Immunoprotection Efficacy of Con A-Purified Proteins against Haemonchus contortus in Goats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Parasites
2.3. Antigen Preparation
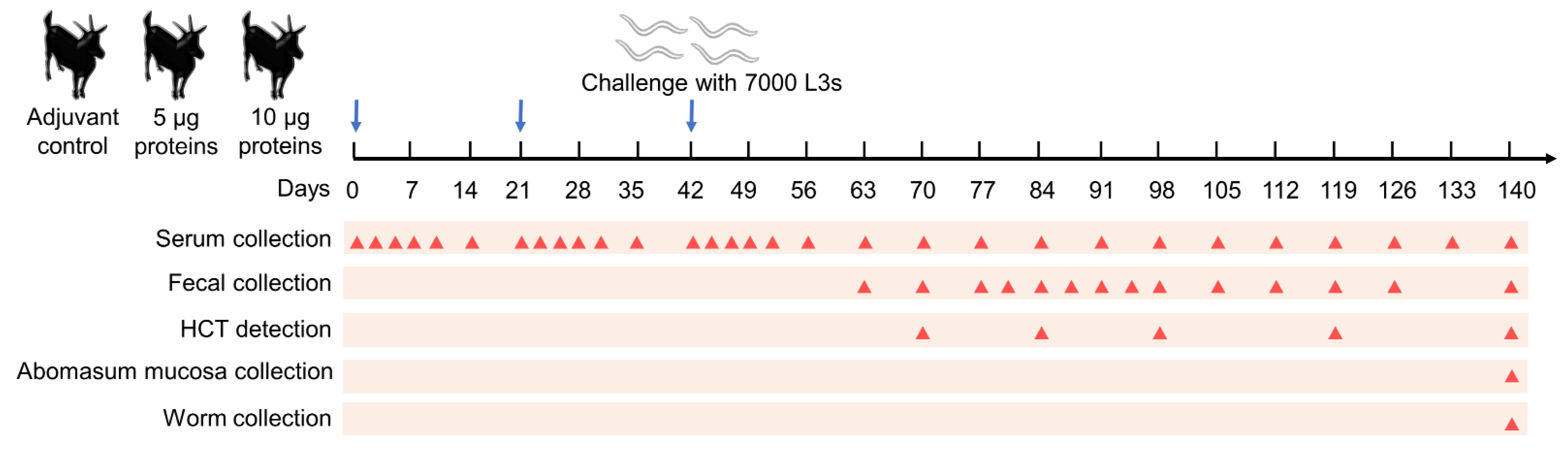
2.4. Vaccination Trials
2.5. Parasitology
2.6. Antibody Levels
2.7. Hematocrit (HCT)
2.8. Statistical Analysis
3. Results
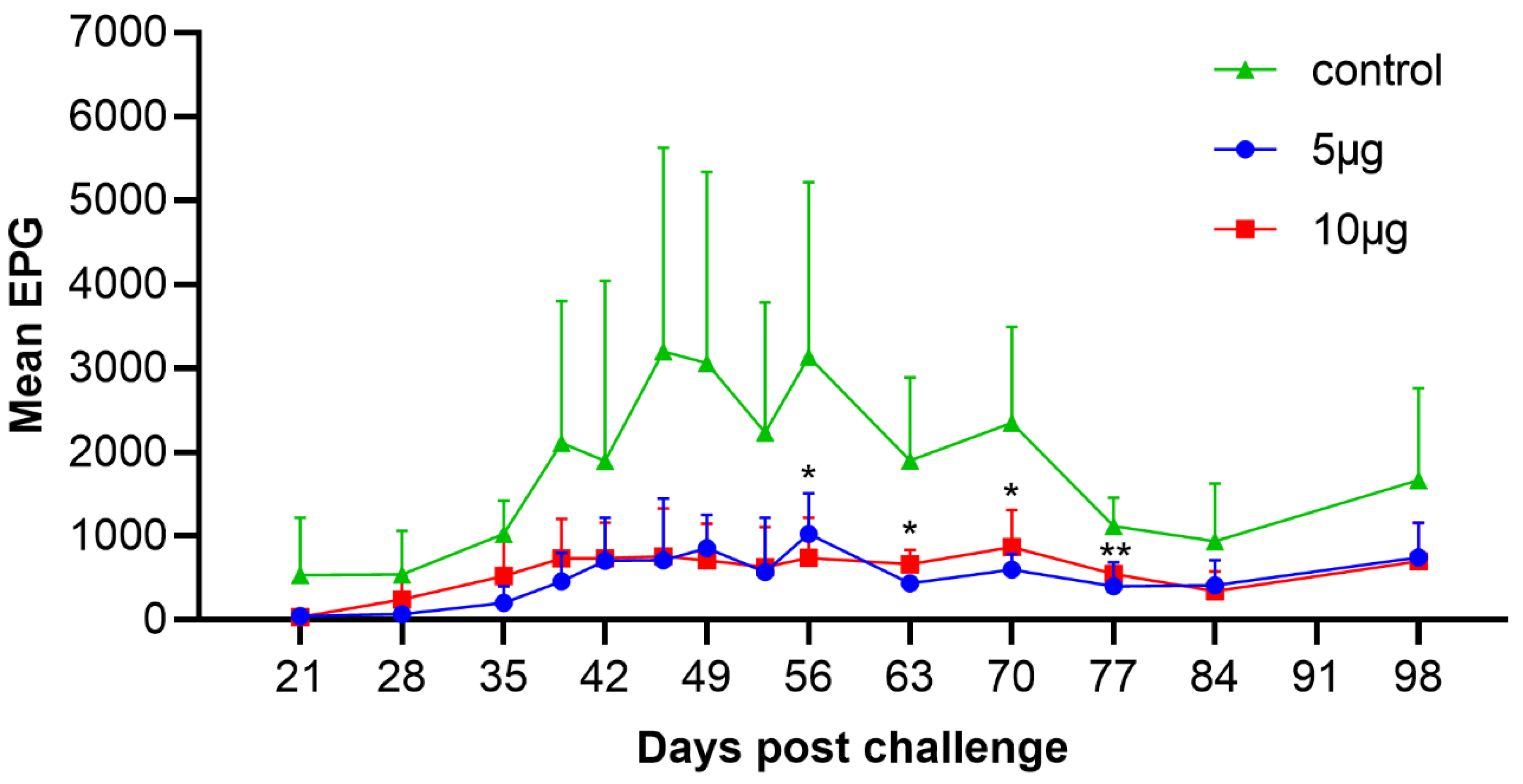
3.1. Fecal Egg Count (FEC)
3.2. Worm Burdens
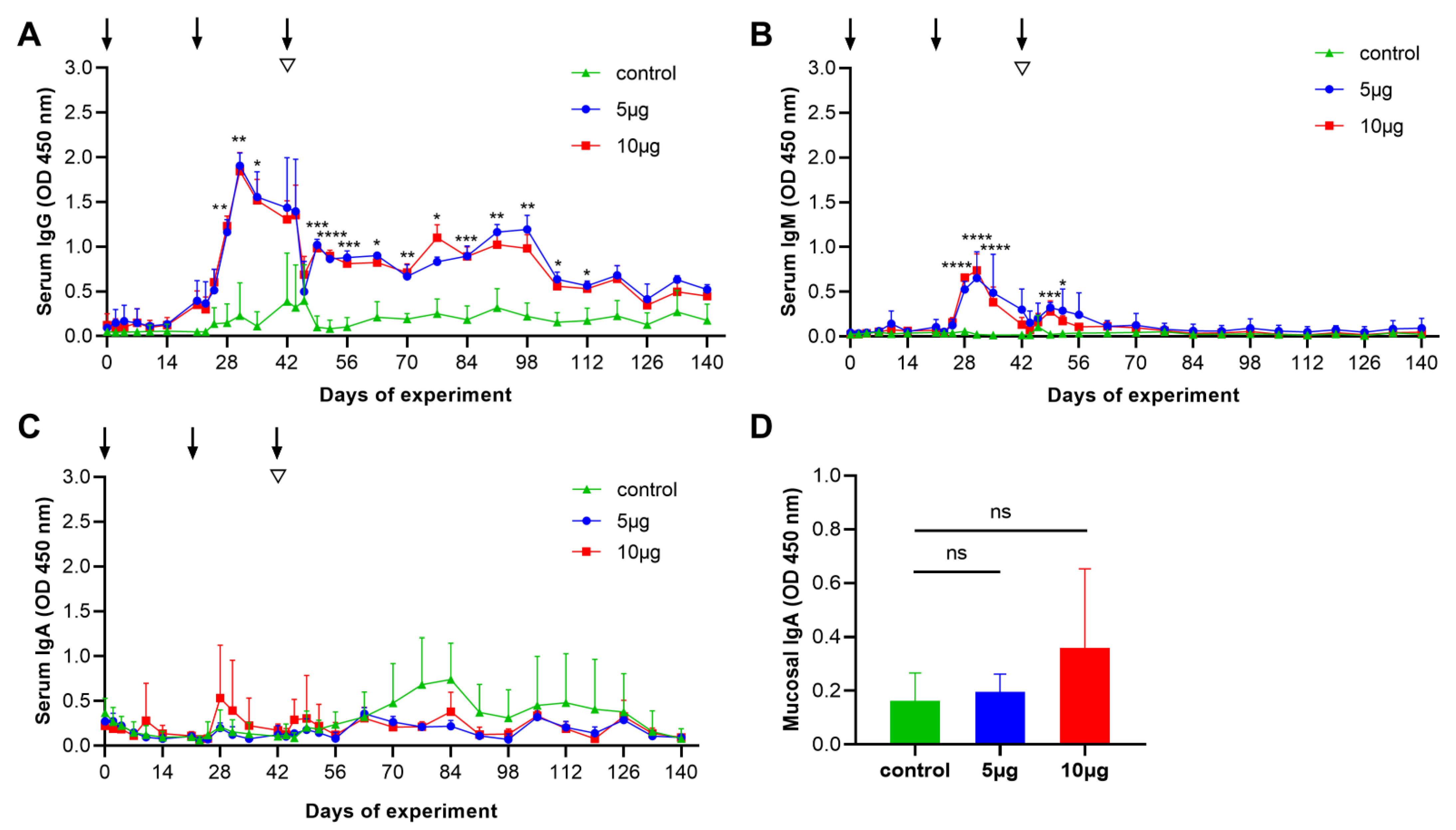
3.3. Antibody Responses to Con A-Purified Proteins
3.4. Degree of Anemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Li, F.; Zhang, Z.; Yang, X.; Ahmad, A.A.; Li, X.; Du, A.; Hu, M. Recent research progress in China on Haemonchus contortus. Front. Microbiol. 2017, 8, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, V.H.; Martínez, G.M.; Micheloud, J.F.; Viñabal, A.E. Epidemiology and effect of gastrointestinal nematodes on beef cattle from tropical Argentina. Trop. Anim. Health Prod. 2018, 50, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, Z.; Getnet, K.; Chanie, M.; Derso, S.; Fentahun, S. Morbidity parameters associated with gastrointestinal tract nematodes in sheep in Dabat District, Northwest Ethiopia. Biomed. Res. Int. 2018, 2018, 9247439. [Google Scholar] [CrossRef] [Green Version]
- Olivas-Salazar, R.; Estrada-Angulo, A.; Mellado, M.; Aguilar-Caballero, A.J.; Castro-Pérez, B.I.; Gutiérrez-Blanco, E.; Ruiz-Zárate, F. Prevalence of gastrointestinal nematode infections in goat flocks on semi-arid rangelands of northeastern Mexico. Trop. Anim. Health Prod. 2018, 50, 807–813. [Google Scholar] [CrossRef]
- Naeem, M.; Iqbal, Z.; Roohi, N. Ovine haemonchosis: A review. Trop. Anim. Health Prod. 2020, 53, 19. [Google Scholar] [CrossRef]
- Mickiewicz, M.; Czopowicz, M.; Kawecka-Grochocka, E.; Moroz, A.; Szaluś-Jordanow, O.; Várady, M.; Königová, A.; Spinu, M.; Górski, P.; Bagnicka, E.; et al. The first report of multidrug resistance in gastrointestinal nematodes in goat population in Poland. BMC Vet. Res. 2020, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Kotze, A.C.; Prichard, R.K. Anthelmintic resistance in Haemonchus contortus: History, mechanisms and diagnosis. Adv. Parasitol. 2016, 93, 397–428. [Google Scholar]
- Jabbar, A.; Iqbal, M.Z.; Ashraf, M.; Durrani, A.Z.; Sajjad, H.; Wana, M.N.; Ullah, A.; Imran, M.; Ghauri, M.S.Z.; Ahmad, H.I. Effects of ferula asafetida, closantel, albendazole, oxfendazole, and ivermectin against Haemonchus contortus in goats and sheep. Trop. Anim. Health Prod. 2022, 54, 107. [Google Scholar] [CrossRef]
- Ehsan, M.; Hu, R.S.; Liang, Q.L.; Hou, J.L.; Song, X.; Yan, R.; Zhu, X.Q.; Li, X. Advances in the development of anti-Haemonchus contortus vaccines: Challenges, opportunities, and perspectives. Vaccines 2020, 8, 555. [Google Scholar] [CrossRef]
- Britton, C.; Emery, D.L.; McNeilly, T.N.; Nisbet, A.J.; Stear, M.J. The potential for vaccines against scour worms of small ruminants. Int. J. Parasitol. 2020, 50, 533–553. [Google Scholar] [CrossRef]
- Reszka, N.; Rijsewijk, F.A.; Zelnik, V.; Moskwa, B.; Bieńkowska-Szewczyk, K. Haemonchus contortus: Characterization of the baculovirus expressed form of aminopeptidase H11. Exp. Parasitol. 2007, 117, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.B.; Geldhof, P.; Tzelos, T.; Claerebout, E. Progress in the development of subunit vaccines for gastrointestinal nematodes of ruminants. Parasite Immunol. 2016, 38, 744–753. [Google Scholar] [CrossRef] [PubMed]
- LeJambre, L.F.; Windon, R.G.; Smith, W.D. Vaccination against Haemonchus contortus: Performance of native parasite gut membrane glycoproteins in Merino lambs grazing contaminated pasture. Vet. Parasitol. 2008, 153, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.D.; Smith, S.K.; Murray, J.M. Protection studies with integral membrane fractions of Haemonchus contortus. Parasite Immunol. 1994, 16, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Scarff, C.A.; Thompson, R.F.; Newlands, G.F.J.; Jamson, A.H.; Kennaway, C.; da Silva, V.J.; Rabelo, E.M.; Song, C.F.; Trinick, J.; Smith, W.D.; et al. Structure of the protective nematode protease complex H-gal-GP and its conservation across roundworm parasites. PLoS Pathog. 2020, 16, e1008465. [Google Scholar] [CrossRef] [Green Version]
- Nisbet, A.J.; Meeusen, E.N.; González, J.F.; Piedrafita, D.M. Immunity to Haemonchus contortus and vaccine development. Adv. Parasitol. 2016, 93, 353–396. [Google Scholar]
- Munn, E.A.; Smith, T.S.; Graham, M.; Tavernor, A.S.; Greenwood, C.A. The potential value of integral membrane proteins in the vaccination of lambs against Haemonchus contortus. Int. J. Parasitol. 1993, 23, 261–269. [Google Scholar] [CrossRef]
- Newton, S.E.; Meeusen, E.N. Progress and new technologies for developing vaccines against gastrointestinal nematode parasites of sheep. Parasite Immunol. 2003, 25, 283–296. [Google Scholar] [CrossRef]
- Knox, D.P.; Redmond, D.L.; Newlands, G.F.; Skuce, P.J.; Pettit, D.; Smith, W.D. The nature and prospects for gut membrane proteins as vaccine candidates for Haemonchus contortus and other ruminant trichostrongyloids. Int. J. Parasitol. 2003, 33, 1129–1137. [Google Scholar] [CrossRef]
- Tavernor, A.S.; Smith, T.S.; Langford, C.F.; Graham, M.; Munn, E.A. Immune response of Clun Forest sheep to vaccination with membrane glycoproteins from Haemonchus contortus. Parasite Immunol. 1992, 14, 671–675. [Google Scholar] [CrossRef]
- Smith, T.S.; Munn, E.A.; Graham, M.; Tavernor, A.S.; Greenwood, C.A. Purification and evaluation of the integral membrane protein H11 as a protective antigen against Haemonchus contortus. Int. J. Parasitol. 1993, 23, 271–280. [Google Scholar] [CrossRef]
- Dicker, A.J.; Inglis, N.F.; Manson, E.D.; Subhadra, S.; Illangopathy, M.; Muthusamy, R.; Knox, D.P. Proteomic analysis of Mecistocirrus digitatus and Haemonchus contortus intestinal protein extracts and subsequent efficacy testing in a vaccine trial. PLoS Negl. Trop. Dis. 2014, 8, e2909. [Google Scholar] [CrossRef] [PubMed]
- Mines, J.J. Modifications of the McMaster worm egg counting method. Aust. Vet. J. 1977, 53, 342–343. [Google Scholar] [CrossRef]
- Kalyanasundaram, A.; Jawahar, S.; Ilangopathy, M.; Palavesam, A.; Raman, M. Comparative immunoprophylactic efficacy of Haemonchus contortus recombinant enolase (rHcENO) and Con A purified native glycoproteins in sheep. Exp. Parasitol. 2015, 154, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Munn, E.A.; Smith, T.S.; Graham, M.; Greenwood, C.A.; Tavernor, A.S.; Coetzee, G. Vaccination of merino lambs against haemonchosis with membrane-associated proteins from the adult parasite. Parasitology 1993, 106, 63–66. [Google Scholar] [CrossRef]
- de Matos, A.F.I.M.; Nobre, C.O.R.; Monteiro, J.P.; Bevilaqua, C.M.L.; Smith, W.D.; Teixeira, M. Attempt to control Haemonchus contortus in dairy goats with Barbervax®, a vaccine derived from the nematode gut membrane glycoproteins. Small Ruminant Res. 2017, 151, 1–4. [Google Scholar] [CrossRef]
- Nobre, C.O.R.; de Matos, A.F.; Monteiro, J.P.; de Souza, V.; Smith, W.D.; Teixeira, M. Benefits of vaccinating goats against Haemonchus contortus during gestation and lactation. Small Rumin. Res. 2020, 182, 46–51. [Google Scholar] [CrossRef]
- Meier, L.; Torgerson, P.R.; Hertzberg, H. Vaccination of goats against Haemonchus contortus with the gut membrane proteins H11/H-gal-GP. Vet. Parasitol. 2016, 229, 15–21. [Google Scholar] [CrossRef]
- Broomfield, M.A.; Doyle, E.K.; Kahn, L.P.; Smith, W.D.; Walkden-Brown, S.W. A simplified Barbervax® vaccination regimen in lambs to evoke immunological protection to Haemonchus contortus. Vet. Parasitol. 2020, 287, 109243. [Google Scholar] [CrossRef]
- Benavides, M.V.; Souza, C.J.H.; Smith, W.D.; Moraes, J.C.F. Evaluation of protection in grazing lambs immunised with different doses of Haemonchus contortus gut membrane glycoproteins in Southern Brazil. Vet. Parasitol. 2021, 290, 109360. [Google Scholar] [CrossRef]
- Bassetto, C.C.; Almeida, F.A.; Newlands, G.F.J.; Smith, W.D.; Castilhos, A.M.; Fernandes, S.; Siqueira, E.R.; Amarante, A.F.T. Trials with the Haemonchus vaccine, Barbervax®, in ewes and lambs in a tropical environment: Nutrient supplementation improves protection in periparturient ewes. Vet. Parasitol. 2018, 264, 52–57. [Google Scholar] [CrossRef]
- Bassetto, C.C.; Almeida, F.A.; Newlands, G.F.J.; Smith, W.D.; Amarante, A.F.T. Repeated vaccination against Haemonchus contortus provides continuous protection to young grazing sheep. Vet. Parasitol. 2020, 287, 109273. [Google Scholar] [CrossRef] [PubMed]
- Munn, E.A.; Smith, T.S.; Smith, H.; James, F.M.; Smith, F.C.; Andrews, S.J. Vaccination against Haemonchus contortus with denatured forms of the protective antigen H11. Parasite Immunol. 1997, 19, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Picece, V.; Ou, B.S.; Luo, W.; Pulendran, B.; Appel, E.A. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 2022, 7, 174–195. [Google Scholar] [CrossRef]
- Jewell, C.M.; López, S.C.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Liu, Z.; Xu, W.; Li, Q.; Han, T.; Pan, D.; Yue, N.; Wu, M.; Liu, Q.; Yuan, W.; et al. Versatile functionalization of ferritin nanoparticles by intein-mediated trans-splicing for antigen/adjuvant co-delivery. Nano Lett. 2019, 19, 5469–5475. [Google Scholar] [CrossRef]
- Sanders, M.T.; Brown, L.E.; Deliyannis, G.; Pearse, M.J. ISCOM-based vaccines: The second decade. Immunol. Cell Biol. 2005, 83, 119–128. [Google Scholar] [CrossRef]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Curley, S.M.; Putnam, D. Biological nanoparticles in vaccine development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Roth, G.A.; Saouaf, O.M.; Smith, A.A.A.; Gale, E.C.; Hernández, M.A.; Idoyaga, J.; Appel, E.A. Prolonged codelivery of hemagglutinin and a TLR7/8 agonist in a supramolecular polymer-nanoparticle hydrogel enhances potency and breadth of influenza vaccination. ACS Biomater. Sci. Eng. 2021, 7, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Lebre, F.; Hearnden, C.H.; Lavelle, E.C. Modulation of immune responses by particulate materials. Adv. Mater. 2016, 28, 5525–5541. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.; Antonopoulos, A.; Haslam, S.M.; Dicker, A.J.; McNeilly, T.N.; Johnston, S.L.; Dell, A.; Knox, D.P.; Britton, C. Novel expression of Haemonchus contortus vaccine candidate aminopeptidase H11 using the free-living nematode Caenorhabditis elegans. Vet. Res. 2013, 44, 111. [Google Scholar] [CrossRef] [PubMed]
| Groups | Mean FEC | SD | Reduction (%) a |
|---|---|---|---|
| Control 5 μg | 25,587.3 7211.7 | 14,637.8 3875.8 | – 71.8 * |
| 10 μg | 8039.8 | 4189.2 | 68.6 * |
| Groups | Female | Male | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Reduction (%) a | Mean | SD | Reduction (%) a | Mean | SD | Reduction (%) a | |
| Control 5 μg | 133.3 43.3 | 70.1 18.9 | – 67.5 * | 99.2 27 | 40.6 17.1 | – 72.8 * | 232.5 70.3 | 109.4 35 | – 69.8 * |
| 10 μg | 47 | 15.6 | 64.7 * | 42.2 | 25.8 | 57.5 * | 89.2 | 41.1 | 61.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Zhang, Y.; Wu, S.; Wang, Z.; Liu, F.; Wang, C.; Hu, M. Immunoprotection Efficacy of Con A-Purified Proteins against Haemonchus contortus in Goats. Vaccines 2022, 10, 1891. https://doi.org/10.3390/vaccines10111891
Ye L, Zhang Y, Wu S, Wang Z, Liu F, Wang C, Hu M. Immunoprotection Efficacy of Con A-Purified Proteins against Haemonchus contortus in Goats. Vaccines. 2022; 10(11):1891. https://doi.org/10.3390/vaccines10111891
Chicago/Turabian StyleYe, Lisha, Yao Zhang, Simin Wu, Zhiheng Wang, Feng Liu, Chunqun Wang, and Min Hu. 2022. "Immunoprotection Efficacy of Con A-Purified Proteins against Haemonchus contortus in Goats" Vaccines 10, no. 11: 1891. https://doi.org/10.3390/vaccines10111891
APA StyleYe, L., Zhang, Y., Wu, S., Wang, Z., Liu, F., Wang, C., & Hu, M. (2022). Immunoprotection Efficacy of Con A-Purified Proteins against Haemonchus contortus in Goats. Vaccines, 10(11), 1891. https://doi.org/10.3390/vaccines10111891





