ATAK Complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 Vaccination and Review of the Literature
Abstract
:1. Introduction
2. Case Report
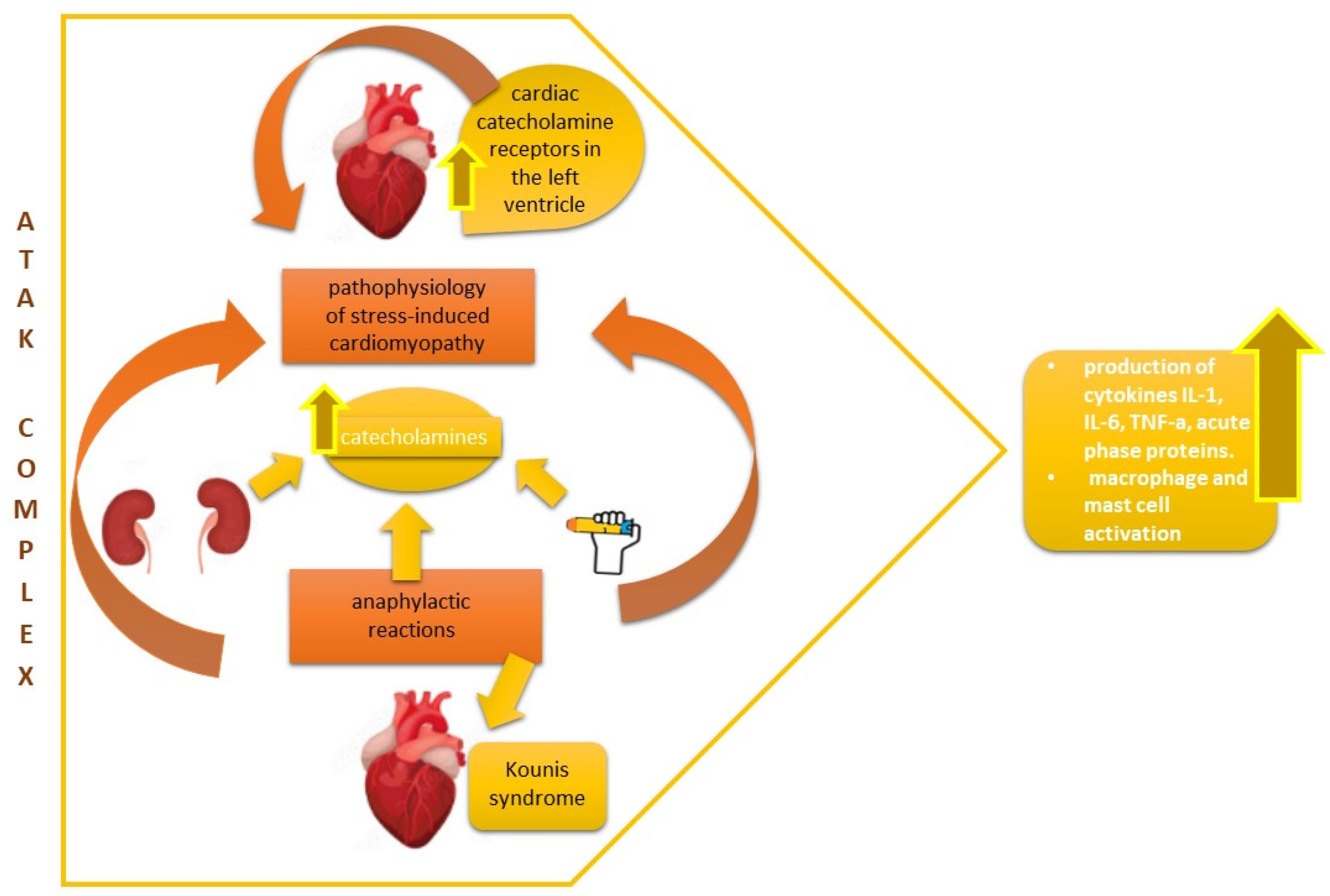
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic reactions to current available covid-19 vaccinations: Pathophysiology, causality, and therapeutic considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Neun, B.W.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro. Molecules 2018, 23, 1700. [Google Scholar] [CrossRef] [Green Version]
- Haq, H.N.; Khan, H.; Chaudhry, H.; Nimmala, S.; Demidovich, J.; Papudesi, B.N.; Potluri, S.D. Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273) COVID-19 mRNA vaccines and hypersensitivity reactions. J. Natl. Med. Assoc. 2022, 114, 601–612. [Google Scholar] [CrossRef]
- Rutkowski, K.; Mirakian, R.; Till, S.; Rutkowski, R.; Wagner, A. Adverse reactions to COVID-19 vaccines: A practical approach. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2021, 51, 770–777. [Google Scholar] [CrossRef]
- Laisuan, W.; Wongsa, C.; Chiewchalermsri, C.; Thongngarm, T.; Rerkpattanapipat, T.; Iamrahong, P.; Ruangwattanachok, C.; Nanthapisal, S.; Sompornrattanaphan, M. CoronaVac COVID-19 Vaccine-Induced Anaphylaxis: Clinical Characteristics and Revaccination Outcomes. J. Asthma Allergy 2021, 14, 1209–1215. [Google Scholar] [CrossRef]
- Lyapina, M.G.; Stoyanova Dencheva, M. Contact sensitization to ingredients of dental materials and cosmetics in dental students: A pilot study. Cent. Eur. J. Public Health 2019, 27, 73–77. [Google Scholar] [CrossRef]
- Chung, E.H. Vaccine allergies. Clin. Exp. Vaccine Res. 2014, 3, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivera Mesa, D.; Hogan, A.B.; Watson, O.J.; Charles, G.D.; Hauck, K.; Ghani, A.C.; Winskill, P. Modelling the impact of vaccine hesitancy in prolonging the need for Non-Pharmaceutical Interventions to control the COVID-19 pandemic. Commun. Med. 2022, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Khan, S. Mast cell tryptase level should be checked in all patients with suspected Kounis syndrome. Eur. Heart J. 2020, 41, 3018. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, M.; Subedi, R.; Shah, S.; Kozman, H. Kounis syndrome: A review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int. J. Cardiol. 2017, 232, 1–4. [Google Scholar] [CrossRef]
- Stone, C.A.J.; Rukasin, C.R.F.; Beachkofsky, T.M.; Phillips, E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019, 85, 2694–2706. [Google Scholar] [CrossRef] [Green Version]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, E.J.; Lee, M.H.; Song, K.B.; Lee, Y.K.; Kim, M.K.; Kim, T.E.; Chung, E.H. Vaccine-related anaphylaxis cases confirmed by KCDC from 2001–2016. J. Korean Med. Sci. 2020, 35, 1–7. [Google Scholar] [CrossRef]
- Greenhawt, M.; Abrams, E.M.; Shaker, M.; Chu, D.K.; Khan, D.; Akin, C.; Alqurashi, W.; Arkwright, P.; Baldwin, J.L.; Ben-Shoshan, M.; et al. The Risk of Allergic Reaction to SARS-CoV-2 Vaccines and Recommended Evaluation and Management: A Systematic Review, Meta-Analysis, GRADE Assessment, and International Consensus Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 3546–3567. [Google Scholar] [CrossRef]
- Shimabukuro, T.; Nair, N. Allergic Reactions including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA-J. Am. Med. Assoc. 2021, 325, 780–781. [Google Scholar] [CrossRef]
- del Farmaco, A.I. Rapporto Annuale Sulla Sicurezza dei Vaccini Anti-COVID-19; AIFA: Rome, Italy, 2021.
- Cheng, T.O.; Kounis, N.G. Takotsubo cardiomyopathy, mental stress and the Kounis syndrome. Int. J. Cardiol. 2012, 161, 65–67. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kagawa, Y.; Endo, A.; Tanabe, K. Intense Emotional Stress Over Potential Coronavirus Disease Vaccination Side Effects Leads to Takotsubo Cardiomyopathy. Circ. Rep. 2021, 3, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.J.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, C.M. Managing immunization stress-related response: A contributor to sustaining trust in vaccines. Can. Commun. Dis. Rep. 2020, 46, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; Mplani, V.; Kouni, S.; Velissaris, D.; Plotas, P.; Tsigkas, G. Rare Hypersensitivity Myocardial Reactions Following COVID-19 Vaccination: Hypersensitivity Myocardial Infarction (Kounis Syndrome) and Hypersensitivity Myocarditis. Anatol. J. Cardiol. 2022, 26, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Hahalis, G. Serum IgE levels in coronary artery disease. Atherosclerosis 2016, 251, 498–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Yuan, S.; Liu, Y.; Zeng, Y.; Xie, H.; Liu, Z.; Zhang, S.; Fang, Q.; Wang, J.; Shen, Z. Serum IgE levels are associated with coronary artery disease severity. Atherosclerosis 2016, 251, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Zavras, G.M. Histamine-induced coronary artery spasm: The concept of allergic angina. Br. J. Clin. Pract. 1991, 45, 121–128. [Google Scholar]
- Kounis, N.G.; Soufras, G.D. Shoulder arthroscopy and ATAK (adrenaline, Takotsubo, anaphylaxis, and Kounis hypersensitivty-associated syndrome). Orthop. Traumatol. Surg. Res. 2016, 102, 273–274. [Google Scholar] [CrossRef]
- Giovannini, M.; Koniari, I.; Mori, F.; Barni, S.; Novembre, E.; Kounis, N.G. Kounis syndrome: Towards a new classification. Int. J. Cardiol. 2021, 341, 13–14. [Google Scholar] [CrossRef]
- Allam, C.; Kounis, N.G.; Chlawit, R.; Saouma, M.; Badaoui, G. Kounis syndrome following COVID-19 vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon-on-Thames, UK, 2022; Volume 35, pp. 369–370. [Google Scholar]
- Özdemir, İ.H.; Özlek, B.; Özen, M.B.; Gündüz, R.; Bayturan, Ö. Type 1 Kounis syndrome induced by inactivated SARS-COV-2 vaccine. J. Emerg. Med. 2021, 61, e71–e76. [Google Scholar] [CrossRef]
- Tajstra, M.; Jaroszewicz, J.; Gąsior, M. Acute coronary tree thrombosis after vaccination for COVID-19. Cardiovasc. Interv. 2021, 14, e103–e104. [Google Scholar] [CrossRef] [PubMed]
- Maadarani, O.; Bitar, Z.; Elzoueiry, M.; Nader, M.; Abdelfatah, M.; Zaalouk, T.; Mohsen, M.; Elhabibi, M. Myocardial infarction post COVID-19 vaccine—Coincidence, Kounis syndrome or other explanation—Time will tell. JRSM Open 2021, 12, 205427042110252. [Google Scholar] [CrossRef] [PubMed]
- Boivin, Z.; Martin, J. Untimely Myocardial Infarction or COVID-19 Vaccine Side Effect. Cureus 2021, 13, e13651. [Google Scholar] [CrossRef]
- Amin, H.Z.; Amin, L.Z.; Pradipta, A. Takotsubo Cardiomyopathy: A Brief Review. J. Med. Life 2020, 13, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Koniari, I.; Tzanis, G.; Tsigkas, G.; Soufras, G.; Hahalis, G.; Kounis, N. Attacking the ATAK Complex in Cardiac Anesthesia. J. Cardiothorac. Vasc. Anesth. 2017, 31, e89–e91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazlollahi, A.; Zahmatyar, M.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Shekarriz-Foumani, R.; Kolahi, A.A.; Singh, K.; Safiri, S. Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series. Rev. Med. Virol. 2021, 32, e2318. [Google Scholar] [CrossRef] [PubMed]
- Berto, M.B.; Spano, G.; Wagner, B.; Bernhard, B.; Häner, J.; Huber, A.T.; Gräni, C. Takotsubo cardiomyopathy after mRNA COVID-19 vaccination. Heart Lung Circ. 2021, 30, e119–e120. [Google Scholar] [CrossRef] [PubMed]
- Vidula, M.K.; Ambrose, M.; Glassberg, H.; Chokshi, N.; Chen, T.; Ferrari, V.A.; Han, Y. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus 2021, 13, e15576. [Google Scholar] [CrossRef]
- Fearon, C.; Parwani, P.; Gow-Lee, B.; Abramov, D. Takotsubo syndrome after receiving the COVID-19 vaccine. J. Cardiol. Cases 2021, 24, 223–226. [Google Scholar] [CrossRef]
- Jani, C.; Leavitt, J.; Al Omari, O.; Dimaso, A.; Pond, K.; Gannon, S.; Chandran, A.K.; Dennis, C.; Colgrove, R. COVID-19 Vaccine-Associated Takotsubo Cardiomyopathy. Am. J. Ther. 2021, 28, 361–364. [Google Scholar] [CrossRef]
- Lee, E.; Chew, N.W.; Ng, P.; Yeo, T.J. A spectrum of cardiac manifestations post Pfizer-BioNTech COVID-19 vaccination. QJM 2021, 114, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Camilli, M.; Ianni, U.; Tavecchia, G.; Palazzini, M.; Cartella, I.; Gentile, P.; Quattrocchi, G.; Maria Spanò, F.; Cipriani, M.; et al. Takotsubo syndrome after BNT162b2 mRNA COVID-19 vaccine: Emotional or causative relationship with vaccination? Int. J. Cardiol. Heart Vasc. 2022, 40, 101002. [Google Scholar] [CrossRef] [PubMed]
- Khalid Ahmed, S.; Gamal Mohamed, M.; Abdulrahman Essa, R.; Abdelaziz Ahmed Rashad Dabou, E.; Omar Abdulqadir, S.; Muhammad Omar, R. Global reports of takotsubo (stress) cardiomyopathy following COVID-19 vaccination: A systematic review and meta-analysis. IJC Heart Vasc. 2022, 43, 101108. [Google Scholar] [CrossRef]
- Fialho, I.; Mateus, C.; Martins-dos-Santos, G.; Pita, J.; Cabanelas, N.; Baptista, S.B.; Roque, D. Recurrent Kounis syndrome—A life-threatening event after COVID-19 vaccine administration. J. Cardiol. Cases 2022, 25, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Şancı, E.; Örçen, C.; Çelik, O.M.; Özen, M.T.; Bozyel, S. Kounis syndrome associated with BNT162b2 mRNA COVID-19 vaccine presenting as ST-elevation acute myocardial infarction. Anatol. J. Cardiol. 2022, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Margonato, D.; Abete, R.; Di Giovine, G.; Delfino, P.; Grillo, M.; Mazzetti, S.; Poggio, D.; Rossi, J.; Khouri, T.; Mortara, A. Takotsubo cardiomyopathy associated with Kounis syndrome: A clinical case of the “ATAK complex”. J. Cardiol. Cases 2019, 20, 52–56. [Google Scholar] [CrossRef]
- Badami, K.G. Transfusion double whammy? Adrenaline-takotsubo-anaphylaxis-Kounis complex post transfusion? Vox Sang. 2022, 117, 862–865. [Google Scholar] [CrossRef]
- Mustehsan, M.H.; Jahufar, F.; Arora, S. A Diagnostically Challenging Infusion Reaction-Kounis, Takotsubo, or the ATAK! JAMA Intern. Med. 2019, 179, 99–100. [Google Scholar] [CrossRef]
- Gicquel-Schlemmer, B.; Beller, J.-P.; Mchalwat, A.; Gicquel, P. Fatal Takotsubo cardiomyopathy due to epinephrine in shoulder arthroscopy. Orthop. Traumatol. Surg. Res. 2015, 101, 981–982. [Google Scholar] [CrossRef] [Green Version]
- Soufras, G.D.; Kounis, N.G. Adrenaline administration for anaphylaxis and the risk of takotsubo and Kounis syndrome. Int. J. Cardiol. 2013, 166, 281–282. [Google Scholar] [CrossRef]
| Reference | Age | Sex | Diagnosis | mRNA Vaccine Dose | Time from Vaccination to Symptom | Symptoms | Cardiac Test | Treatment |
|---|---|---|---|---|---|---|---|---|
| Year | ||||||||
| Authors | ||||||||
| [39] | 63 Y | female | Takotsubo cardiomyopathy | 1 | 1 day | Fever and dyspnea. | ECG: Negative T waves over the inferior/anterior leads. Angiography: normal. | N/A |
| 2021 | ||||||||
| Berto et al. | ||||||||
| [40] | 60 Y | female | Takotsubo cardiomyopathy | 2 | 4 days | Chest pain. | ECG: Inferolateral T wave inversions. Angiography: normal. | Metoprolol and Lisinopril |
| 2021 | ||||||||
| Vidula et al | ||||||||
| [41] | 73 Y | male | Takotsubo cardiomyopathy | 2 | 17 h | Dyspnea, fatigue, chest pain, shortness of breath, and orthopnea. | ECG: ST changes in inferolateral leads, poor anterior R wave progression. Angiograph: normal. | Furosemide IV diuresis, metoprolol, and Losartan |
| 2021 | ||||||||
| Fearon et al. | ||||||||
| [42] | 65 Y | female | Takotsubo cardiomyopathy | 1 | 1 day | Chest pain, myalgia, nausea, and headache. | ECG: abnormal | Aspirin, atorvastatin, lisinopril, and metoprolol succinate |
| 2021 | ||||||||
| Jani et al. | ||||||||
| [43] | 44 Y | female | Takotsubo cardiomyopathy | 1 | 15 min | Chest pain palpitation. | ECG: ST elevations in the inferolateral leads. Angiograph: normal. | Conservative treatment |
| 2021 | ||||||||
| Lee et al. | ||||||||
| [44] | 71 Y | female | Takotsubo cardiomyopathy | 1 | 5 h | Chest pain and shortness of breath. | ECG: abnormal. | N/A |
| 2022 | ||||||||
| Tedeschi et al. | ||||||||
| [31] | 64 Y | male | Kounis III | 1 | immediately | Chills, chest pain, pallor, diaphoresis, and hypotension. | ECG: ST segment elevation in the anteroseptal precordial leads. Angiography: stent thrombosis in the proximal segment of the left anterior descending artery and TIMI grade 0 flow. | N/A |
| 2022 | ||||||||
| Chadi Allam et al. | ||||||||
| [46] | 59 Y | male | Kounis III | 1 | 20 min | Precordial pain, sweat, and discrete micropapular rash on chest. No exanthema, pruritus, dyspnea, wheezing, diarrhea, or abdominal pain. | ECG: showed sinus rhythm, pathological Q waves and T wave inversion in V2–V5 leads and ST segment elevation in II, III, and aVF leads. An ST elevation myocardial infarction (STEMI) was admitted. Angiography: evidence of stent thrombosis of right coronary artery. | Clopidogrel and Rivaroxaban |
| 2022 | ||||||||
| Fihalo et al. | ||||||||
| [32] | 41 Y | female | Kounis I | 1 (CoronaVac) | 15 min | Flushing, palpitation, lip and tongue swelling, shortness of breath, and chest pain. | ECG: poor R wave progression in precordial leads, V4–6 T wave inversion, and fragmented QRS in aVL. Angiography: no sign of coronary atherosclerosis. | Aspirin, oral antihistamines, diltiazem, and corticosteroid |
| 2021 | ||||||||
| Ozdemir et al. | ||||||||
| [47] | 22 Y | female | Kounis I | 1 | 15 min | On admission, vital signs were stable besides a mild tachycardia; during follow-up, the patient had increased complaints including shortness of breath and chest pain. | ECG: ST segment elevations in the inferior and anterior derivations (D2, D3, avF, and V3–6). Angiography: no abnormalities. | Acetyl salicylic acid (300 mg), pheniramine maleate (45.5 Mg), and dexamethasone (8 mg) |
| 2022 | ||||||||
| Şancı E. et al |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minciullo, P.L.; Amato, G.; Vita, F.; Pioggia, G.; Gangemi, S. ATAK Complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 Vaccination and Review of the Literature. Vaccines 2023, 11, 322. https://doi.org/10.3390/vaccines11020322
Minciullo PL, Amato G, Vita F, Pioggia G, Gangemi S. ATAK Complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 Vaccination and Review of the Literature. Vaccines. 2023; 11(2):322. https://doi.org/10.3390/vaccines11020322
Chicago/Turabian StyleMinciullo, Paola Lucia, Giuliana Amato, Federica Vita, Giovanni Pioggia, and Sebastiano Gangemi. 2023. "ATAK Complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 Vaccination and Review of the Literature" Vaccines 11, no. 2: 322. https://doi.org/10.3390/vaccines11020322
APA StyleMinciullo, P. L., Amato, G., Vita, F., Pioggia, G., & Gangemi, S. (2023). ATAK Complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 Vaccination and Review of the Literature. Vaccines, 11(2), 322. https://doi.org/10.3390/vaccines11020322






