The Influence of Introducing Free Vaccination against Streptococcus pneumoniae on the Uptake of Recommended Vaccination in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Obtaining Data
2.3. Study Group Settings
2.4. Used Assumptions
2.5. Statistical Methods
3. Results
3.1. Characteristics of the Study Group
3.2. Differences in Recommended Vaccination before and after Introducing Compulsory Pneumococcal Vaccination
3.3. Differences in Recommended Vaccination Depenging on Vaccination with Chargeable PCV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legal Act—Journal of Laws of the Republic of Poland No. 234, Item 1570, 2008. Polish Sejm Act of 5 December 2008 on Preventing and Combating Infections and Infectious Diseases in Humans. 2008. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20082341570 (accessed on 13 November 2023).
- Legal Act—Journal of Laws of the Republic of Poland No. 182, I. 1068, 2011. Minister of Health Regulation of the Minister of Health of 29 September 2023 on Mandatory Preventive Vaccinations. 2023. Available online: https://dziennikmz.mz.gov.pl/legalact/2023/88/ (accessed on 13 November 2023).
- Legal Act—Official Journal of the Ministry of Health No. 35, 2016 Chief Sanitary Inspector Announcement of October 16, 2015 on the Preventive Vaccination Program for 2016. 2015. Available online: https://dziennikmz.mz.gov.pl/legalact/2015/63/ (accessed on 13 November 2023).
- Legal Act—Official Journal of the Ministry of Health No. 133, 2022 Chief Sanitary Inspector Announcement of October 28, 2022 on the Preventive Vaccination Program for 2023. 2022. Available online: https://dziennikmz.mz.gov.pl/legalact/2022/113/ (accessed on 13 November 2023).
- Ophinni, Y.; Frediansyah, A.; Sirinam, S.; Megawati, D.; Stoian, A.M.; Enitan, S.S.; Akele, R.Y.; Sah, R.; Pongpirul, K.; Abdeen, Z.; et al. Monkeypox: Immune Response, Vaccination and Preventive Efforts. Narra J 2022, 2. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Ahmed, M.; Hussain, S. The Anti-Vaccination Movement: A Regression in Modern Medicine. Cureus 2018, 10, e2919. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, G.; Costantino, C.; Napoli, G.; Marchese, V.; Cracchiolo, M.; Casuccio, A.; Vitale, F.; on behalf of the ESCULAPIO working group; Amicizia, D.; Bechini, A.; et al. Determinants of European Parents’ Decision on the Vaccination of Their Children against Measles, Mumps and Rubella: A Systematic Review and Meta-Analysis. Hum. Vaccin. Immunother. 2016, 12, 1909. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, S.C.; Eaton, L.A. The Emergence and Persistence of the Anti-Vaccination Movement. Health Psychol. 2023, 42, 516–520. [Google Scholar] [CrossRef] [PubMed]
- McNeil, D.A.; Mueller, M.; MacDonald, S.; McDonald, S.; Saini, V.; Kellner, J.D.; Tough, S. Maternal Perceptions of Childhood Vaccination: Explanations of Reasons for and against Vaccination. BMC Public Health 2019, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Malchrzak, W.; Babicki, M.; Pokorna-Kałwak, D.; Doniec, Z.; Mastalerz-Migas, A. COVID-19 Vaccination and Ukrainian Refugees in Poland during Russian–Ukrainian War—Narrative Review. Vaccines 2022, 10, 955. [Google Scholar] [CrossRef]
- Miśkiewicz, K.; Kuchar, E.; Nitsch-Osuch, A.; Preisner, K.; Szenborn, L. Immunizations against Respiratory Infections in Children in Primary Health Care in Poland: Coverage and Delays. Adv. Exp. Med. Biol. 2015, 836, 9–17. [Google Scholar] [CrossRef]
- Isaacman, D.J.; Strutton, D.R.; Kalpas, E.A.; Horowicz-Mehler, N.; Stern, L.S.; Casciano, R.; Ciuryla, V. The Impact of Indirect (Herd) Protection on the Cost-Effectiveness of Pneumococcal Conjugate Vaccine. Clin. Ther. 2008, 30, 341–357. [Google Scholar] [CrossRef]
- Saokaew, S.; Rayanakorn, A.; Wu, D.B.C.; Chaiyakunapruk, N. Cost Effectiveness of Pneumococcal Vaccination in Children in Low- and Middle-Income Countries: A Systematic Review. Pharmacoeconomics 2016, 34, 1211–1225. [Google Scholar] [CrossRef]
- Rosiello, D.F.; Anwar, S.; Yufika, A.; Adam, R.Y.; Ismaeil, M.I.; Ismail, A.Y.; Dahman, N.B.; Hafsi, M.; Ferjani, M.; Sami, F.S.; et al. Acceptance of COVID-19 Vaccination at Different Hypothetical Efficacy and Safety Levels in Ten Countries in Asia, Africa, and South America. Narra J 2021, 1. [Google Scholar] [CrossRef]
- Abu Seir, R.; Azmi, K.; Hamdan, A.; Namouz, H.; Jaar, F.; Jaber, H.; Rubin, C.; Doron, D.; Rahav, G.; Abdeen, Z.; et al. Comparison of Early Effects of Pneumococcal Conjugate Vaccines: PCV7, PCV10 and PCV13 on Streptococcus pneumoniae Nasopharyngeal Carriage in a Population Based Study; The Palestinian-Israeli Collaborative Research (PICR). PLoS ONE 2018, 13, e0206927. [Google Scholar] [CrossRef] [PubMed]
- Malchrzak, W.; Babicki, M.; Mastalerz-Migas, A. Vaccination against Streptococcus pneumoniae in Children Born between 2015 and 2018 in Poland—How Has the Introduction of Free Compulsory Pneumococcal Vaccination Affected Its Uptake? Vaccines 2023, 11, 1654. [Google Scholar] [CrossRef]
- Lee, C.; Robinson, J.L. Systematic Review of the Effect of Immunization Mandates on Uptake of Routine Childhood Immunizations. J. Infect. 2016, 72, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Neufeind, J.; Schmid-Küpke, N.; Rehfuess, E.; Betsch, C.; Wichmann, O. How a Generally Well-Accepted Measles Vaccine Mandate May Lead to Inequities and Decreased Vaccine Uptake: A Preregistered Survey Study in Germany. BMC Public Health 2022, 22, 1846. [Google Scholar] [CrossRef] [PubMed]
- Mahameed, H.; Al-Mahzoum, K.; Alraie, L.A.; Aburumman, R.; Al-Naimat, H.; Alhiary, S.; Barakat, M.; Al-Tammemi, A.B.; Salim, N.A.; Sallam, M. Previous Vaccination History and Psychological Factors as Significant Predictors of Willingness to Receive Mpox Vaccination and a Favorable Attitude towards Compulsory Vaccination. Vaccines 2023, 11, 897. [Google Scholar] [CrossRef]
- Central Statistical Office. Demographic Yearbook of Poland 2015; Central Statistical Office: Warsaw, Poland, 2015.
- Central Statistical Office. Demographic Yearbook of Poland 2016; Central Statistical Office: Warsaw, Poland, 2016.
- Central Statistical Office. Demographic Yearbook of Poland 2017; Central Statistical Office: Warsaw, Poland, 2017.
- Statistics Poland. Demographic Yearbook of Poland 2018; Statistics Poland: Warsaw, Poland, 2018.
- Schellenberg, N.; Crizzle, A.M. Vaccine Hesitancy among Parents of Preschoolers in Canada: A Systematic Literature Review. Can. J. Public Health 2020, 111, 562. [Google Scholar] [CrossRef] [PubMed]
- Ebi, S.J.; Deml, M.J.; Jafflin, K.; Buhl, A.; Engel, R.; Picker, J.; Häusler, J.; Wingeier, B.; Krüerke, D.; Huber, B.M.; et al. Original Research: Parents’ Vaccination Information Seeking, Satisfaction with and Trust in Medical Providers in Switzerland: A Mixed-Methods Study. BMJ Open 2022, 12, 53267. [Google Scholar] [CrossRef]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.D.; Paterson, P. Understanding Vaccine Hesitancy around Vaccines and Vaccination from a Global Perspective: A Systematic Review of Published Literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef]
- Malerba, V.; Costantino, C.; Napoli, G.; Marchese, V.; Casuccio, A.; Tabacchi, G.; Vitale, F.; ESCULAPIO Working Group. Antimeningococcal and Antipneumococcal Vaccination Determinants: A European Systematic Literature Review. Epidemiol. Prev. 2015, 39, 59–64. [Google Scholar]
- McNamara, L.A.; Shumate, A.M.; Johnsen, P.; MacNeil, J.R.; Patel, M.; Bhavsar, T.; Cohn, A.C.; Dinitz-Sklar, J.; Duffy, J.; Finnie, J.; et al. First Use of a Serogroup B Meningococcal Vaccine in the US in Response to a University Outbreak. Pediatrics 2015, 135, 798–804. [Google Scholar] [CrossRef]
- infoWire.pl Editorial Office. New Vaccine against Meningococci. Available online: https://infowire.pl/generic/release/286359/nowa-szczepionka-przeciw-meningokokom/ (accessed on 13 November 2023).
- GSK Commercial Sp. z o.o. Outrun Meningococci. Available online: https://wyprzedzmeningokoki.pl/ (accessed on 13 November 2023).
- Jawień, M.; Jurewicz, A. Meningococci–Symptoms, Management, Meningococcal Vaccine. Available online: https://www.medonet.pl/ciaza-i-dziecko/choroby-dzieciece,meningokoki---objawy--leczenie--szczepionka-na-meningokoki,artykul,1632217.html (accessed on 13 November 2023).
- Hardt, K.; Bonanni, P.; King, S.; Santos, J.I.; El-Hodhod, M.; Zimet, G.D.; Preiss, S. Vaccine Strategies: Optimising Outcomes. Vaccine 2016, 34, 6691–6699. [Google Scholar] [CrossRef] [PubMed]
- Mc Kenna, P.; Broadfield, L.A.; Willems, A.; Masyn, S.; Pattery, T.; Draghia-Akli, R. Digital Health Technology Used in Emergency Large-Scale Vaccination Campaigns in Low- and Middle-Income Countries: A Narrative Review for Improved Pandemic Preparedness. Expert Rev. Vaccines 2023, 22, 243–255. [Google Scholar] [CrossRef]
- Collins, J.; Westerveld, R.; Nelson, K.A.; Rohan, H.; Bower, H.; Lazenby, S.; Ikilezi, G.; Bartlein, R.; Bausch, D.G.; Kennedy, D.S. ‘Learn from the Lessons and Don’t Forget Them’: Identifying Transferable Lessons for COVID-19 from Meningitis A, Yellow Fever and Ebola Virus Disease Vaccination Campaigns. BMJ Glob. Health 2021, 6, 6951. [Google Scholar] [CrossRef] [PubMed]
- pwx/Rynek Zdrowia. Rotaviruses Are the Most Common Cause of Hospitalization in Young Children. Available online: https://www.rynekzdrowia.pl/Serwis-Szczepienia/Rotawirusy-sa-najczestsza-przyczyna-hospitalizacji-malych-dzieci,196596,1018.html (accessed on 13 November 2023).
- Lode, H.; Ludwig, E.; Kassianos, G. Pneumococcal Infection—Low Awareness as a Potential Barrier to Vaccination: Results of a European Survey. Adv. Ther. 2013, 30, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Chang, J.; Yue, D.; Fang, H.; Meng, Q.; Zhang, Y. Determinants of Willingness to Pay for Self-Paid Vaccines in China. Vaccine 2014, 32, 4471–4477. [Google Scholar] [CrossRef] [PubMed]
- Martinón-Torres, F.; Banzhoff, A.; Azzari, C.; De Wals, P.; Marlow, R.; Marshall, H.; Pizza, M.; Rappuoli, R.; Bekkat-Berkani, R. Recent Advances in Meningococcal B Disease Prevention: Real-World Evidence from 4CMenB Vaccination. J. Infect. 2021, 83, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Waśko, I.; Gołębiewska, A.; Kiedrowska, M.; Ronkiewicz, P.; Wróbel-Pawelczyk, I.; Kuch, A.; Hong, E.; Skoczyńska, A. Genetic Variability of Polish Serogroup B Meningococci (2010–2016) Including the 4CMenB Vaccine Component Genes. Vaccine 2020, 38, 1943–1952. [Google Scholar] [CrossRef]
- National Institute of Public Health NIH—National Research Institute Department of Epidemiology and Surveillance of Infectious Diseases; Chief Sanitary Inspectorate—Department of Epidemic Prevention and Border Sanitary Protection. Infectious Diseases and Poisonings in Poland in 2021; National Institute of Public Health: Warsaw, Poland, 2022. [Google Scholar]
- National Institute of Public Health—National Institute of Hygiene Department of Epidemiology and Surveillance of Infectious Diseases; Chief Sanitary Inspectorate—Department for Communicable Disease and Infection Prevention and Control. Infectious Diseases and Poisonings in Poland in 2017; National Institute of Public Health: Warsaw, Poland, 2018. [Google Scholar]
- National Institute of Public Health—National Institute of Hygiene—Department of Epidemiology; Chief Sanitary Inspectorate—Department for Communicable Disease and Infection Prevention and Control. Infectious Diseases and Poisonings in Poland in 2012; National Institute of Public Health: Warsaw, Poland, 2013. [Google Scholar]
- Skoczyńska, A.; Wróbel-Pawelczyk, I.; Gołębiewska, A.; Kiedrowska, M.; Ronkiewicz, P.; Błaszczyk, K.; Kuch, A.; Hryniewicz, W. Invasive Meningococcal Disease in Poland in 2022 (KOROUN Data). Available online: https://koroun.nil.gov.pl/wp-content/uploads/2023/06/Inwazyjna-choroba-meningokokowa-ICHM-w-Polsce-w-2022-roku-uzupelnienie.pdf (accessed on 13 November 2023).
- Kuchar, E.; Czajka, H.; Gowin, E.; Nitsch-Osuch, A.; Skoczyńska, A.; Szenborn, L.; Wrotek, A.; Wysocki, J.; Mastalerz-Migas, A.; Peregud-Pogorzelski, J.; et al. Recommendations on Meningococcal Vaccination in Children and Adults. Przegląd Pediatryczny 2022, 51, 8–20. [Google Scholar]
- Campbell, H.; Andrews, N.; Parikh, S.R.; White, J.; Edelstein, M.; Bai, X.; Lucidarme, J.; Borrow, R.; Ramsay, M.E.; Ladhani, S.N. Impact of an Adolescent Meningococcal ACWY Immunisation Programme to Control a National Outbreak of Group W Meningococcal Disease in England: A National Surveillance and Modelling Study. Lancet Child Adolesc. Health 2022, 6, 96–105. [Google Scholar] [CrossRef]
- Liao, S.L.; Huang, T.; Huang, Y.C.; Jiang, D.D.-S. Survey of the Status of Self-Paid Varicella Vaccination among Children One to Six Years of Age in Taiwan. J. Microbiol. Immunol. Infect. 2007, 40, 112–115. [Google Scholar]
- Ganczak, M.; Dmytrzyk-Daniłów, G.; Karakiewicz, B.; Korzeń, M.; Szych, Z. Determinants Influencing Self-Paid Vaccination Coverage, in 0–5 Years Old Polish Children. Vaccine 2013, 31, 5687–5692. [Google Scholar] [CrossRef]
- Theeten, H.; Hens, N.; Aerts, M.; Vandermeulen, C.; Roelants, M.; Hoppenbrouwers, K.; Van Damme, P.; Beutels, P. Caregivers’ Willingness to Pay to Reduce the Number of Vaccine Injections in Infants. Pediatr. Infect. Dis. J. 2009, 28, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Matson, D.O.; Dennehy, P.; Van Damme, P.; Santosham, M.; Rodriguez, Z.; Dallas, M.J.; Heyse, J.F.; Goveia, M.G.; Black, S.B.; et al. Safety and Efficacy of a Pentavalent Human–Bovine (WC3) Reassortant Rotavirus Vaccine. N. Engl. J. Med. 2006, 354, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Parashar, U.D. Rotavirus Vaccines in Routine Use. Clin. Infect. Dis. 2014, 59, 1291–1301. [Google Scholar] [CrossRef]
- Legal Act–Official Journal of the Ministry of Health No. 117, 2020 Chief Sanitary Inspector Announcement of December 22, 2020 on the Preventive Vaccination Program for 2021. 2020. Available online: https://dziennikmz.mz.gov.pl/legalact/2020/117/ (accessed on 13 November 2023).
- Marshall, H.; Ryan, P.; Roberton, D.; Beilby, J. Varicella Immunisation Practice: Implications for Provision of a Recommended, Non-Funded Vaccine. J. Paediatr. Child Health 2009, 45, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; Ryan, P.; Roberton, D. Uptake of Varicella Vaccine—A Cross Sectional Survey of Parental Attitudes to Nationally Recommended but Unfunded Varicella Immunisation. Vaccine 2005, 23, 5389–5397. [Google Scholar] [CrossRef]
- Grose, C. Varicella Vaccination of Children in the United States: Assessment after the First Decade 1995–2005. J. Clin. Virol. 2005, 33, 89–95. [Google Scholar] [CrossRef]
- Vezzosi, L.; Santagati, G.; Angelillo, I.F. Knowledge, Attitudes, and Behaviors of Parents towards Varicella and Its Vaccination. BMC Infect. Dis. 2017, 17, 172. [Google Scholar] [CrossRef]


| Vaccine | Until 2016 | From 2017 |
|---|---|---|
| PCV10 1 | Recommended; chargeable | Mandatory; free of charge |
| PCV13 2 | Recommended; chargeable | Recommended; chargeable |
| Patient | Total Population N (%) | 2015 and 2016 Age Group N (%) | 2017 and 2018 Age Group N (%) | p | |
|---|---|---|---|---|---|
| Sex | Male | 763 (47.8) | 387 (46.5) | 445 (53.5) | 0.525 |
| Female | 832 (52.2) | 368 (48.2) | 395 (51.8) | ||
| Place | Urban area | 1318 (82.6) | 630 (47.8) | 688 (52.2) | 0.417 |
| Rural area | 277 (17.4) | 125 (45.1) | 152 (54.9) | ||
| Recommended Vaccination | Total Population N (%) | 2015 and 2016 Age Group N (%) | 2017 and 2018 Age Group N (%) | p | |
|---|---|---|---|---|---|
| 5-in-1 or 6-in-1 conjugate vaccines | Yes | 1288 (80.8) | 608 (80.5) | 680 (81.0) | 0.831 |
| No | 307 (19.2) | 147 (19.5) | 160 (19.0) | ||
| Against rotavirus | Yes | 823 (51.6) | 366 (48.5) | 457 (54.4) | 0.018 |
| No | 772 (48.4) | 389 (51.5) | 383 (45.6) | ||
| Against chickenpox | Yes | 516 (32.4) | 231 (30.6) | 285 (33.9) | 0.156 |
| No | 1079 (67.6) | 524 (69.4) | 555 (66.1) | ||
| Against N. meningitidis B | Yes | 179 (11.2) | 36 (4.8) | 143 (17.0) | <0.001 |
| No | 1416 (88.8) | 719 (95.2) | 697 (83.0) | ||
| Against N. meningitidis C | Yes | 173 (10.8) | 82 (10.9) | 91 (10.8) | 0.986 |
| No | 1422 (89.2) | 673 (89.1) | 749 (89.2) | ||
| Against tick-borne encephalitis | Yes | 21 (1.3) | 6 (0.8) | 15 (1.8) | 0.083 |
| No | 1574 (98.7) | 749 (99.2) | 825 (98.2) | ||
| Against hepatitis A | Yes | 15 (0.9) | 8 (1.1) | 7 (0.8) | 0.640 |
| No | 1580 (99.1) | 747 (98.9) | 833 (99.2) | ||
| Any recommended vaccinations | Yes | 1021 (64.0) | 462 (61.2) | 559 (66.6) | 0.026 |
| No | 574 (36.0) | 293 (38.8) | 281 (33.5) | ||
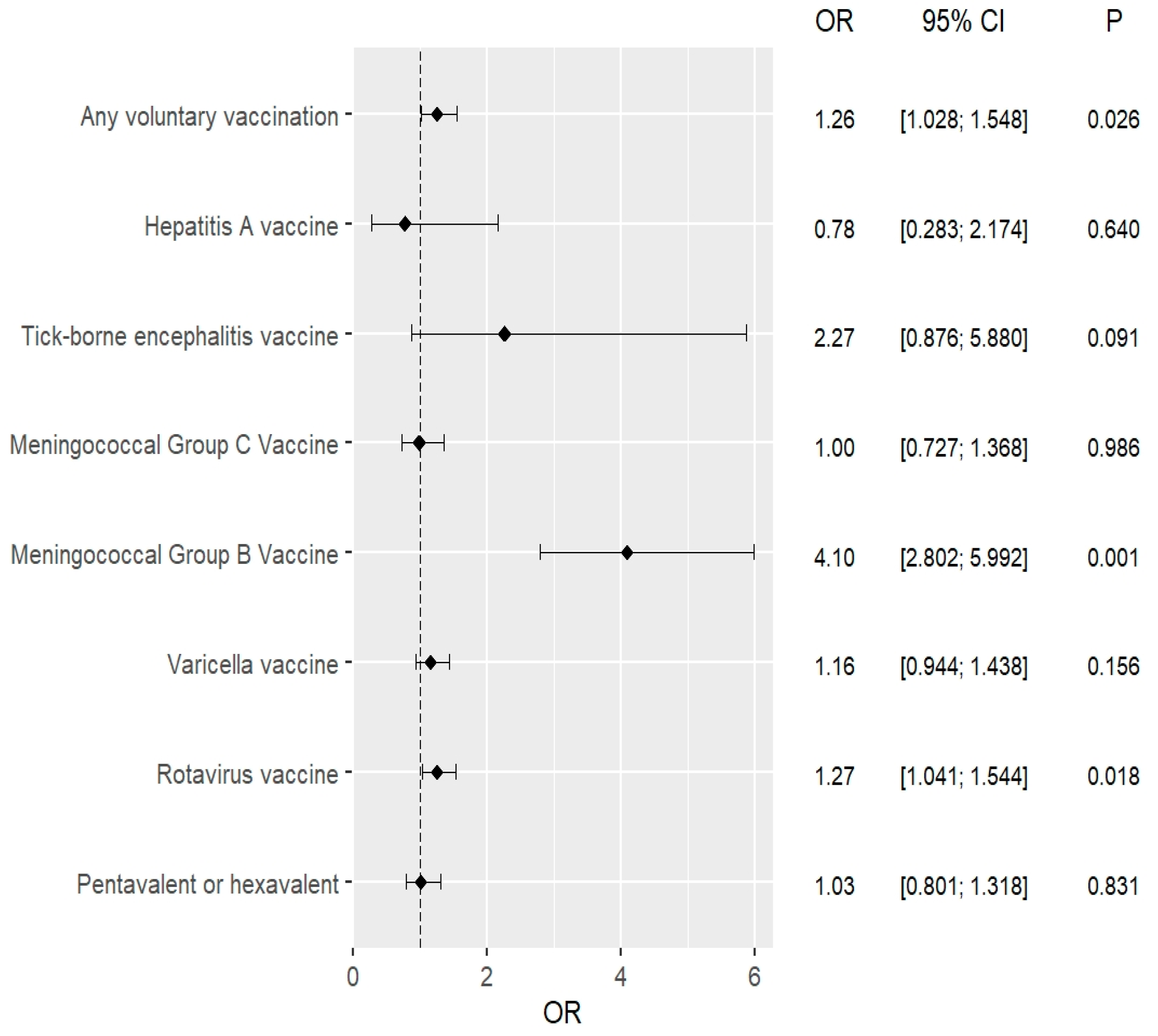
| Recommended Vaccinations | Univariate Analysis 2015–2016 vs. 2017–2018 | Multivariate Analysis * 2015–2016 vs. 2017–2018 | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Conjugate (5-in-1 or 6-in-1) vaccines | 1.028 (0.801–1.318) | 0.831 | 1.022 (0.796–1.311) | 0.867 |
| Against rotavirus | 1.268 (1.041–1.544) | 0.018 | 1.271 (1.044–1.549) | 0.017 |
| Against chickenpox | 1.165 (0.944–1.438) | 0.156 | 1.166 (0.944–1.439) | 0.154 |
| Against N. meningitidis B | 4.098 (2.802–5.992) | <0.001 | 4.099 (2.803–5.995) | <0.001 |
| Against N. meningitidis C | 0.997 (0.727–1.368) | 0.986 | 1.000 (0.728–1.372) | 0.998 |
| Against tick-borne encephalitis | 2.270 (0.876–5.880) | 0.091 | 2.278 (0.879–5.905) | 0.090 |
| Against hepatitis A | 0.784 (0.283–2.174) | 0.640 | 0.773 (0.278–2.148) | 0.621 |
| Any recommended vaccinations | 1.262 (1.028–1.548) | 0.026 | 1.265 (1.030–1.552) | 0.025 |
| Recommended Vaccination | Total Population N (%) | Free PCV or no PCV N (%) | Chargeable PVC N (%) | p | |
|---|---|---|---|---|---|
| 5-in-1 or 6-in-1 conjugate vaccines | Yes | 1288 (80.8) | 651 (73.4) | 637 (90.0) | <0.001 |
| No | 307 (19.2) | 236 (26.6) | 71 (10.0) | ||
| Against rotavirus | Yes | 823 (51.6) | 376 (42.4) | 447 (63.1) | <0.001 |
| No | 772 (48.4) | 511 (57.6) | 261 (36.9) | ||
| Against chickenpox | Yes | 516 (32.4) | 249 (28.1) | 267 (37.7) | <0.001 |
| No | 1079 (67.6) | 638 (71.9) | 441 (62.3) | ||
| Against N. meningitidis B | Yes | 179 (11.2) | 97 (10.9) | 82 (11.6) | 0.685 |
| No | 1416 (88.8) | 790 (89.1) | 626 (88.4) | ||
| Against N. meningitidis C | Yes | 173 (10.8) | 63 (7.1) | 110 (15.5) | <0.001 |
| No | 1422 (89.2) | 824 (92.9) | 598 (84.5) | ||
| Against tick-borne encephalitis | Yes | 21 (1.3) | 11 (1.2) | 10 (1.4) | 0.764 |
| No | 1574 (98.7) | 876 (98.8) | 698 (98.6) | ||
| Against hepatitis A | Yes | 15 (0.9) | 5 (0.6) | 10 (1.4) | 0.081 |
| No | 1580 (99.1) | 882 (99.4) | 698 (98.6) | ||
| Any recommended vaccinations | Yes | 1021 (64.0) | 484 (54.6) | 537 (75.9) | <0.001 |
| No | 574 (36.0) | 403 (45.4) | 171 (24.2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malchrzak, W.; Babicki, M.; Pokorna-Kałwak, D.; Mastalerz-Migas, A. The Influence of Introducing Free Vaccination against Streptococcus pneumoniae on the Uptake of Recommended Vaccination in Poland. Vaccines 2023, 11, 1838. https://doi.org/10.3390/vaccines11121838
Malchrzak W, Babicki M, Pokorna-Kałwak D, Mastalerz-Migas A. The Influence of Introducing Free Vaccination against Streptococcus pneumoniae on the Uptake of Recommended Vaccination in Poland. Vaccines. 2023; 11(12):1838. https://doi.org/10.3390/vaccines11121838
Chicago/Turabian StyleMalchrzak, Wojciech, Mateusz Babicki, Dagmara Pokorna-Kałwak, and Agnieszka Mastalerz-Migas. 2023. "The Influence of Introducing Free Vaccination against Streptococcus pneumoniae on the Uptake of Recommended Vaccination in Poland" Vaccines 11, no. 12: 1838. https://doi.org/10.3390/vaccines11121838
APA StyleMalchrzak, W., Babicki, M., Pokorna-Kałwak, D., & Mastalerz-Migas, A. (2023). The Influence of Introducing Free Vaccination against Streptococcus pneumoniae on the Uptake of Recommended Vaccination in Poland. Vaccines, 11(12), 1838. https://doi.org/10.3390/vaccines11121838







