Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy
Abstract
:1. Introduction
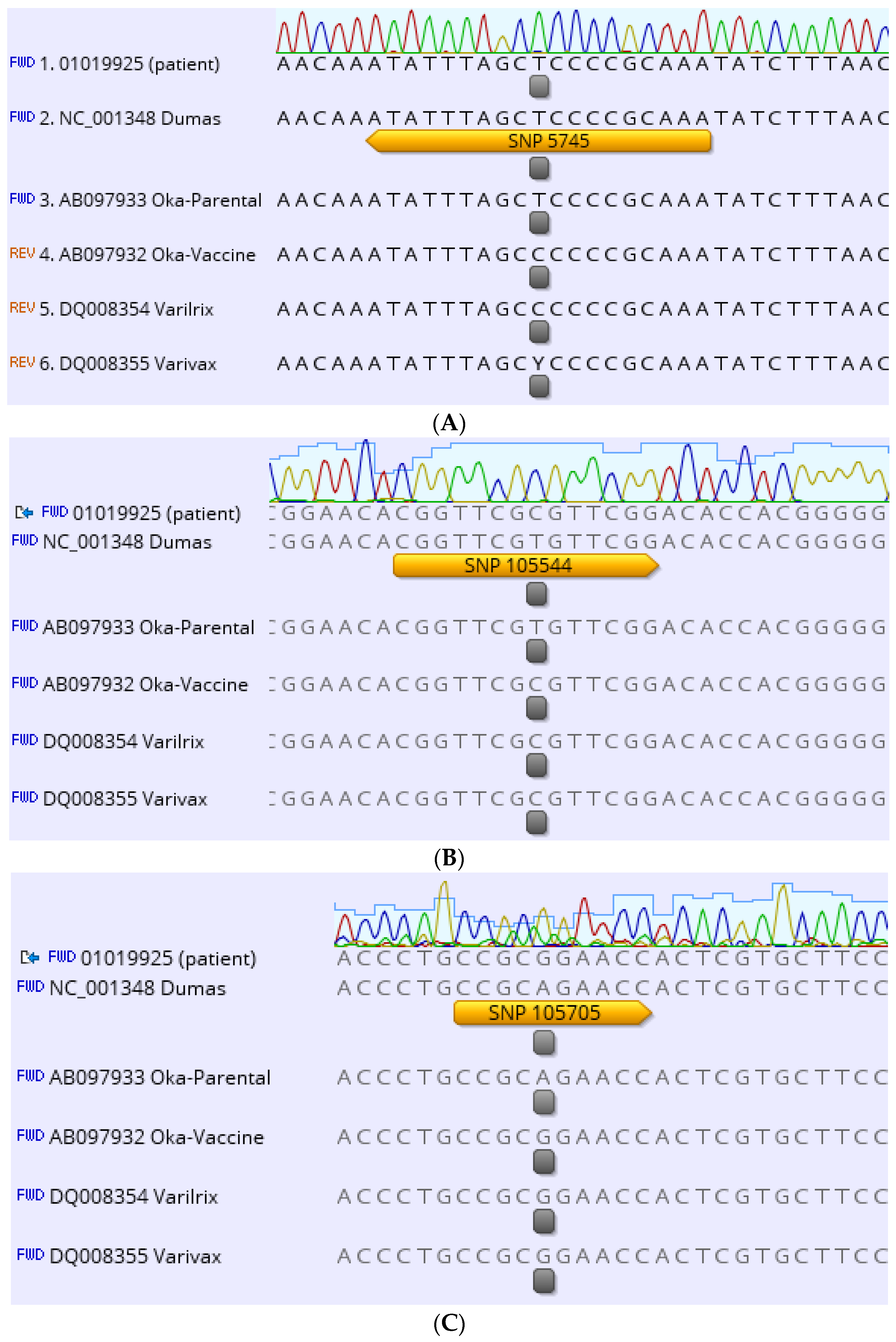
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VZV | Varicella-Zoster-Virus |
| CSF | Cerebrospinal fluid |
| SNP | single nucleotide polymorphism |
| ORF | open reading frame |
| wt | wildtype |
References
- Weibel, R.E.; Neff, B.J.; Kuter, B.J.; Guess, H.A.; Rothenberger, C.A.; Fitzgerald, A.J.; Connor, K.A.; McLean, A.A.; Hilleman, M.R.; Buynak, E.B.; et al. Live attenuated varicella virus vaccine. Efficacy trial in healthy children. N. Engl. J. Med. 1984, 310, 1409–1415. [Google Scholar] [CrossRef]
- Marin, M.; Meissner, H.C.; Seward, J.F. Varicella prevention in the United States: A review of successes and challenges. Pediatrics 2008, 122, e744–e751. [Google Scholar] [CrossRef]
- Bialek, S.R.; Perella, D.; Zhang, J.; Mascola, L.; Viner, K.; Jackson, C.; Lopez, A.S.; Watson, B.; Civen, R. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics 2013, 132, e1134–e1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heywood, A.E.; Wang, H.; Macartney, K.K.; McIntyre, P. Varicella and herpes zoster hospitalizations before and after implementation of one-dose varicella vaccination in Australia: An ecological study. Bull. World Health Organ. 2014, 92, 593–604. [Google Scholar] [CrossRef]
- Siedler, A.; Arndt, U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveill. 2010, 15, 19530. [Google Scholar] [CrossRef]
- Bonanni, P.; Breuer, J.; Gershon, A.; Gershon, M.; Hryniewicz, W.; Papaevangelou, V.; Rentier, B.; Rumke, H.; Sadzot-Delvaux, C.; Senterre, J.; et al. Varicella vaccination in Europe—Taking the practical approach. BMC Med. 2009, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Dollard, S.; Chen, M.H.; Lindstrom, S.; Marin, M.; Rota, P.A. Diagnostic and Immunologic Testing for Varicella in the Era of High-Impact Varicella Vaccination: An Evolving Problem. J. Infect. Dis. 2022, 226, S450–S455. [Google Scholar] [CrossRef]
- Weinmann, S.; Chun, C.; Schmid, D.S.; Roberts, M.; Vandermeer, M.; Riedlinger, K.; Bialek, S.R.; Marin, M. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J. Infect. Dis. 2013, 208, 1859–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinmann, S.; Naleway, A.L.; Koppolu, P.; Baxter, R.; Belongia, E.A.; Hambidge, S.J.; Irving, S.A.; Jackson, M.L.; Klein, N.P.; Lewin, B.; et al. Incidence of Herpes Zoster Among Children: 2003–2014. Pediatrics 2019, 144, e20182917. [Google Scholar] [CrossRef] [PubMed]
- Zoch-Lesniak, B.; Tolksdorf, K.; Siedler, A. Trends in herpes zoster epidemiology in Germany based on primary care sentinel surveillance data, 2005–2016. Hum. Vaccines Immunother. 2018, 14, 1807–1814. [Google Scholar] [CrossRef] [Green Version]
- Kleinschmidt-DeMasters, B.K.; Amlie-Lefond, C.; Gilden, D.H. The patterns of varicella zoster virus encephalitis. Hum. Pathol. 1996, 27, 927–938. [Google Scholar] [CrossRef]
- Chaves, S.S.; Haber, P.; Walton, K.; Wise, R.P.; Izurieta, H.S.; Schmid, D.S.; Seward, J.F. Safety of varicella vaccine after licensure in the United States: Experience from reports to the vaccine adverse event reporting system, 1995–2005. J. Infect. Dis. 2008, 197, S170–S177. [Google Scholar] [CrossRef] [Green Version]
- Levin, M.J.; DeBiasi, R.L.; Bostik, V.; Schmid, D.S. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J. Infect. Dis. 2008, 198, 1444–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, S.; Mittal, M.K.; Hodinka, R.L. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann. Emerg. Med. 2009, 53, 792–795. [Google Scholar] [CrossRef]
- Chen, Y.-C.; James, A.; Kung, E.; Madhavan, V. A case of herpes zoster and meningitis in a twice-vaccinated healthy adolescent. J. Pediatr. Infect. Dis. 2017, 12, 142–144. [Google Scholar] [CrossRef] [Green Version]
- Harrington, W.E.; Mato, S.; Burroughs, L.; Carpenter, P.A.; Gershon, A.; Schmid, D.S.; Englund, J.A. Vaccine Oka Varicella Meningitis in Two Adolescents. Pediatrics 2019, 144, e20191522. [Google Scholar] [CrossRef]
- Ramachandran, V.; Elliott, S.C.; Rogers, K.L.; Cohrs, R.J.; Weinberger, M.; Jackson, W.; Carpenter, J.E.; Grose, C.; Bonthius, D.J. Varicella Vaccine Meningitis as a Complication of Herpes Zoster in Twice-Immunized Immunocompetent Adolescents. J. Child Neurol. 2020, 35, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Loparev, V.N.; McCaustland, K.; Holloway, B.P.; Krause, P.R.; Takayama, M.; Schmid, D.S. Rapid genotyping of varicella-zoster virus vaccine and wild-type strains with fluorophore-labeled hybridization probes. J. Clin. Microbiol. 2000, 38, 4315–4319. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Xu, S.; Maple, P.A.C.; Xu, W.; Brown, K.E. Differentiation between wild-type and vaccines strains of varicella zoster virus (VZV) based on four single nucleotide polymorphisms. Epidemiol. Infect. 2017, 145, 2618–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, P.L.; Leung, J.; Marquez, P.; Kim, Y.; Wei, S.; Su, J.R.; Marin, M. Safety Surveillance of Varicella Vaccines in the Vaccine Adverse Event Reporting System, United States, 2006–2020. J. Infect. Dis. 2022, 226, S431–S440. [Google Scholar] [CrossRef]
- Heusel, E.H.; Grose, C. Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses 2020, 12, 1078. [Google Scholar] [CrossRef]
- Ramachandran, P.S.; Wilson, M.R.; Catho, G.; Blanchard-Rohner, G.; Schiess, N.; Cohrs, R.J.; Boutolleau, D.; Burrel, S.; Yoshikawa, T.; Wapniarski, A.; et al. Meningitis Caused by the Live Varicella Vaccine Virus: Metagenomic Next Generation Sequencing, Immunology Exome Sequencing and Cytokine Multiplex Profiling. Viruses 2021, 13, 2286. [Google Scholar] [CrossRef]
- Kawamura, Y.; Suzuki, D.; Kono, T.; Miura, H.; Kozawa, K.; Mizuno, H.; Yoshikawa, T. A Case of Aseptic Meningitis without Skin Rash Caused by Oka Varicella Vaccine. Pediatr. Infect. Dis. J. 2022, 41, 78–79. [Google Scholar] [CrossRef]
- Schmid, D.S.; Jumaan, A.O. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin. Microbiol. Rev. 2010, 23, 202–217. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.H.; Dohner, D.E.; Wellinghoff, W.J.; Gelb, L.D. Restriction endonuclease analysis of varicella-zoster vaccine virus and wild-type DNAs. J. Med. Virol. 1982, 9, 69–76. [Google Scholar] [CrossRef]
- Adams, S.G.; Dohner, D.E.; Gelb, L.D. Restriction fragment differences between the genomes of the Oka varicella vaccine virus and American wild-type varicella-zoster virus. J. Med. Virol. 1989, 29, 38–45. [Google Scholar] [CrossRef]
- LaRussa, P.; Lungu, O.; Hardy, I.; Gershon, A.; Steinberg, S.P.; Silverstein, S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 1992, 66, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- LaRussa, P.; Steinberg, S.; Arvin, A.; Dwyer, D.; Burgess, M.; Menegus, M.; Rekrut, K.; Yamanishi, K.; Gershon, A. Polymerase chain reaction and restriction fragment length polymorphism analysis of varicella-zoster virus isolates from the United States and other parts of the world. J. Infect. Dis. 1998, 178, S64–S66. [Google Scholar] [CrossRef] [Green Version]
- Toi, C.S.; Dwyer, D.E. Differentiation between vaccine and wild-type varicella-zoster virus genotypes by high-resolution melt analysis of single nucleotide polymorphisms. J. Clin. Virol. 2008, 43, 18–24. [Google Scholar] [CrossRef]
- Takayama, M.; Takayama, N.; Inoue, N.; Kameoka, Y. Application of long PCR method of identification of variations in nucleotide sequences among varicella-zoster virus isolates. J. Clin. Microbiol. 1996, 34, 2869–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, C.; Takahara, R.; Toriyama, T.; Nagai, T.; Takahashi, M.; Yamanishi, K. Identification of the Oka strain of the live attenuated varicella vaccine from other clinical isolates by molecular epidemiologic analysis. J. Infect. Dis. 1998, 178, 35–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawrami, K.; Breuer, J. Development of a fluorogenic polymerase chain reaction assay (TaqMan) for the detection and quantitation of varicella zoster virus. J. Virol. Methods 1999, 79, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hawrami, K.; Breuer, J. Analysis of United Kingdom wild-type strains of varicella-zoster virus: Differentiation from the Oka vaccine strain. J. Med. Virol. 1997, 53, 60–62. [Google Scholar] [CrossRef]
- Hawrami, K.; Hart, I.J.; Pereira, F.; Argent, S.; Bannister, B.; Bovill, B.; Carrington, D.; Ogilvie, M.; Rawstorne, S.; Tryhorn, Y.; et al. Molecular epidemiology of varicella-zoster virus in East London, England, between 1971 and 1995. J. Clin. Microbiol. 1997, 35, 2807–2809. [Google Scholar] [CrossRef] [Green Version]
- Sauerbrei, A.; Rubtcova, E.; Wutzler, P.; Schmid, D.S.; Loparev, V.N. Genetic profile of an Oka varicella vaccine virus variant isolated from an infant with zoster. J. Clin. Microbiol. 2004, 42, 5604–5608. [Google Scholar] [CrossRef] [Green Version]
- Depledge, D.P.; Yamanishi, K.; Gomi, Y.; Gershon, A.A.; Breuer, J. Deep Sequencing of Distinct Preparations of the Live Attenuated Varicella-Zoster Virus Vaccine Reveals a Conserved Core of Attenuating Single-Nucleotide Polymorphisms. J. Virol. 2016, 90, 8698–8704. [Google Scholar] [CrossRef] [Green Version]
- Tillieux, S.L.; Halsey, W.S.; Thomas, E.S.; Voycik, J.J.; Sathe, G.M.; Vassilev, V. Complete DNA sequences of two oka strain varicella-zoster virus genomes. J. Virol. 2008, 82, 11023–11044. [Google Scholar] [CrossRef] [Green Version]
- Gilden, D.H.; Kleinschmidt-DeMasters, B.K.; LaGuardia, J.J.; Mahalingam, R.; Cohrs, R.J. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 2000, 342, 635–645. [Google Scholar] [CrossRef]
- Persson, A.; Bergstrom, T.; Lindh, M.; Namvar, L.; Studahl, M. Varicella-zoster virus CNS disease—Viral load, clinical manifestations and sequels. J. Clin. Virol. Off. Publ. Pan. Am. Soc. Clin. Virol. 2009, 46, 249–253. [Google Scholar] [CrossRef]
- Science, M.; MacGregor, D.; Richardson, S.E.; Mahant, S.; Tran, D.; Bitnun, A. Central nervous system complications of varicella-zoster virus. J. Pediatr. 2014, 165, 779–785. [Google Scholar] [CrossRef]
- Moffat, J.F.; Zerboni, L.; Kinchington, P.R.; Grose, C.; Kaneshima, H.; Arvin, A.M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 1998, 72, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Hambleton, S.; Steinberg, S.P.; Larussa, P.S.; Shapiro, E.D.; Gershon, A.A. Risk of herpes zoster in adults immunized with varicella vaccine. J. Infect. Dis. 2008, 197, S196–S199. [Google Scholar] [CrossRef] [Green Version]
- Civen, R.; Chaves, S.S.; Jumaan, A.; Wu, H.; Mascola, L.; Gargiullo, P.; Seward, J.F. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr. Infect. Dis. J. 2009, 28, 954–959. [Google Scholar] [CrossRef]
- Galil, K.; Brown, C.; Lin, F.; Seward, J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 931–935. [Google Scholar] [CrossRef]
- Rawson, H.; Crampin, A.; Noah, N. Deaths from chickenpox in England and Wales 1995–1997: Analysis of routine mortality data. BMJ 2001, 323, 1091–1093. [Google Scholar] [CrossRef] [Green Version]
- Galea, S.A.; Sweet, A.; Beninger, P.; Steinberg, S.P.; Larussa, P.S.; Gershon, A.A.; Sharrar, R.G. The safety profile of varicella vaccine: A 10-year review. J. Infect. Dis. 2008, 197, S165–S169. [Google Scholar] [CrossRef]
- Jeon, J.S.; Won, Y.H.; Kim, I.K.; Ahn, J.H.; Shin, O.S.; Kim, J.H.; Lee, C.H. Analysis of single nucleotide polymorphism among Varicella-Zoster Virus and identification of vaccine-specific sites. Virology 2016, 496, 277–286. [Google Scholar] [CrossRef]
- Thiele, S.; Borschewski, A.; Kuchler, J.; Bieberbach, M.; Voigt, S.; Ehlers, B. Molecular analysis of varicella vaccines and varicella-zoster virus from vaccine-related skin lesions. Clin. Vaccine Immunol. CVI 2011, 18, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Kanda, R.K.; Quinlivan, M.L.; Gershon, A.A.; Nichols, R.A.; Breuer, J. Population diversity in batches of the varicella Oka vaccine. Vaccine 2011, 29, 3293–3298. [Google Scholar] [CrossRef] [Green Version]
- Spackova, M.; Wiese-Posselt, M.; Dehnert, M.; Matysiak-Klose, D.; Heininger, U.; Siedler, A. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine 2010, 28, 686–691. [Google Scholar] [CrossRef]
- Depledge, D.P.; Kundu, S.; Jensen, N.J.; Gray, E.R.; Jones, M.; Steinberg, S.; Gershon, A.; Kinchington, P.R.; Schmid, D.S.; Balloux, F.; et al. Deep sequencing of viral genomes provides insight into the evolution and pathogenesis of varicella zoster virus and its vaccine in humans. Mol. Biol. Evol. 2014, 31, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, G.; Depledge, D.P.; Brown, J.R.; Hale, A.D.; Tutil, H.; Williams, R.; Breuer, J. Acute Retinal Necrosis Caused by the Zoster Vaccine Virus. Clin. Infect. Dis. 2017, 65, 2122–2125. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Patient | Reference Range |
|---|---|---|
| White Cell Count | 5700 cells/µl | 4500–13,500 cells/µL |
| Lymphocytes | 32.3% | 20.4–51.1% |
| Monocytes | 9.1% | 1–14% |
| Neutrophil granulocytes | 50.1% | 42.2–75.2% |
| B-lymphocytes (CD19+) | 18.6% | 8–24% |
| T-lymphocytes (CD3+) | 73.1% | 52–78% |
| T-helper cells (CD3+CD4+) | 43.6% | 25–48% |
| Cytotoxic T cells (CD3+CD8+) | 24% | 9–35% |
| CD4/CD8 ratio | 1.8 | 0.9–3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierbaum, S.; Fischer, V.; Briedigkeit, L.; Werner, C.; Hengel, H.; Huzly, D. Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy. Vaccines 2023, 11, 309. https://doi.org/10.3390/vaccines11020309
Bierbaum S, Fischer V, Briedigkeit L, Werner C, Hengel H, Huzly D. Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy. Vaccines. 2023; 11(2):309. https://doi.org/10.3390/vaccines11020309
Chicago/Turabian StyleBierbaum, Sibylle, Veronika Fischer, Lutz Briedigkeit, Claudius Werner, Hartmut Hengel, and Daniela Huzly. 2023. "Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy" Vaccines 11, no. 2: 309. https://doi.org/10.3390/vaccines11020309
APA StyleBierbaum, S., Fischer, V., Briedigkeit, L., Werner, C., Hengel, H., & Huzly, D. (2023). Meningitis without Rash after Reactivation of Varicella Vaccine Strain in a 12-Year-Old Immunocompetent Boy. Vaccines, 11(2), 309. https://doi.org/10.3390/vaccines11020309





