A Head-to-Head Comparative Study of the Replication-Competent Vaccinia Virus and AAV1-Based Malaria Vaccine versus RTS,S/AS01 in Murine Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasites and Animals
2.2. Vaccines
2.3. Immunization
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Parasite Challenge Test
2.6. Transgenic Sporozoite Neutralization Assay
2.7. In Vivo Bioluminescence Imaging System
2.8. Histopathology and Immunohistochemistry
2.9. TB Assay
2.10. Statistical Analysis
3. Results
3.1. Schematic Representation of m8∆/AAV1 and RTS,S Vaccines
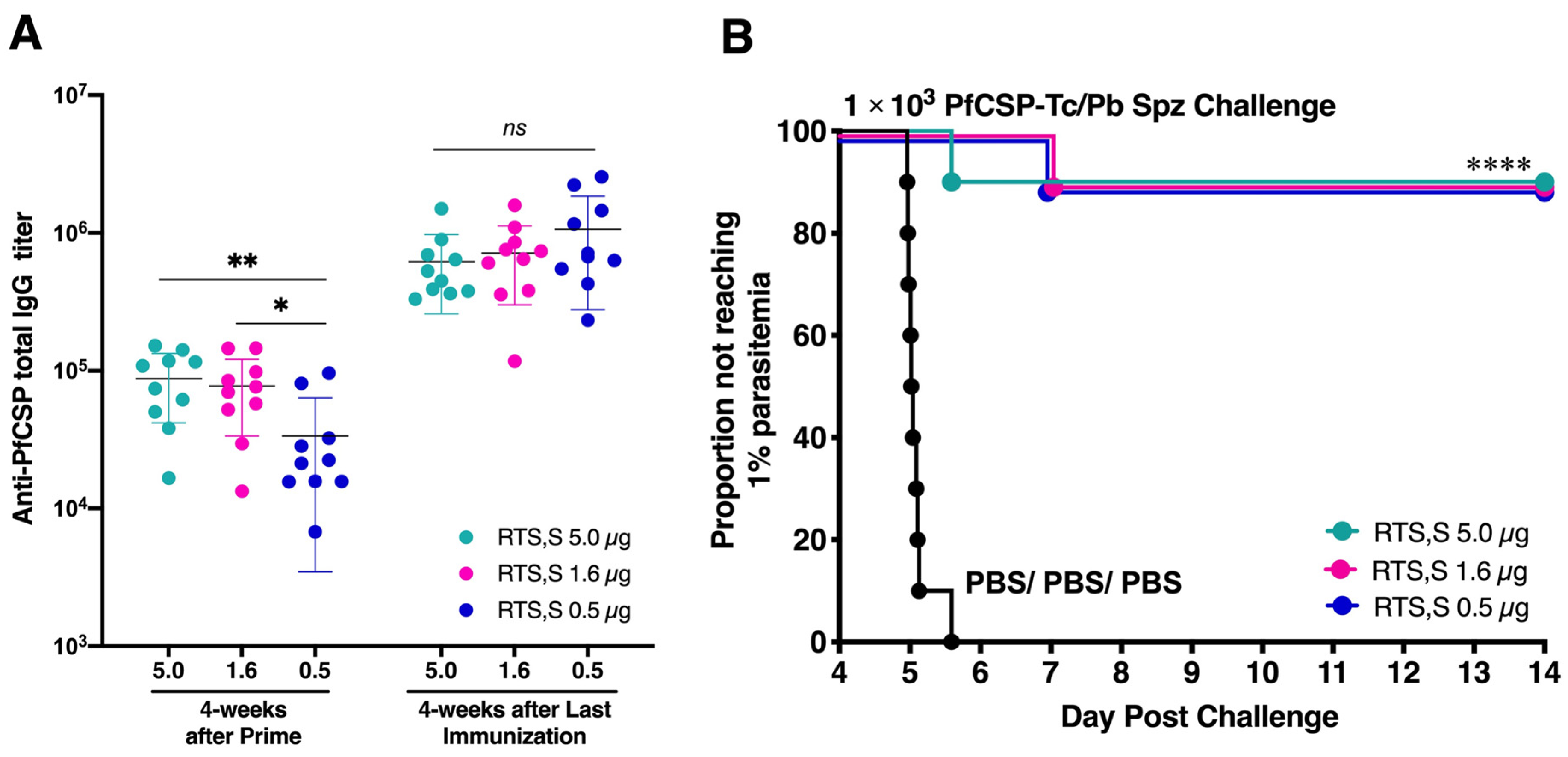
3.2. Low-Dose RTS,S Elicits Robust Humoral Immune Response and Sterile Protection
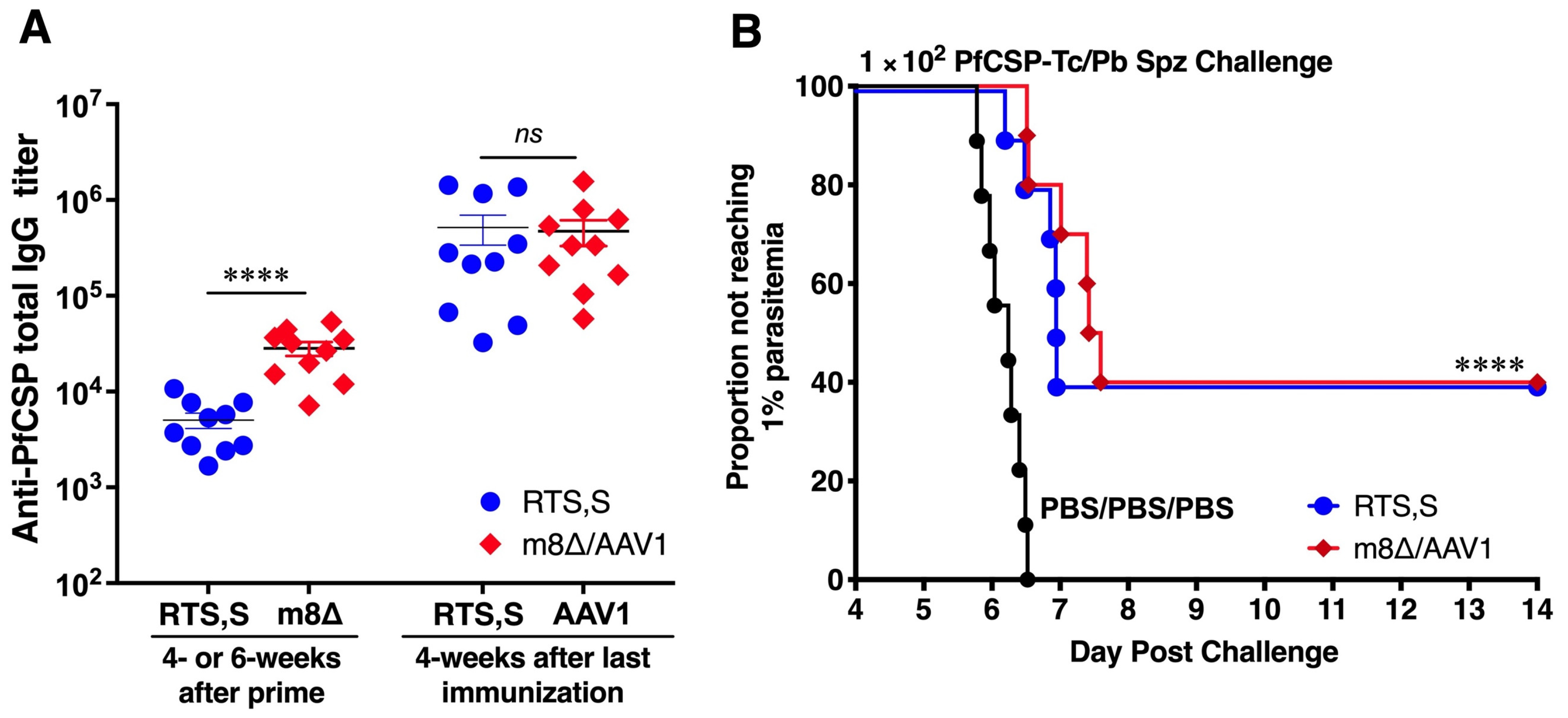
3.3. Long-Lasting Humoral Responses and Sterile Protection
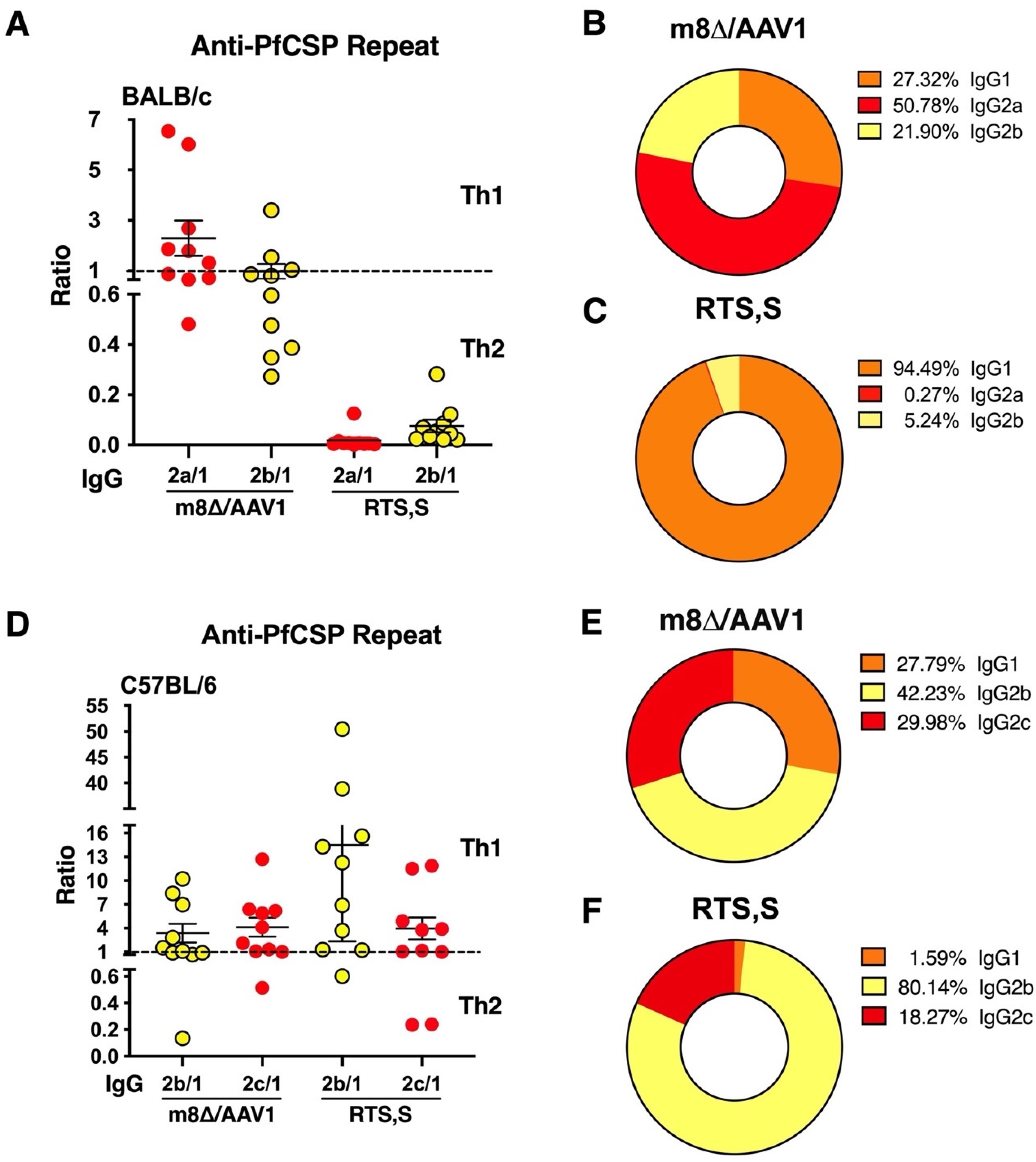
3.4. A Broad Humoral Immune Response across Truncated PfCSP
3.5. IgG Subclass Distribution with m8∆/AAV1 Vaccine
3.6. Vaccine-Induced Immune Sera Neutralize Sporozoite Invasion of Liver Cells
3.7. Vaccines Provide Moderate Protection in C57BL/6 Mice
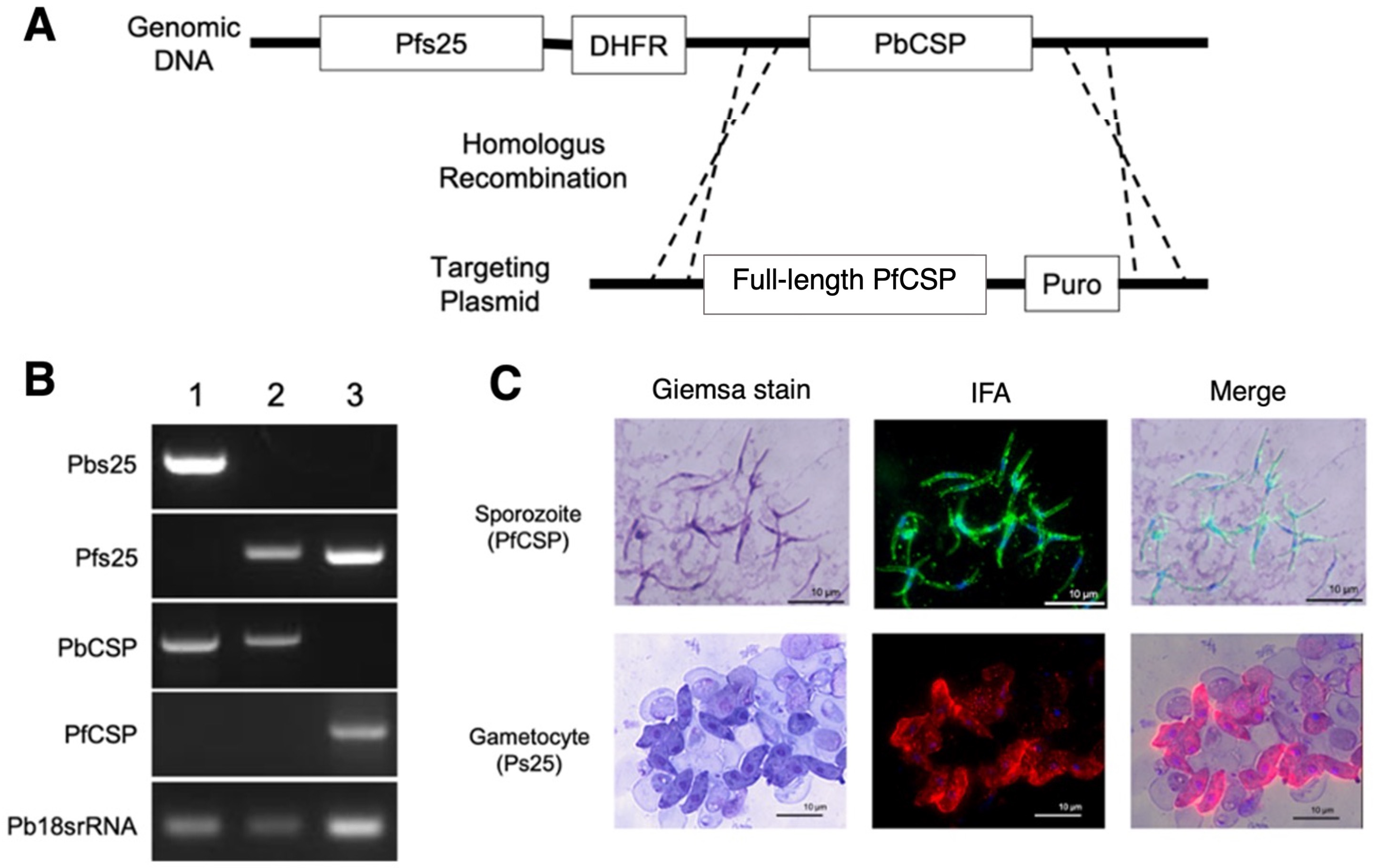
3.8. Double-Transgenic Pfs25-PfCSP/Pb Parasite Construction
3.9. M8∆/AAV1 Vaccine Matches RTS,S’s Efficacy in Preventing Cerebral Malaria
3.10. TB Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| Ab | Antibody |
| BSA | Bovine Serum Albumin |
| CSP | Circumsporozoite protein |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DFA | Direct-feeding assay |
| ELISA | Enzyme-linked immunosorbent assay |
| ICAM-1 | Intracellular Adhesion Molecule-1 |
| IFAs | Immunofluorescence Assays |
| IgG | Immunoglobulin G |
| IgG1 | Immunoglobulin G1 |
| IgG2a | Immunoglubulin G2a |
| IgG2b | Immunoglobulin G2b |
| i.m. | intramuscular |
| i.p. | intraperitoneal |
| iRBCs | infected Red Blood Cells |
| i.v. | intravenous |
| IVIS | in vivo imaging system |
| GSK | Glaxo Smith Kline |
| LC16m8 | Japanese replication competent Vaccinia virus strain used for smallpox vaccination |
| LC16m8Δ | Highly attenuated and genetically stable variant of LC16m8 |
| m8Δ | Simple abbreviations of LC16m8Δ |
| mAb | monoclonal antibody |
| MPL | monophosphoryl lipid |
| Pb | Plasmodium berghei |
| PBS | Phosphate Buffer Saline |
| PBST | Phosphate Buffer Saline containing 0.1% tween-20 |
| PCR | Polymerase Chain Reaction |
| Pf | Plasmodium falciparum |
| PfCSP | Plasmodium falciparum circumsporozoite |
| PfCSP-Tc/Pb | Transgenic P. berghei ANKA parasite line that express P. falciparum CSP under the control of the P. berghei CSP promoter |
| Pfs25 | P. falciparum P25 protein expressed on the surface of zygote and ookinete forms of malaria parasites |
| Pfs25-PfCSP/Pb | Double-transgenic P. berghei parasites that carried the Pfs25-PfCSP fusion gene |
| Pfs25DR3 | Transgenic P. berghei ANKA parasite line that express P. falciparum P25 antigen |
| PFU | plaque-forming unit |
| RPMI | Roswell Park Memorial Institute medium used in cell culture and tissue culture |
| R.T. | Room Temperature |
| s.c. | scarification |
| SD | Standard Deviation |
| SEM | Standard Error Mean |
| Spz | Sporozoite |
| TB | Transmission-blocking |
| TBA | Transmission-blocking activity |
| TBV | Transmission-blocking Vaccine |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| TRA | Transmission-reducing Activity |
| TSNA | Transgenic Sporozoite Neutralization Assay |
| t.s. | tail scarification |
| vg | viral genome |
| VV | Vaccinia Virus |
References
- Jamison, D.T.; Feachem, R.G.; Makgoba, M.W.; Bos, E.R.; Baingana, F.K.; Hofman, K.J.; Rogo, K.O. Disease and Mortality in Sub-Saharan Africa, 2nd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023; pp. xviii–xix. [Google Scholar]
- El-Moamly, A.A.; El-Sweify, M.A. Malaria vaccines: The 60-year journey of hope and final success-lessons learned and future prospects. Trop. Med. Health 2023, 51, 29. [Google Scholar] [CrossRef]
- Rts, S.C.T.P.; Agnandji, S.T.; Lell, B.; Soulanoudjingar, S.S.; Fernandes, J.F.; Abossolo, B.P.; Conzelmann, C.; Methogo, B.G.; Doucka, Y.; Flamen, A.; et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011, 365, 1863–1875. [Google Scholar] [CrossRef]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Leroux-Roels, I.; Clement, F.; Ofori-Anyinam, O.; Lievens, M.; Jongert, E.; Moris, P.; Ballou, W.R.; Cohen, J. Evaluation of the immune response to RTS,S/AS01 and RTS,S/AS02 adjuvanted vaccines: Randomized, double-blind study in malaria-naive adults. Hum. Vaccin. Immunother. 2014, 10, 2211–2219. [Google Scholar] [CrossRef]
- Tinto, H.; D’Alessandro, U.; Sorgho, H.; Valea, I.; Tahita, M.C.; Kabore, W.; Kiemde, F.; Lompo, P.; Ouedraogo, S.; Derra, K.; et al. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef]
- Collins, K.A.; Brod, F.; Snaith, R.; Ulaszewska, M.; Longley, R.J.; Salman, A.M.; Gilbert, S.C.; Spencer, A.J.; Franco, D.; Ballou, W.R.; et al. Ultra-low dose immunization and multi-component vaccination strategies enhance protection against malaria in mice. Sci. Rep. 2021, 11, 10792. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Aly, A.S.; Kappe, S.H. Malaria parasite pre-erythrocytic stage infection: Gliding and hiding. Cell Host Microbe 2008, 4, 209–218. [Google Scholar] [CrossRef]
- Ewer, K.J.; Sierra-Davidson, K.; Salman, A.M.; Illingworth, J.J.; Draper, S.J.; Biswas, S.; Hill, A.V. Progress with viral vectored malaria vaccines: A multi-stage approach involving “unnatural immunity”. Vaccine 2015, 33, 7444–7451. [Google Scholar] [CrossRef]
- Duffy, P.E.; Sahu, T.; Akue, A.; Milman, N.; Anderson, C. Pre-erythrocytic malaria vaccines: Identifying the targets. Expert Rev. Vaccines 2012, 11, 1261–1280. [Google Scholar] [CrossRef]
- Tran, T.M.; Portugal, S.; Draper, S.J.; Crompton, P.D. Malaria Vaccines: Moving Forward After Encouraging First Steps. Curr Trop. Med. Rep. 2015, 2, 1–3. [Google Scholar] [CrossRef]
- Molina-Franky, J.; Cuy-Chaparro, L.; Camargo, A.; Reyes, C.; Gomez, M.; Salamanca, D.R.; Patarroyo, M.A.; Patarroyo, M.E. Plasmodium falciparum pre-erythrocytic stage vaccine development. Malar. J. 2020, 19, 56. [Google Scholar] [CrossRef]
- Yusuf, Y.; Yoshii, T.; Iyori, M.; Mizukami, H.; Fukumoto, S.; Yamamoto, D.S.; Emran, T.B.; Amelia, F.; Islam, A.; Syafira, I.; et al. A Viral-Vectored Multi-Stage Malaria Vaccine Regimen with Protective and Transmission-Blocking Efficacies. Front. Immunol. 2019, 10, 2412. [Google Scholar] [CrossRef]
- Miura, K. Progress and prospects for blood-stage malaria vaccines. Expert Rev. Vaccines 2016, 15, 765–781. [Google Scholar] [CrossRef]
- Birkett, A.J. Status of vaccine research and development of vaccines for malaria. Vaccine 2016, 34, 2915–2920. [Google Scholar] [CrossRef]
- Churcher, T.S.; Blagborough, A.M.; Delves, M.; Ramakrishnan, C.; Kapulu, M.C.; Williams, A.R.; Biswas, S.; Da, D.F.; Cohuet, A.; Sinden, R.E. Measuring the blockade of malaria transmission--an analysis of the Standard Membrane Feeding Assay. Int. J. Parasitol. 2012, 42, 1037–1044. [Google Scholar] [CrossRef]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef]
- Richie, T.L.; Saul, A. Progress and challenges for malaria vaccines. Nature 2002, 415, 694–701. [Google Scholar] [CrossRef]
- Alonso, P.L.; Tanner, M. Public health challenges and prospects for malaria control and elimination. Nat. Med. 2013, 19, 150–155. [Google Scholar] [CrossRef]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Bliss, C.M.; Drammeh, A.; Bowyer, G.; Sanou, G.S.; Jagne, Y.J.; Ouedraogo, O.; Edwards, N.J.; Tarama, C.; Ouedraogo, N.; Ouedraogo, M.; et al. Viral Vector Malaria Vaccines Induce High-Level T Cell and Antibody Responses in West African Children and Infants. Mol. Ther. 2017, 25, 547–559. [Google Scholar] [CrossRef]
- Iyori, M.; Blagborough, A.M.; Mizuno, T.; Abe, Y.I.; Nagaoka, M.; Hori, N.; Yamagoshi, I.; Da, D.F.; Gregory, W.F.; Hasyim, A.A.; et al. Sterile protection and transmission blockade by a multistage anti-malarial vaccine in the pre-clinical study. Front. Immunol. 2022, 13, 1005476. [Google Scholar] [CrossRef]
- Hasyim, A.A.; Iyori, M.; Mizuno, T.; Abe, Y.I.; Yamagoshi, I.; Yusuf, Y.; Syafira, I.; Sakamoto, A.; Yamamoto, Y.; Mizukami, H.; et al. Adeno-associated virus-based malaria booster vaccine following attenuated replication-competent vaccinia virus LC16m8Delta priming. Parasitol. Int. 2023, 92, 102652. [Google Scholar] [CrossRef]
- Iyori, M.; Blagborough, A.M.; Sala, K.A.; Nishiura, H.; Takagi, K.; Yoshida, S. Protective efficacy of an IL-12-expressing baculoviral malaria vaccine. Parasite Immunol. 2017, 39, e12498. [Google Scholar] [CrossRef]
- Yusuf, Y.; Yoshii, T.; Iyori, M.; Yoshida, K.; Mizukami, H.; Fukumoto, S.; Yamamoto, D.S.; Alam, A.; Emran, T.B.; Amelia, F.; et al. Adeno-Associated Virus as an Effective Malaria Booster Vaccine Following Adenovirus Priming. Front. Immunol. 2019, 10, 730. [Google Scholar] [CrossRef]
- Goodman, A.L.; Blagborough, A.M.; Biswas, S.; Wu, Y.; Hill, A.V.; Sinden, R.E.; Draper, S.J. A viral vectored prime-boost immunization regime targeting the malaria Pfs25 antigen induces transmission-blocking activity. PLoS ONE 2011, 6, e29428. [Google Scholar] [CrossRef]
- Sumitani, M.; Kasashima, K.; Yamamoto, D.S.; Yagi, K.; Yuda, M.; Matsuoka, H.; Yoshida, S. Reduction of malaria transmission by transgenic mosquitoes expressing an antisporozoite antibody in their salivary glands. Insect Mol. Biol. 2013, 22, 41–51. [Google Scholar] [CrossRef]
- Iyori, M.; Nakaya, H.; Inagaki, K.; Pichyangkul, S.; Yamamoto, D.S.; Kawasaki, M.; Kwak, K.; Mizukoshi, M.; Goto, Y.; Matsuoka, H.; et al. Protective efficacy of baculovirus dual expression system vaccine expressing Plasmodium falciparum circumsporozoite protein. PLoS ONE 2013, 8, e70819. [Google Scholar] [CrossRef]
- Mizutani, M.; Fukumoto, S.; Soubeiga, A.P.; Soga, A.; Iyori, M.; Yoshida, S. Development of a Plasmodium berghei transgenic parasite expressing the full-length Plasmodium vivax circumsporozoite VK247 protein for testing vaccine efficacy in a murine model. Malar. J. 2016, 15, 251. [Google Scholar] [CrossRef]
- Yoshida, K.; Iyori, M.; Blagborough, A.M.; Salman, A.M.; Dulal, P.; Sala, K.A.; Yamamoto, D.S.; Khan, S.M.; Janse, C.J.; Biswas, S.; et al. Adenovirus-prime and baculovirus-boost heterologous immunization achieves sterile protection against malaria sporozoite challenge in a murine model. Sci. Rep. 2018, 8, 3896. [Google Scholar] [CrossRef]
- Olufadewa, I.; Akinrinde, D.; Adesina, M.; Oladele, R.; Ayorinde, T.; Omo-Sowho, U. The approval of the first malaria vaccine: The beginning of the end of the malaria epidemic. J. Glob. Health 2022, 12, 03087. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Locke, E.; Flores-Garcia, Y.; Mayer, B.T.; MacGill, R.S.; Borate, B.; Salgado-Jimenez, B.; Gerber, M.W.; Mathis-Torres, S.; Shapiro, S.; King, C.R.; et al. Establishing RTS,S/AS01 as a benchmark for comparison to next-generation malaria vaccines in a mouse model. NPJ Vaccines 2024, 9, 29. [Google Scholar] [CrossRef]
- Amelia, F.; Iyori, M.; Emran, T.B.; Yamamoto, D.S.; Genshi, K.; Otsuka, H.; Onoue, Y.; Yusuf, Y.; Islam, A.; Yoshida, S. Down-selecting circumsporozoite protein-based malaria vaccine: A comparison of malaria sporozoite challenge model. Parasite Immunol. 2019, 41, e12624. [Google Scholar] [CrossRef]
- Miura, K.; Takashima, E.; Deng, B.; Tullo, G.; Diouf, A.; Moretz, S.E.; Nikolaeva, D.; Diakite, M.; Fairhurst, R.M.; Fay, M.P.; et al. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect. Immun. 2013, 81, 4377–4382. [Google Scholar] [CrossRef]
- Emran, T.B.; Iyori, M.; Ono, Y.; Amelia, F.; Yusuf, Y.; Islam, A.; Alam, A.; Tamura, M.; Ogawa, R.; Matsuoka, H.; et al. Baculovirus-Induced Fast-Acting Innate Immunity Kills Liver-Stage Plasmodium. J. Immunol. 2018, 201, 2441–2451. [Google Scholar] [CrossRef]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim, B.K.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M.; et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef]
- Iyori, M.; Yamamoto, D.S.; Sakaguchi, M.; Mizutani, M.; Ogata, S.; Nishiura, H.; Tamura, T.; Matsuoka, H.; Yoshida, S. DAF-shielded baculovirus-vectored vaccine enhances protection against malaria sporozoite challenge in mice. Malar. J. 2017, 16, 390. [Google Scholar] [CrossRef]
- Miura, K.; Swihart, B.J.; Deng, B.; Zhou, L.; Pham, T.P.; Diouf, A.; Burton, T.; Fay, M.P.; Long, C.A. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine 2016, 34, 4145–4151. [Google Scholar] [CrossRef]
- Crompton, P.D.; Pierce, S.K.; Miller, L.H. Advances and challenges in malaria vaccine development. J. Clin. Investig. 2010, 120, 4168–4178. [Google Scholar] [CrossRef]
- Beutler, N.; Pholcharee, T.; Oyen, D.; Flores-Garcia, Y.; MacGill, R.S.; Garcia, E.; Calla, J.; Parren, M.; Yang, L.; Volkmuth, W.; et al. A novel CSP C-terminal epitope targeted by an antibody with protective activity against Plasmodium falciparum. PLoS Pathog. 2022, 18, e1010409. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; MacGill, R.S.; Early, A.M.; Bolton, J.S.; King, C.R.; Locke, E.; Pierson, T.; Wirth, D.F.; Neafsey, D.E.; Bergmann-Leitner, E.S. Breadth of humoral immune responses to the C-terminus of the circumsporozoite protein is associated with protective efficacy induced by the RTS,S malaria vaccine. Vaccine 2021, 39, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Spaccapelo, R.; Bistoni, F.; Holder, A.A.; Crisanti, A. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J. Biol. Chem. 2002, 277, 47613–47618. [Google Scholar] [CrossRef]
- Graves, P.; Gelband, H. Vaccines for preventing malaria (blood-stage). Cochrane Database Syst. Rev. 2006, 2006, CD006199. [Google Scholar] [CrossRef]
- Ademolue, T.W.; Awandare, G.A. Evaluating antidisease immunity to malaria and implications for vaccine design. Immunology 2018, 153, 423–434. [Google Scholar] [CrossRef]
- Long, C.A.; Zavala, F. Immune Responses in Malaria. Cold Spring Harb. Perspect. Med. 2017, 7, a025577. [Google Scholar] [CrossRef]
- Huang, H.Y.; Liang, X.Y.; Lin, L.Y.; Chen, J.T.; Ehapo, C.S.; Eyi, U.M.; Li, J.; Jiang, T.T.; Zheng, Y.Z.; Zha, G.C.; et al. Genetic polymorphism of Plasmodium falciparum circumsporozoite protein on Bioko Island, Equatorial Guinea and global comparative analysis. Malar. J. 2020, 19, 245. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Ali Albsheer, M.M.; Abdelbagi, H.; Siddig, E.E.; Mohamed, M.A.; Ahmed, A.E.; Omer, R.A.; Muneer, M.S.; Ahmed, A.; Osman, H.A.; et al. Genetic polymorphism of the N-terminal region in circumsporozoite surface protein of Plasmodium falciparum field isolates from Sudan. Malar. J. 2019, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.A.; Gutierrez, G.M.; Rojas-Lopez, M.; Noe, A.R.; Shi, L.; Tse, S.W.; Sinnis, P.; Zavala, F. Proteolytic Cleavage of the Plasmodium falciparum Circumsporozoite Protein Is a Target of Protective Antibodies. J. Infect. Dis. 2015, 212, 1111–1119. [Google Scholar] [CrossRef]
- Yoshida, S.; Kawasaki, M.; Hariguchi, N.; Hirota, K.; Matsumoto, M. A baculovirus dual expression system-based malaria vaccine induces strong protection against Plasmodium berghei sporozoite challenge in mice. Infect. Immun. 2009, 77, 1782–1789. [Google Scholar] [CrossRef]
- Wu, X.; Brombacher, F.; Chroneos, Z.C.; Norbury, C.C.; Gowda, D.C. IL-4Ralpha signaling by CD8alpha(+) dendritic cells contributes to cerebral malaria by enhancing inflammatory, Th1, and cytotoxic CD8(+) T cell responses. J. Biol. Chem. 2021, 296, 100615. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Waris, A.; Khan, M.A.; Asim, M.; Khan, A.U.; Khan, S.; Zeb, J. Recent advancement, immune responses, and mechanism of action of various vaccines against intracellular bacterial infections. Life Sci. 2023, 314, 121332. [Google Scholar] [CrossRef] [PubMed]
- Dobano, C.; Santano, R.; Vidal, M.; Jimenez, A.; Jairoce, C.; Ubillos, I.; Dosoo, D.; Aguilar, R.; Williams, N.A.; Diez-Padrisa, N.; et al. Differential Patterns of IgG Subclass Responses to Plasmodium falciparum Antigens in Relation to Malaria Protection and RTS,S Vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef]
- Ssewanyana, I.; Rek, J.; Rodriguez, I.; Wu, L.; Arinaitwe, E.; Nankabirwa, J.I.; Beeson, J.G.; Mayanja-Kizza, H.; Rosenthal, P.J.; Dorsey, G.; et al. Impact of a Rapid Decline in Malaria Transmission on Antimalarial IgG Subclasses and Avidity. Front. Immunol. 2020, 11, 576663. [Google Scholar] [CrossRef]
- Rogers, K.J.; Vijay, R.; Butler, N.S. Anti-malarial humoral immunity: The long and short of it. Microbes Infect. 2021, 23, 104807. [Google Scholar] [CrossRef] [PubMed]
- Kaba, S.A.; Price, A.; Zhou, Z.; Sundaram, V.; Schnake, P.; Goldman, I.F.; Lal, A.A.; Udhayakumar, V.; Todd, C.W. Immune responses of mice with different genetic backgrounds to improved multiepitope, multitarget malaria vaccine candidate antigen FALVAC-1A. Clin. Vaccine Immunol. 2008, 15, 1674–1683. [Google Scholar] [CrossRef]
- Furtado, R.; Paul, M.; Zhang, J.; Sung, J.; Karell, P.; Kim, R.S.; Caillat-Zucman, S.; Liang, L.; Felgner, P.; Bauleni, A.; et al. Cytolytic circumsporozoite-specific memory CD4(+) T cell clones are expanded during Plasmodium falciparum infection. Nat. Commun. 2023, 14, 7726. [Google Scholar] [CrossRef] [PubMed]
- Flores-Garcia, Y.; Herrera, S.M.; Jhun, H.; Perez-Ramos, D.W.; King, C.R.; Locke, E.; Raghunandan, R.; Zavala, F. Optimization of an in vivo model to study immunity to Plasmodium falciparum pre-erythrocytic stages. Malar. J. 2019, 18, 426. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Suzue, K.; Ngo-Thanh, H.; Shimokawa, C.; Hisaeda, H. Potential and Limitations of Cross-Protective Vaccine against Malaria by Blood-Stage Naturally Attenuated Parasite. Vaccines 2020, 8, 375. [Google Scholar] [CrossRef]
- Mura, M.; Ruffie, C.; Combredet, C.; Aliprandini, E.; Formaglio, P.; Chitnis, C.E.; Amino, R.; Tangy, F. Recombinant measles vaccine expressing malaria antigens induces long-term memory and protection in mice. NPJ Vaccines 2019, 4, 12. [Google Scholar] [CrossRef]
- Hadjilaou, A.; Brandi, J.; Riehn, M.; Friese, M.A.; Jacobs, T. Pathogenetic mechanisms and treatment targets in cerebral malaria. Nat. Rev. Neurol. 2023, 19, 688–709. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommends R21/Matrix-M Vaccine for Malaria Prevention in Updated Advice on Immunization; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Beeson, J.G.; Chan, J.A. A step forward for Plasmodium falciparum malaria transmission-blocking vaccines. Lancet Infect. Dis. 2023, 23, 1210–1212. [Google Scholar] [CrossRef] [PubMed]



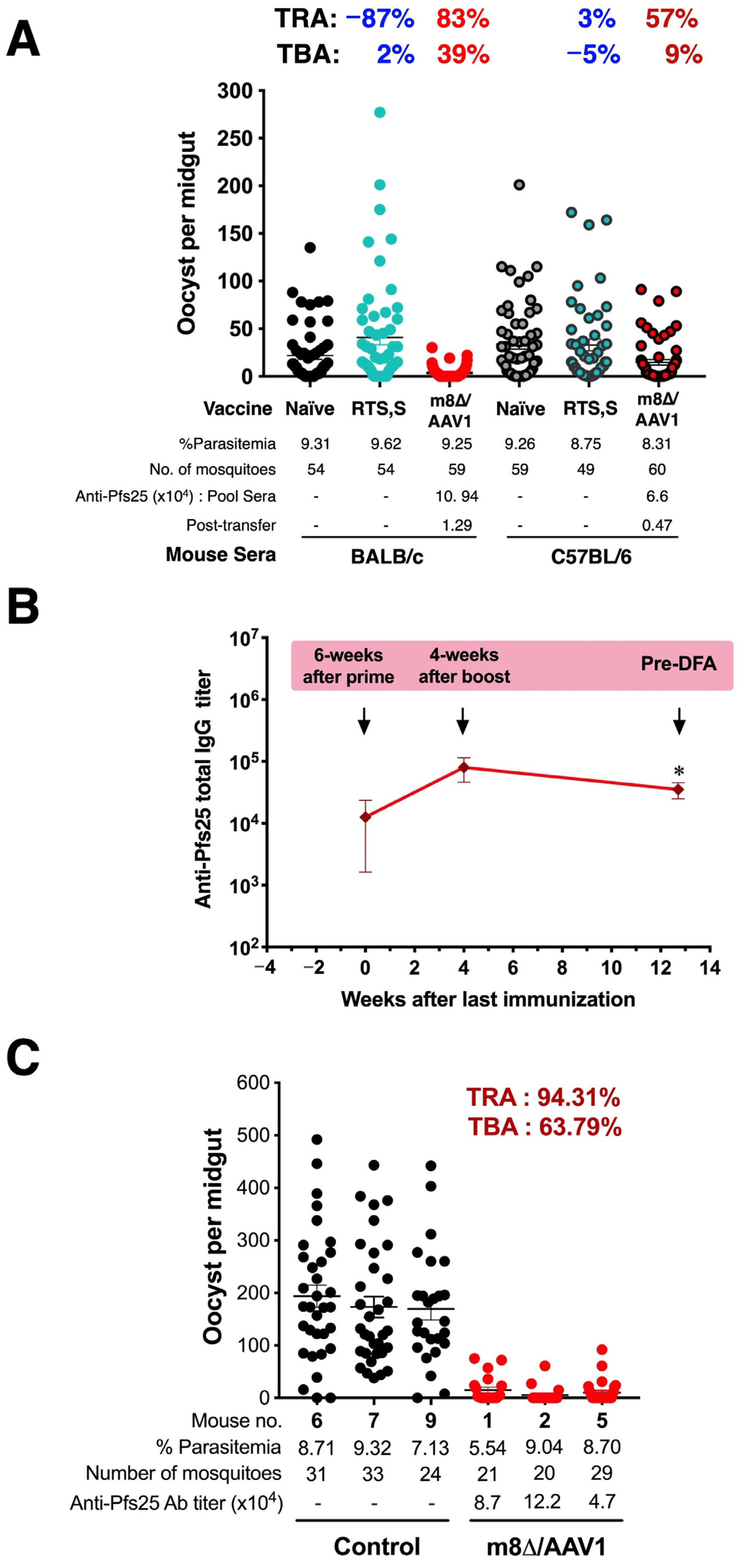
| Group | Vaccine Administration | Challenge | ||
|---|---|---|---|---|
| Day 0 a | Day 28 b | Day 56 c | Day 84 | |
| 1 | PBS | PBS | PBS | 1 × 103 PfCSP-Tc/Pb Sporozoites |
| 2 | RTS,S 5 μg | RTS,S 5 μg | RTS,S 5 μg | |
| 3 | RTS,S 1.6 μg | RTS,S 1.6 μg | RTS,S 1.6 μg | |
| 4 | RTS,S 0.5 μg | RTS,S 0.5 μg | RTS,S 0.5 μg | |
| Mice | Group | Vaccine Administration | Challenge | |||
|---|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 28 | Day 56 | Day 84 | ||
| BALB/c | 1 | PBS | N/A | PBS | PBS | 1 × 103 PfCSP-Tc/Pb Sporozoites |
| 2 | RTS,S 0.5 μg a | N/A | RTS,S 0.5 μg b | RTS,S 0.5 μg c | ||
| 3 | N/A | m8∆ 107 PFU d | N/A | AAV1 1010 vg e | ||
| C57BL/6 | 1 | PBS | N/A | PBS | PBS | 1 × 102 PfCSP-Tc/Pb Sporozoites |
| 2 | RTS,S 0.5 μg a | N/A | RTS,S 0.5 μg b | RTS,S 0.5 μg c | ||
| 3 | N/A | m8∆ 107 PFU d | N/A | AAV1 1010 vg e | ||
| Source of Sera | Group | Donor Serum | Oocysts Intensity (STDEV) | Infected Mosquitoes Prevalence (%) | TRA a,b (%) | TBA c,d (%) |
|---|---|---|---|---|---|---|
| BALB/c | G.1 | Naïve | 21.85 (29.04) | 83.33 | ||
| G.2 | RTS,S | 40.94 (56.64) | 81.48 | −87.37 ns | 2.22 ns | |
| G.3 | m8∆/AAV1 | 3.71 (6.17) | 50.85 | 83.02 **** | 38.98 **** | |
| C57BL/6 | G.4 | Naïve | 33.86 (39.79) | 87.93 | ||
| G.5 | RTS,S | 32.92 (43.15) | 91.84 | 2.78 ns | −4.45 ns | |
| G.6 | m8∆/AAV1 | 14.72 (22.56) | 80.00 | 56.53 ** | 9.02 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainal, K.H.; Hasyim, A.A.; Yamamoto, Y.; Mizuno, T.; Sato, Y.; Rasyid, S.H.; Niikura, M.; Abe, Y.-i.; Iyori, M.; Mizukami, H.; et al. A Head-to-Head Comparative Study of the Replication-Competent Vaccinia Virus and AAV1-Based Malaria Vaccine versus RTS,S/AS01 in Murine Models. Vaccines 2024, 12, 1155. https://doi.org/10.3390/vaccines12101155
Zainal KH, Hasyim AA, Yamamoto Y, Mizuno T, Sato Y, Rasyid SH, Niikura M, Abe Y-i, Iyori M, Mizukami H, et al. A Head-to-Head Comparative Study of the Replication-Competent Vaccinia Virus and AAV1-Based Malaria Vaccine versus RTS,S/AS01 in Murine Models. Vaccines. 2024; 12(10):1155. https://doi.org/10.3390/vaccines12101155
Chicago/Turabian StyleZainal, Kartika Hardianti, Ammar Abdurrahman Hasyim, Yutaro Yamamoto, Tetsushi Mizuno, Yuna Sato, Sani Hadiyan Rasyid, Mamoru Niikura, Yu-ichi Abe, Mitsuhiro Iyori, Hiroaki Mizukami, and et al. 2024. "A Head-to-Head Comparative Study of the Replication-Competent Vaccinia Virus and AAV1-Based Malaria Vaccine versus RTS,S/AS01 in Murine Models" Vaccines 12, no. 10: 1155. https://doi.org/10.3390/vaccines12101155
APA StyleZainal, K. H., Hasyim, A. A., Yamamoto, Y., Mizuno, T., Sato, Y., Rasyid, S. H., Niikura, M., Abe, Y.-i., Iyori, M., Mizukami, H., Shida, H., & Yoshida, S. (2024). A Head-to-Head Comparative Study of the Replication-Competent Vaccinia Virus and AAV1-Based Malaria Vaccine versus RTS,S/AS01 in Murine Models. Vaccines, 12(10), 1155. https://doi.org/10.3390/vaccines12101155







