particles as a Vaccine against Foot-and-Mouth Disease. Vaccines 2021, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.H.; Chathuranga, W.A.G.; Shim, Y.J.; Haluwana, D.K.; Kim, E.H.; Yoon, I.J.; Lim, Y.T.; Shin, S.H.; Jo, H.; Hwang, S.Y.; et al. The Potential Adjuvanticity of CAvant((R))SOE for Foot-and-Mouth Disease Vaccine. Vaccines 2021, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, J.; Hou, L.; Yu, X.; Zheng, Q.; Hou, J. Long-term humoral immunity induced by CVC1302-adjuvanted serotype O foot-and-mouth disease inactivated vaccine correlates with promoted T follicular helper cells and thus germinal center responses in mice. Vaccine 2017, 35, 7088–7094. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Fuchs, K. A Decision-support System for Real-time Risk Assessment of Airborne Spread of the Foot-and-Mouth Disease Virus. Methods Inf. Med. 2005, 44, 590–595. [Google Scholar] [PubMed]

| Category | Locus | Allele | Protein |
|---|---|---|---|
| SLA class I (classical) | SLA-1 | 90 | 88 |
| SLA-2 | 97 | 94 | |
| SLA-3 | 41 | 39 | |
| SLA class I (nonclassical) | SLA-6 | 10 | 10 |
| SLA-7 | 3 | 3 | |
| SLA-8 | 5 | 5 | |
| SLA class I (unclassified) | SLA-12 | 6 | 6 |
| SLA class I (pseudogene) | SLA-4 | 3 | 0 |
| SLA-5 | 4 | 0 | |
| SLA-9 | 5 | 0 | |
| SLA-11 | 2 | 0 | |
| Total class I alleles | 266 | 245 | |
| SLA class II | DRA | 14 | 6 |
| DRB1 | 99 | 92 | |
| DQA | 26 | 24 | |
| DQB1 | 53 | 48 | |
| DMA | 7 | 5 | |
| DMB | 1 | 1 | |
| DOA | 2 | 2 | |
| DOB1 | 3 | 3 | |
| SLA class II (pseudogene) | DRB2 | 12 | 0 |
| DRB3 | 5 | 0 | |
| DRB4 | 1 | 0 | |
| DRB5 | 1 | 0 | |
| DQB2 | 1 | 0 | |
| DQB2 | 1 | 0 | |
| DYB | 1 | 0 | |
| Total class II alleles | 227 | 181 | |
| Other non-SLA genes | MIC-1 | 1 | 0 |
| MIC-2 | 1 | 1 | |
| TAP1 | 1 | 1 | |
| TAP2 | 1 | 1 | |
| Total SLA-related alleles | 4 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Yu, S.; Wang, W.; Chen, W.; Wang, X.; Wu, K.; Li, X.; Fan, S.; Ding, H.; Yi, L.; et al. Development of Foot-and-Mouth Disease Vaccines in Recent Years. Vaccines 2022, 10, 1817. https://doi.org/10.3390/vaccines10111817
Lu Z, Yu S, Wang W, Chen W, Wang X, Wu K, Li X, Fan S, Ding H, Yi L, et al. Development of Foot-and-Mouth Disease Vaccines in Recent Years. Vaccines. 2022; 10(11):1817. https://doi.org/10.3390/vaccines10111817
Chicago/Turabian StyleLu, Zhimin, Shu Yu, Weijun Wang, Wenxian Chen, Xinyan Wang, Keke Wu, Xiaowen Li, Shuangqi Fan, Hongxing Ding, Lin Yi, and et al. 2022. "Development of Foot-and-Mouth Disease Vaccines in Recent Years" Vaccines 10, no. 11: 1817. https://doi.org/10.3390/vaccines10111817
APA StyleLu, Z., Yu, S., Wang, W., Chen, W., Wang, X., Wu, K., Li, X., Fan, S., Ding, H., Yi, L., & Chen, J. (2022). Development of Foot-and-Mouth Disease Vaccines in Recent Years. Vaccines, 10(11), 1817. https://doi.org/10.3390/vaccines10111817




