Exposure to Pollutants and Vaccines’ Effectiveness: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion and Exclusion Criteria
- Population: all people (individuals of all gender, age, ethnicity and health conditions) vaccinated against any vaccine-preventable disease.
- Intervention: exposure to environmental pollutants.
- Control: age-, gender- and condition-matched not vaccinated or vaccinated but differently exposed to pollutant(s).
- Outcomes: effects of exposure to environmental pollutants on vaccine-induced immune response, assessed through vaccine-induced antibody levels.
- Study: observational studies and semi-experimental and experimental studies on humans.
2.3. Risk of Bias Assessment
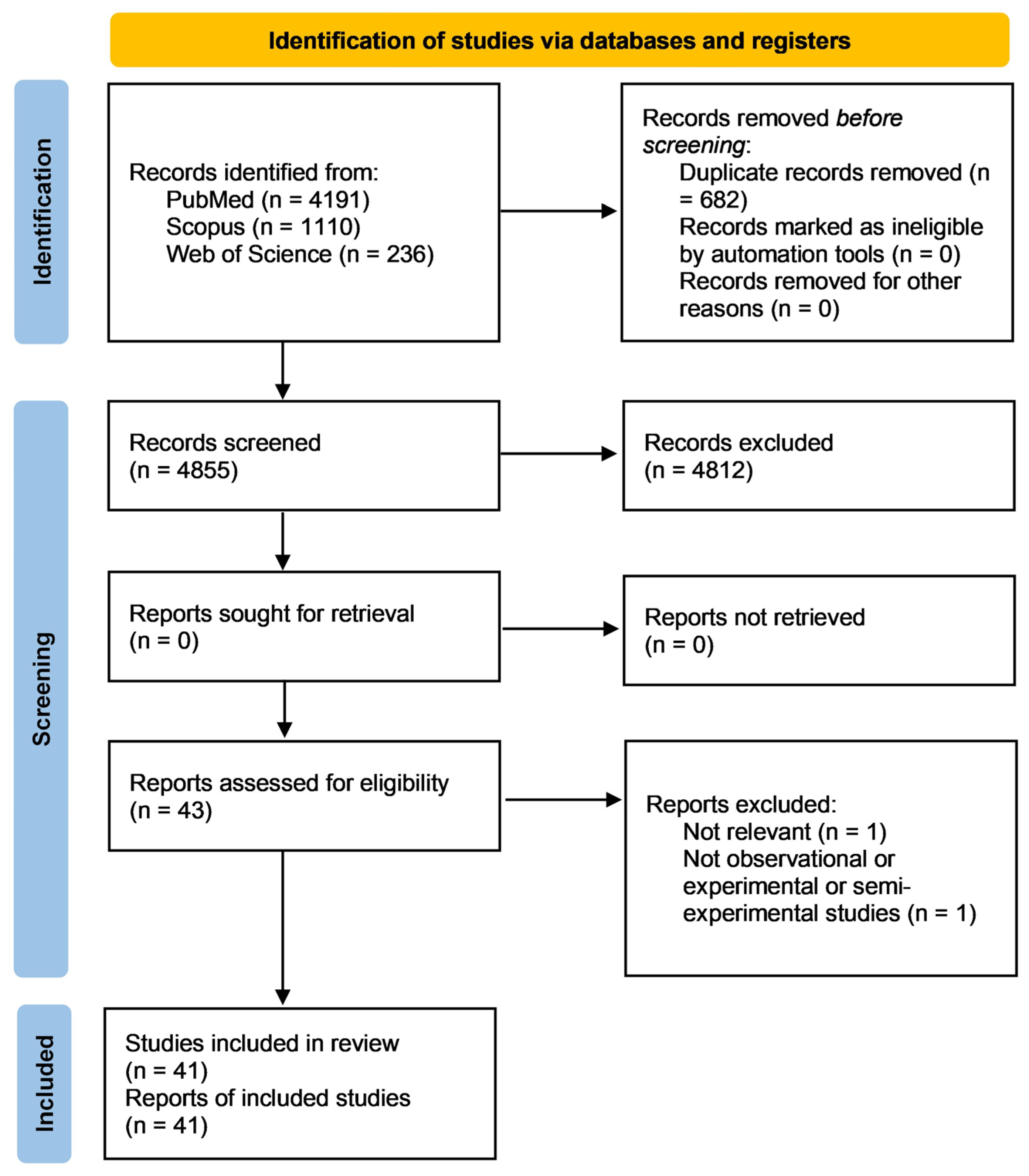
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammershaimb, E.A.D.; Campbell, J.D. Vaccine Development. Pediatr. Clin. N. Am. 2024, 71, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Vidal, R.M.; Velasco, J.; Carreño, L.J.; Torres, J.P.; Benachi, O.M.A.; Tovar-Rosero, Y.Y.; Oñate, A.A.; O’Ryan, M. Two centuries of vaccination: Historical and conceptual approach and future perspectives. Front. Public Health 2024, 11, 1326154. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.C.; Sarsaiya, S.; Gupta, A. A systematic review on the impact of cement industries on the natural environment. Environ. Sci. Pollut. Res. Int. 2022, 29, 18440–18451. [Google Scholar] [CrossRef]
- Kundu, D.; Dutta, D.; Joseph, A.; Jana, A.; Samanta, P.; Bhakta, J.N.; Alreshidi, M.A. Safeguarding drinking water: A brief insight on characteristics, treatments and risk assessment of contamination. Environ. Monit. Assess. 2024, 196, 180. [Google Scholar] [CrossRef]
- Vašíčková, J.; Hvězdová, M.; Kosubová, P.; Hofman, J. Ecological risk assessment of pesticide residues in arable soils of the Czech Republic. Chemosphere 2019, 216, 479–487. [Google Scholar] [CrossRef]
- Fletcher, C.; Ripple, W.J.; Newsome, T.; Barnard, P.; Beamer, K.; Behl, A.; Bowen, J.; Cooney, M.; Crist, E.; Field, C.; et al. Earth at risk: An urgent call to end the age of destruction and forge a just and sustainable future. PNAS Nexus 2024, 3, 106. [Google Scholar] [CrossRef]
- Rojas-Rueda, D.; Morales-Zamora, E.; Alsufyani, W.A.; Herbst, C.H.; AlBalawi, S.M.; Alsukait, R.; Alomran, M. Environmental Risk Factors and Health: An Umbrella Review of Meta-Analyses. Int. J. Environ. Res. Public Health 2021, 18, 704. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, 535–547. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality, Energy and Health, Type of Pollutants. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts/types-of-pollutants (accessed on 6 September 2024).
- Farhadi, Z.; Abulghasem Gorgi, H.; Shabaninejad, H.; Aghajani Delavar, M.; Torani, S. Association between PM2.5 and risk of hospitalization for myocardial infarction: A systematic review and a meta-analysis. BMC Public Health 2020, 20, 314. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Li, T.; Yan, Y.; Xian, H.; Al-Aly, Z. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. J. Am. Soc. Nephrol. 2018, 29, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Debelu, D.; Mengistu, D.A.; Aschalew, A.; Mengistie, B.; Deriba, W. Global Public Health Implications of Traffic Related Air Pollution: Systematic Review. Environ. Health Insights 2024, 18, 11786302241272403. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Memon, A.N.; Sheikh, S.A.; Rouq, F.A.; Usmani, A.M.; Hassan, A.; Arian, S.A. Effect of environmental air pollution on type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 123–128. [Google Scholar]
- Jalili, C.; Kazemi, M.; Taheri, E.; Mohammadi, H.; Boozari, B.; Hadi, A.; Moradi, S. Exposure to heavy metals and the risk of osteopenia or osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1671–1682. [Google Scholar] [CrossRef]
- Keleb, A.; Daba, C.; Asmare, L.; Bayou, F.D.; Arefaynie, M.; Mohammed, A.; Tareke, A.A.; Kebede, N.; Tsega, Y.; Endawkie, A.; et al. The association between children’s exposure to pesticides and asthma, wheezing, and lower respiratory tract infections. A systematic review and meta-analysis. Front. Public Health 2024, 12, 1402908. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustieles, V.; Korevaar, T.I.M.; Martin, L.; Sun, Y.; Bibi, Z.; Torres, N.; Coburn-Sanderson, A.; First, O.; Souter, I.; et al. Association between per- and polyfluoroalkyl substances exposure and thyroid function biomarkers among females attending a fertility clinic. Environ. Pollut. 2024, 346, 123513. [Google Scholar] [CrossRef]
- Arif, I.; Adams, M.D.; Johnson, M.T.J. A meta-analysis of the carcinogenic effects of particulate matter and polycyclic aromatic hydrocarbons. Environ. Pollut. 2024, 351, 123941. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 6 September 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Military Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Weisglas-Kuperus, N.; Sas, T.C.; Koopman-Esseboom, C.; van der Zwan, C.W.; De Ridder, M.A.; Beishuizen, A.; Hooijkaas, H.; Sauer, P.J. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatr. Res. 1995, 38, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.M.; Wilson, T.J.; Ireland, J.; Jones, A.L.; Gorman, J.S.; Gale, N.L.; Johnson, J.C.; Hewett, J.E. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology 1999, 134, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Weisglas-Kuperus, N.; Patandin, S.; Berbers, G.A.; Sas, T.C.; Mulder, P.G.; Sauer, P.J.; Hooijkaas, H. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ. Health Perspect. 2000, 108, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef]
- Steerenberg, P.; van Amelsvoort, L.; Colosio, C.; Corsini, E.; Fustinoni, S.; Vergieva, T.; Zaikov, C.; Pennanen, S.; Liesivuori, J.; Van Loveren, H. Toxicological evaluation of the immune function of pesticide workers, a European wide assessment. Hum. Exp. Toxicol. 2008, 27, 701–707. [Google Scholar] [CrossRef]
- Baranska, M.; Van Amelsvoort, L.; Birindelli, S.; Fustinoni, S.; Corsini, E.; Liesivuori, J.; Van Loveren, H. Association of pesticide exposure, vaccination response, and interleukin-1 gene polymorphisms. Hum. Exp. Toxicol. 2008, 27, 709–713. [Google Scholar] [CrossRef]
- Heilmann, C.; Budtz-Jørgensen, E.; Nielsen, F.; Heinzow, B.; Weihe, P.; Grandjean, P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ. Health Perspect. 2010, 118, 1434–1438. [Google Scholar] [CrossRef]
- Jusko, T.A.; De Roos, A.J.; Schwartz, S.M.; Lawrence, B.P.; Palkovicova, L.; Nemessanyi, T.; Drobna, B.; Fabisikova, A.; Kocan, A.; Sonneborn, D.; et al. A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6-months of age. Environ. Res. 2010, 110, 388–395. [Google Scholar] [CrossRef]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012, 307, 391–397, Erratum in JAMA 2012, 307, 1142. [Google Scholar] [CrossRef]
- Stølevik, S.B.; Nygaard, U.C.; Namork, E.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Knutsen, H.K.; Aaberge, I.; Vainio, K.; van Loveren, H.; et al. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem. Toxicol. 2013, 51, 165–172. [Google Scholar] [CrossRef]
- Gallagher, C.M.; Smith, D.M.; Golightly, M.G.; Meliker, J.R. Total blood mercury and rubella antibody concentrations in US children aged 6-11 years, NHANES 2003–2004. Sci. Total Environ 2013, 442, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Looker, C.; Luster, M.I.; Calafat, A.M.; Johnson, V.J.; Burleson, G.R.; Burleson, F.G.; Fletcher, T. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci. 2014, 138, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, X.; Zhang, J.; Guo, P.; Fu, T.; Dai, Y.; Lin, S.L.; Huo, X. Decreased blood hepatitis B surface antibody levels linked to e-waste lead exposure in preschool children. J. Hazard. Mater. 2015, 298, 122–128. [Google Scholar] [CrossRef]
- Mogensen, U.B.; Grandjean, P.; Heilmann, C.; Nielsen, F.; Weihe, P.; Budtz-Jørgensen, E. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environ. Health 2015, 14, 47. [Google Scholar] [CrossRef]
- Cardenas, A.; Smit, E.; Bethel, J.W.; Houseman, E.A.; Kile, M.L. Arsenic exposure and the seroprevalence of total hepatitis A antibodies in the US population: NHANES, 2003–2012. Epidemiol. Infect. 2016, 144, 1641–1651. [Google Scholar] [CrossRef]
- Jusko, T.A.; De Roos, A.J.; Lee, S.Y.; Thevenet-Morrison, K.; Schwartz, S.M.; Verner, M.A.; Murinova, L.P.; Drobná, B.; Kočan, A.; Fabišiková, A.; et al. A Birth Cohort Study of Maternal and Infant Serum PCB-153 and DDE Concentrations and Responses to Infant Tuberculosis Vaccination. Environ. Health Perspect. 2016, 124, 813–821. [Google Scholar] [CrossRef]
- Kielsen, K.; Shamim, Z.; Ryder, L.P.; Nielsen, F.; Grandjean, P.; Budtz-Jørgensen, E.; Heilmann, C. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J. Immunotoxicol. 2016, 13, 270–273. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, X.; Dai, Y.; Zhang, Y.; Li, W.; Huo, X. Considerable decrease of antibody titers against measles, mumps, and rubella in preschool children from an e-waste recycling area. Sci. Total Environ. 2016, 573, 760–766. [Google Scholar] [CrossRef]
- Stein, C.R.; Ge, Y.; Wolff, M.S.; Ye, X.; Calafat, A.M.; Kraus, T.; Moran, T.M. Perfluoroalkyl substance serum concentrations and immune response to FluMist vaccination among healthy adults. Environ. Res. 2016, 149, 171–178. [Google Scholar] [CrossRef]
- Lin, X.; Xu, X.; Zeng, X.; Xu, L.; Zeng, Z.; Huo, X. Decreased vaccine antibody titers following exposure to multiple metals and metalloids in e-waste-exposed preschool children. Environ. Pollut. 2017, 220, 354–363. [Google Scholar] [CrossRef]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Timmermann, A.; Budtz-Jørgensen, E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 2017, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Budtz-Jørgensen, E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2017, 125, 077018. [Google Scholar] [CrossRef]
- Raqib, R.; Ahmed, S.; Ahsan, K.B.; Kippler, M.; Akhtar, E.; Roy, A.K.; Lu, Y.; Arifeen, S.E.; Wagatsuma, Y.; Vahter, M. Humoral Immunity in Arsenic-Exposed Children in Rural Bangladesh: Total Immunoglobulins and Vaccine-Specific Antibodies. Environ. Health Perspect. 2017, 125, 067006. [Google Scholar] [CrossRef] [PubMed]
- Pilkerton, C.S.; Hobbs, G.R.; Lilly, C.; Knox, S.S. Rubella immunity and serum perfluoroalkyl substances: Sex and analytic strategy. PLoS ONE 2018, 13, e0203330. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.; Permar, S.R.; Ortiz, E.; Berky, A.; Woods, C.W.; Amouou, G.F.; Itell, H.; Hsu-Kim, H.; Pan, W. Mercury Exposure and Poor Nutritional Status Reduce Response to Six Expanded Program on Immunization Vaccines in Children: An Observational Cohort Study of Communities Affected by Gold Mining in the Peruvian Amazon. Int. J. Environ. Res. Public Health 2019, 16, 638. [Google Scholar] [CrossRef]
- Di Lenardo, T.Z.; Ward, B.J.; Pillet, S.; Mann, K.; Bornman, R.; Obida, M.; Chevrier, J. Exposure to lead and vaccine-specific IgG titers in South African children participating in the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE): A longitudinal study. Environ. Res. 2020, 180, 108794. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Jensen, K.J.; Nielsen, F.; Budtz-Jørgensen, E.; van der Klis, F.; Benn, C.S.; Grandjean, P.; Fisker, A.B. Serum Perfluoroalkyl Substances, Vaccine Responses, and Morbidity in a Cohort of Guinea-Bissau Children. Environ. Health Perspect. 2020, 128, 87002. [Google Scholar] [CrossRef]
- Abraham, K.; Mielke, H.; Fromme, H.; Völkel, W.; Menzel, J.; Peiser, M.; Zepp, F.; Willich, S.N.; Weikert, C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020, 94, 2131–2147. [Google Scholar] [CrossRef]
- Welch, B.M.; Branscum, A.; Geldhof, G.J.; Ahmed, S.M.; Hystad, P.; Smit, E.; Afroz, S.; Megowan, M.; Golam, M.; Sharif, O.; et al. Evaluating the effects between metal mixtures and serum vaccine antibody concentrations in children: A prospective birth cohort study. Environ. Health 2020, 19, 41. [Google Scholar] [CrossRef]
- Wen, H.J.; Guo, Y.L.; Su, P.H.; Sun, C.W.; Wang, S.J. Prenatal and childhood exposure to phthalic acid esters and vaccination antibodies in children: A 15-year follow-up birth cohort study. Environ. Int. 2020, 145, 106134. [Google Scholar] [CrossRef]
- Prahl, M.; Odorizzi, P.; Gingrich, D.; Muhindo, M.; McIntyre, T.; Budker, R.; Jagannathan, P.; Farrington, L.; Nalubega, M.; Nankya, F.; et al. Exposure to pesticides in utero impacts the fetal immune system and response to vaccination in infancy. Nat. Commun. 2021, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Blomberg, A.J.; Bind, M.A.; Holm, D.; Nielsen, F.; Heilmann, C.; Weihe, P.; Grandjean, P. Serum vaccine antibody concentrations in adults exposed to per- and polyfluoroalkyl substances: A birth cohort in the Faroe Islands. J. Immunotoxicol. 2021, 18, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, C.A.G.; Pedersen, H.S.; Weihe, P.; Bjerregaard, P.; Nielsen, F.; Heilmann, C.; Grandjean, P. Concentrations of tetanus and diphtheria antibodies in vaccinated Greenlandic children aged 7–12 years exposed to marine pollutants, a cross sectional study. Environ. Res. 2022, 203, 111712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, S.; Xiao, G.; Zhao, M.; Li, J.; Dong, W.; Hu, J.; Yuan, T.; Li, Y.; Liu, L. The associations between air pollutant exposure and neutralizing antibody titers of an inactivated SARS-CoV-2 vaccine. Environ. Sci. Pollut. Res. Int. 2022, 29, 13720–13728. [Google Scholar] [CrossRef]
- Hammel, S.C.; Nordone, S.; Zhang, S.; Lorenzo, A.M.; Eichner, B.; Moody, M.A.; Harrington, L.; Gandee, J.; Schmidt, L.; Smith, S.; et al. Infants’ diminished response to DTaP vaccine is associated with exposure to organophosphate esters. Sci. Total Environ. 2022, 837, 155782. [Google Scholar] [CrossRef]
- Porter, A.K.; Kleinschmidt, S.E.; Andres, K.L.; Reusch, C.N.; Krisko, R.M.; Taiwo, O.A.; Olsen, G.W.; Longnecker, M.P. Antibody response to COVID-19 vaccines among workers with a wide range of exposure to per- and polyfluoroalkyl substances. Environ. Int. 2022, 169, 107537. [Google Scholar] [CrossRef]
- Hollister, J.; Caban-Martinez, A.J.; Ellingson, K.D.; Beitel, S.; Fowlkes, A.L.; Lutrick, K.; Tyner, H.L.; Naleway, A.L.; Yoon, S.K.; Gaglani, M.; et al. Serum per- and polyfluoroalkyl substance concentrations and longitudinal change in post-infection and post-vaccination SARS-CoV-2 antibodies. Environ. Res. 2023, 239, 117297. [Google Scholar] [CrossRef]
- Kogevinas, M.; Karachaliou, M.; Espinosa, A.; Aguilar, R.; Castaño-Vinyals, G.; Garcia-Aymerich, J.; Carreras, A.; Cortés, B.; Pleguezuelos, V.; Papantoniou, K.; et al. Long-Term Exposure to Air Pollution and COVID-19 Vaccine Antibody Response in a General Population Cohort (COVICAT Study, Catalonia). Environ. Health Perspect. 2023, 131, 47001. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustieles, V.; Wang, Y.X.; Sun, Q.; Coull, B.; Sun, Y.; Slitt, A.; Messerlian, C. Red Blood Cell Folate Modifies the Association between Serum Per- and Polyfluoroalkyl Substances and Antibody Concentrations in U.S. Adolescents. Environ. Sci. Technol. 2023, 57, 2445–2456. [Google Scholar] [CrossRef]
- Roh, T.; Regan, A.K.; Johnson, N.M.; Hasan, N.T.; Trisha, N.F.; Aggarwal, A.; Han, D. Association of arsenic exposure with measles antibody titers in US children: Influence of sex and serum folate levels. Environ. Int. 2024, 183, 108329. [Google Scholar] [CrossRef]
- Sigvaldsen, A.; Højsager, F.D.; Paarup, H.M.; Beck, I.H.; Timmermann, C.A.G.; Boye, H.; Nielsen, F.; Halldorsson, T.I.; Nielsen, C.; Möller, S.; et al. Early-life exposure to perfluoroalkyl substances and serum antibody concentrations towards common childhood vaccines in 18-month-old children in the Odense Child Cohort. Environ. Res. 2024, 242, 117814. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Li, Z.; Zhao, W.; Hu, X.; Wang, H.; Wang, J.; Han, M.; Xu, L.; Sun, H.; Qin, C.; et al. Molecular mechanism of immunotoxicity: Binding interaction between perfluorinated compounds and human immunoglobulin G. Environ. Pollut. 2024, 362, 125032. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef]
- de Gomensoro, E.; Del Giudice, G.; Doherty, T.M. Challenges in adult vaccination. Ann. Med. 2018, 50, 181–192. [Google Scholar] [CrossRef]
- Dobrescu, A.I.; Nussbaumer-Streit, B.; Klerings, I.; Wagner, G.; Persad, E.; Sommer, I.; Herkner, H.; Gartlehner, G. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: A systematic review. J. Clin. Epidemiol. 2021, 137, 209–217. [Google Scholar] [CrossRef]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef]
- Moher, D.; Fortin, P.; Jadad, A.R.; Jüni, P.; Klassen, T.; Le Lorier, J.; Liberati, A.; Linde, K.; Penna, A. Completeness of reporting of trials published in languages other than English: Implications for conduct and reporting of systematic reviews. Lancet 1996, 347, 363–366. [Google Scholar] [CrossRef]
| Author, Year, Country, Sponsor | Main Results | Quality Assessment |
|---|---|---|
| Weisglas-Kuperus et al. [23], 1995, Netherlands | No significant correlations between mumps, measles, and rubella antibodies and pre-/postnatal PCB/dioxin exposure. | Good |
| Lutz et al. [24], 1999, USA | A statistically significant relationship between IgE and blood lead level. Statistically non-significant negative association between Rubella index in serum and blood lead values (correlation coefficient 0.09, p < 0.15). | Good |
| Weisglas-Kuperus et al. [25], 2000, Netherlands | Negative correlation between mumps antibodies and PCB maternal levels (r = −0.17, p = 0.04) and between rubella antibodies and PCB cord levels (r = −0.19, p = 0.03). | Good |
| Heilmann et al. [26], 2006, Denmark | Decrease in diphtheria antibodies at 18 months by 24.4% (p = 0.04) for each doubling of cumulative PCB exposure. At 7 years, lower diphtheria response, not associated with the exposure. Tetanus toxoid antibodies affected mainly at 7 years, decreased by 16.5% (p = 0.03) for each doubling of prenatal exposure. | Good |
| Steerenberg et al. [27], 2008, Netherlands | No differences in HBV antibodies at day 49 after the first vaccination. Similar levels of HBV IgG in exposed (mean ± SD, 2.1 ± 1.9 IU/L) and non-exposed (mean ± SD, 2.3 ± 2.0 IU/L). | Good |
| Baranska et al. [28], 2008, Poland | No statistically significant differences in HBV antibodies (exposed and control groups: 2.28 ± 2.01 vs. 2.12 ± 1.85). | Fair |
| Heilmann et al. [29], 2010, Denmark | Relative change in serum tetanus antibody concentrations associated with a doubling of PCB exposure during development: statistically non-significant reduction of 6% at age 5 years and reduction of 21.7% (p < 0.003) at age 7 years. Relative change in serum diphtheria antibody concentrations associated with a doubling of PCB exposure during development: 15.4% reduction (p < 0.01) at age 5 years and 18.3% reduction (p < 0.03) at age 7 years. | Good |
| Jusko et al. [30], 2010, USA | No significant association between pre- or postnatal PCB levels and antibody levels at 6 months of age. | Good |
| Grandjean et al. [31], 2012, USA | A 2-fold increase in PFOS exposure associated with a −39% (95% CI, −55% to −17%) difference in antibody levels at 5 years before the booster. At 7 years, 2-fold increase in PFOA exposure associated with differences of −36% (95% CI, −52% to −14%) and −25% (95% CI, −43% to −2%) for tetanus and diphtheria, respectively. PFOS exposure associated with a difference in diphtheria antibody levels of −28% (95% CI, −46% to −3%). | Good |
| Stølevik et al. [32], 2013, Norway | Maternal exposure to dioxins and dl-PCBs or ndl-PCBs associated with reduced levels of measles antibodies (p = 0.032, p = 0.036). No significant associations between antibody levels from other vaccines and exposure to dioxins and dl-PCBs or ndl-PCBs. | Good |
| Gallagher et al. [33], 2013, USA | Exposure to mercury significantly positively associated with rubella antibody levels (0.24% increase per 1% increase of blood mercury; β = 0.24; 95% CI = 0.11, 0.38) in children with nutritional susceptibility (higher methylmalonic acid, lower folate, and higher homocysteine) and inversely associated in others (0.18% decrease per 1% increase of blood mercury; β = −0.18; 95% CI = −0.34, −0.03). | Good |
| Looker et al. [34], 2014, UK, Supported by C8 Class Action Settlement Agreement DuPont—Plaintiffs. | A/H3N2 antibody levels were negatively associated with PFOA concentrations (adjusted coefficient −0.12 [95% CI: −0.25, 0.02, p = 0.009]). Largest reduction in A/H3N2 antibody levels resulted for the highest quartile of concentrations of PFOA (adjusted coefficient −0.22 [95% CI: −0.43, −0.01]). | Good |
| Xu et al. [35], 2015, China | For the 2011 group: higher blood levels of Pb and lower titers of HBsAb in were found in exposed children respect to the reference group (8.76 µg/dL vs. 7.89 µg/dL; 0.83 s/co vs. 4.64 s/co, respectively). For the 2012 group: higher blood levels of Pb and lower titers of HBsAb were recovered in exposed children compared to the reference group, (5.83 µg/dL vs. 4.61 µg/dL and 1.31 s/co vs. 3.80 s/co, respectively). | Good |
| Mogensen et al. [36], 2015, Denmark | Higher PFAS concentrations measured after 7 years were associated with lower levels of antibodies. Diphtheria antibody levels decreased respectively by 30.3% (95% CI: 7.8%, 47.3%) and 25.4% (95% CI: 5.8%, 40.9%) for double concentration of PFOS and PFOA. Tetanus antibodies decreased by 22.3% (95% CI: 5.2%, 36.3%) for double concentration of PFHxS. | Good |
| Cardenas et al. [37], 2016, USA | HAV antibodies associated with As levels, in relationship with immunization status (P = 0.03). In participants that received ≥ 2 vaccine doses or did not know if they had received any doses, positive dose-response association with anti-HAV (OR 1.75, 95% CI: 1.22–2.2); in those who received < 2 doses or no dose, positive but not statistically significant association (OR 1.46, 95% CI: 0.83–2.59 and OR 1.12, 95% CI: 0.98–1.30, respectively). | Poor |
| Jusko et al. [38], 2016, USA | Higher PCB-153 and DDE concentrations were strongly associated with lower antibody levels specific for BCG: IgG levels specific for BCG were 37% lower for infants with PCB-153 concentrations at the 75th percentile compared to the 25th percentile (95% CI: −42, −32; p < 0.001). Similar findings were recovered for DDE. The exposure to both pollutants more reduced anti-BCG. | Good |
| Kielsen et al. [39], 2016, Denmark | Antibody levels negatively affected by the majority of PFASs. Diphtheria antibodies titer/exposure to PFHxS −13.3% p < 0.05. PFOS −11.9% p < 0.04. PFNA −17.9% p < 0.004. PFDA −18.2% p < 0.009. PFUnDA −12.1% p < 0.03. PFDoDA −15.64% p < 0.03. Tetanus antibodies titer/exposure to PFUnDA −7.9% p < 0.03. PFDoDA −10.8% p < 0.03. | Fair |
| Lin et al. [40], 2016, China | Median titer of measles IgG of exposed subjects decreased of about 40% (669.64 mIU/mL, IQR 372.88–1068.42 mIU/mL) with respect to the reference individuals (median 1046.79 mIU/mL, interquartile range 603.29–1733.10 mIU/mL). The mumps IgG levels decreased by about 45% in the exposed group (median = 272.24 U/mL, IQR = 95.19–590.16 U/mL) with respect to the reference group (median = 491.78 U/mL, IQR = 183.38–945.96 U/mL). Rubella antibodies decreased by about 44% in the exposed group compared to the reference. Anti-rubella antibody levels were below the protective level in 26% of the exposed subjects and in 15% of the reference individuals. | Good |
| Stein et al. [41], 2016, USA | PFOS, PFOA, PFHxS, or PFNA concentrations were not associated with antibody levels. PFOS, PFOA, PFHxS, or PFNA tertile concentrations were not associated with increasing or decreasing levels of immune markers. | Fair |
| Lin et al. [42], 2017, China | The OR of blood concentrations of Zn for diphtheria antibody levels was equal to 0.477 (p = 0.002). The OR for high levels of blood Pb (Pb levels equal to 5–10 μg/dL) and pertussis and diphtheria levels of antibodies was, respectively, 0.361 (95% CI: 0.160–0.818, p = 0.015) and 0.54 (95% CI: 0.247–1.180, p = 0.05). The ORs for higher blood levels of Pb (>10 μg/dL) and levels antibodies were in the range of 0.31–0.454 (diphtheria p = 0.031, pertussis p = 0.01, tetanus p = 0.058, hepatitis B p = 0.025, Japanese encephalitis p = 0.041, polio p = 0.04, and measles p = 0.08). The ORs for high blood concentrations of Cu and the levels of antibodies were in the range of 0.471-0.598 (diphtheria p = 0.081, pertussis p = 0.07, tetanus p = 0.049, hepatitis B p = 0.097, and Japanese encephalitis p = 0.014). The ORs for high blood concentrations of Zn and antibody levels were in the range of 0.483–0.563 (Japanese encephalitis p= 0.021, polio p = 0.076, and measles p = 0.062). High blood concentrations of As, Hg, Se, and Cr were not associated with all seven types of studied antibodies. | Fair |
| Grandjean et al. [43], 2017, USA | At PFAS exposure increase, reduction in tetanus antibody levels [−9.1% (PFOS, p < 0.43), −25.3% (PFOA, p < 0.031), −4.4% (PFHxs, p < 0.65), −10.3% (PFNA, p < 0.21), and −1.75% (PFDA, p < 0.83)] and in diphtheria antibody levels [−8.8% (PFNA, p < 0.32) and −9% (PFDA, p < 0.29%)]. Increases in diphtheria antibody levels were also reported: +17.17% (PFOS, p < 0.21), +18.31 (PFOA, p < 0.24), and +4.26% (PFHxS, p < 0.69). | Good |
| Grandjean et al. [44], 2017, Denmark/USA | For diphtheria antibodies, there were statistically significant inverse associations with all PFASs (PFOS p < 0.002, PFOA p < 0.045, PFHxS p < 0.042, PFNA p < 0.002, and PFDA p < 0.001). For tetanus, there were no significant inverse associations. Over 7 years, there was a similar indirect effect for diphtheria; for tetanus, there were statistically significant indirect effects both for PFOA (–24.2; 95% CI: −41.1, −2.4) and for PFHxS (–25.1; 95% CI: −38.9, −8.3). | Good |
| Raqib et al. [45], 2017, Bangladesh | The levels of IgG against mumps decreased with the increasing of urinary concentrations of As at 4.5 and 9 years of age (β = −0.16; 95% CI: −0.33, 0.01; p = 0.064 and β = −0.12; 95% CI: −0.27, −0.029; p = 0.113, respectively) in 25% of subjects with the lowest preexisting titers of IgG against mumps. | Good |
| Pilkerton et al. [46], 2018, USA | In subjects aged 12–18 years, rubella antibody levels were not associated with either PFOA or PFOS (PFOA p < 0.79 e PFOS p < 0.25). In adults, rubella antibodies were significantly associated with both PFOA (p = 0.0016) and PFOS (p = 0.0295) quartile concentrations. | Good |
| Wyatt et al. [47], 2019, USA, Supported by Hunt Oil Peru LLC | One-unit increases in hair Hg concentrations in younger children were associated with an increase of IgG respectively equal to 0.68 IU/mL (95% CI: 0.18–1.17) for pertussis and 0.79 IU/mL (95% CI: 0.18–1.70) for diphtheria. Hair Hg concentrations exceeding 1.2 µg/g in older children were associated with 73.7 higher odds (95% CI: 2.7–1984.3) of becoming a non-responder against measles and hair Hg concentrations exceeding 2.0 µg/g with a decrease of IgG equal to 0.32 IU/mL (95% CI: 0.10–0.69) for measles. Older children with poor nutrition presented a reduction of measles antibodies from 1.40 to 0.43 for low exposure (<1.2 µg/g) with respect to high Hg exposure, while children with good nutritional status presented minimal change in measles antibodies for low exposure with respect to high Hg exposure (0.72 vs. 0.81, respectively). | Good |
| Di Lenardo et al. [48], 2020, Canada | Blood levels of Pb (BLLs) at 1 year were related to vaccine IgG titers at 3.5 years: BLLs were inversely associated with levels of antibodies against tetanus. A 10-fold increase in BLL was associated with a decrease in tetanus IgG eual to 28% (95% CI = −52.16–8.72) and risk of presenting tetanus IgG titers below the protection levels (RR= 1.88; 95% CI = 1.08–3.24; p < 0.05). BLLs were not associated with the levels of antibodies against measles or Hib (RR = 1.02; 95% CI = 0.26–3.95, and RR 0.96; 95% CI = 0.54–1.71, respectively). | Good |
| Timmermann et al. [49], 2020, Denmark | Intervention group: doubling in PFOS and PFDA levels was associated with a decrease of measles antibody titers respectively equal to 21% (95% CI: 2, 37%) and 25% (95% CI: 1, 43%) at the 9-month visit. Control group: elevated PFAS concentrations at inclusion of the study were significantly associated with a reduction in measles levels of antibodies at the 2 y visit (for PFHxS, PFOS, PFOA, and PFNA) only after removal of the most influential points. A doubled concentration of PFOS was associated with a reduction equal to 27% of measles antibody levels (95% CI: 4, 44%). | Good |
| Abraham et al. [50], 2020, Germany | PFOA levels were significantly associated with Hib (r = −0.32, p = 0.001), tetanus IgG1 (r = −0.25, p = 0.01), and diphtheria (r = −0.23, p = 0.02) antibody levels. No significant associations observed between PFOS and Hib (r = −0.05, p = 0.66), tetanus IgG1 (r = −0.07, p = 0.52), and diphtheria antibodies (r = −0.02, p = 0.84). No significant correlations between PFHxS and PFNA concentrations and vaccine antibody levels. | Fair |
| Welch et al. [51], 2020, USA | Higher water As during pregnancy was associated with lower concentrations of diphtheria antibodies (−3.4% change per doubling in As, 95% CI: −7.2, 0.6%). Higher blood Pb in pregnancy was associated with higher concentrations of tetanus antibodies (10.2, −0.6%, 22.1%). No associations found between Mn and antibody outcomes. | Good |
| Wen et al. [52], 2020, Taiwan | Maternal phthalate metabolite levels and percent change in antibody concentrations among children at 11–14 years of age were not significantly associated. Urinary concentrations of MnBP, MEHHP, MEOHP, and ∑DEHPm were associated with a percent change in the decrease of the levels of antibodies against HBV in children aged 11–14 years. Doubled urinary levels of MnBP, MEHHP, MEOHP, and ∑DEHPm were associated with a decrease in HBV antibodies respectively equal to 23% (95% CI = 3.96–41.66%), 18% (95% CI = 3.33–33.38%), 11% (95% CI = 2.77–18.99%), and 18% (95% CI = 3.34–32.78%). | Good |
| Prahl et al. [53], 2021, USA | Among bendiocarb-exposed infants, a significantly higher anti-measles IgG (p < 0.0001) and proportion of infants who were measles IgG-positive following vaccination (91.3% vs. 77.8%, p = 0.008) were found. | Good |
| Shih et al. [54] 2021, USA | Inverse trends between HAV antibodies and PFOA levels were recovered at 14 and 28 years (S/CO change: −0.71; 95% CI: −1.52, 0.09; and S/CO change: −0.24; 95% CI: −0.59, 0.10, respectively). Inverse trends between HBV antibodies and PFOA levels were found at 22 and 28 years (% change: −21.24; 95% CI: −42.20, 7.34; and % change: −16.77; 95% CI: −35.47, 7.35, respectively). Diphtheria antibodies, but not tetanus ones, were positively associated with cord-blood PFAS and PFAS assessed at ages 22 and 28 years. | Good |
| Timmermann et al. [55], 2022, Denmark | Each 1 ng/mL increase in serum concentration of PFHxS and PFOS was associated with a decrease diphtheria antibody level respectively equal to 78% (95% CI: 25–94%) and 9% (95% CI: 2–16%). Exposure to PCBs and all PFAS concentrations were associated with increased odds of diphtheria antibody levels below the protective level. For each 1 ng/mL increase in serum levels of PFHxS, PFOS, PFNA, and PFDA, an increased odds of not achieving the protective levels of diphtheria antibodies (6.44, 95% CI: 1.51–27.36; 1.14, 95% CI: 1.04–1.26; 1.96, 95% CI: 1.07–3.60; 5.08, 95% CI: 1.32–19.51, respectively) was found. Maternal pollutant concentrations were not consistently associated with the levels of vaccine antibodies. | Good |
| Zhang et al. [56], 2022, China | Daily exposure dose to air pollutants significantly and negatively associated with plasma-neutralizing antibody titers [B (95% CI): −0.809 (−1.600, −0.019) for PM2.5, −0.486 (−0.960, −0.011) for PM10, −2.427 (−4.800, −0.055) for SO2, −1.139 (−2.211, −0.068) for NO2, −0.335 (−0.662, −0.008) for O3, −0.034 (−0.067, −0.001) for CO, and −0.485 (−0.954, −0016) for combined toxic effects, all p < 0.05]. | Fair |
| Hammel et al. [57], 2022, USA, Supported by Gerber Foundation | In 12-month-old infants, for each increasing of log10-unit of BCIPP levels, there was a decrease of 0.57 IU/mL (95% CI: −1.11, −0.02; p = 0.04) in tetanus antibody concentrations. All of the other log10-biomarker concentrations of OPE were associated with an increase of tetanus antibody titers. In 2-month-old infants, each increase in log10-concentration BDCIPP was negatively associated with a decrease of 0.07 IU/mL in diphtheria titers (95% CI: −0.11, −0.03; p < 0.001 with of BDCIPP). No other significant associations were found between the levels of OPE metabolites and diphtheria titers. | Good |
| Porter et al. [58], 2022, USA, Supported by Ramboll and 3M | IgG concentration decreased by about 4% (95% CI −7.03, 0.26) per 14.5 ng/mL (IQR) increase in levels of PFOS. Similar findings were recovered for PFOS concentrations and neutralizing antibodies and for PFOA, PFHxS, and PFNA levels and both neutralizing and IgG antibodies. | Good |
| Hollister et al. [59], 2023, USA | No statistically significant associations between serum PFAS concentration and antibody levels after vaccination nor between serum PFAS concentration and changes in antibody levels over time. | Good |
| Kogevinas et al. [60], 2023, Spain | Among participants without prior infection, IgM (within 1 month post first vaccine dose) and IgG levels (any time post vaccination) were negatively associated with long-term air pollution; no associations observed for IgA. | Good |
| Zhang et al. [61], 2023, China | Serum PFOA levels were negatively associated with rubella antibody titers (PC: −4.36%, 95% CI: −11.53%, 3.40%) and mumps antibodies (PC: −11.05%, 95% CI: −18.56%, −2.85%). PFAS serum levels were not associated with measles antibodies. The quartile increasing in serum levels of the PFAS mixture was associated with an 8% (95% CI: −13.01%, −2.66%) decrease in antibodies for rubella. The quartile increasing in the serum levels of the PFAS mixture was associated with a 5% (95% CI: −10.44%, −0.16%) decrease in antibodies for mumps. Positive joint effects of the PFAS mixture were produced on antibodies for measles (PC: 7.03%, 95% CI: 0.29%, 14.22%). | Fair |
| Roh et al. [62], 2024, USA | Significant decrease in measles antibody titers (10.8%; p < 0.007) for doubled urinary levels of As among individuals with serum folate concentration < 18.7 ng/mL. Stratifying by sex and serum folate levels, a 16.7% decrease in serum measles antibody levels was found (p < 0.001) for a doubling of urinary As levels in males with serum folate concentrations < 18.7 ng/mL. In other groups, urinary levels of As were not associated with measles antibody titers. | Good |
| Sigvaldsen et al. [63], 2024, Denmark | At 18 months, higher serum concentrations of PFAS were associated with lower levels of IgG for measles, mumps, and rubella. Significant differences were found in mumps antibody levels per doubled concentrations of PFNA (−9.2%; 95% CI: −17.4; −0.2), PFHxS (−8.3%; −15.0; −1.0), and PFOS (−7.9%; −14.8; −0.4), but were not significant for PFOA (−5.7%; −12.6; 1.7) and PFDA (−5.6%; −15.6; 5.7). PFAS exposure was inversely associated with tetanus IgG. Higher PFAS concentrations at 18 months of PFHxS concentrations were the only significant association with higher levels of diphtheria IgG. | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protano, C.; Valeriani, F.; Vitale, K.; Del Prete, J.; Liguori, F.; Liguori, G.; Gallè, F. Exposure to Pollutants and Vaccines’ Effectiveness: A Systematic Review. Vaccines 2024, 12, 1252. https://doi.org/10.3390/vaccines12111252
Protano C, Valeriani F, Vitale K, Del Prete J, Liguori F, Liguori G, Gallè F. Exposure to Pollutants and Vaccines’ Effectiveness: A Systematic Review. Vaccines. 2024; 12(11):1252. https://doi.org/10.3390/vaccines12111252
Chicago/Turabian StyleProtano, Carmela, Federica Valeriani, Katia Vitale, Jole Del Prete, Fabrizio Liguori, Giorgio Liguori, and Francesca Gallè. 2024. "Exposure to Pollutants and Vaccines’ Effectiveness: A Systematic Review" Vaccines 12, no. 11: 1252. https://doi.org/10.3390/vaccines12111252
APA StyleProtano, C., Valeriani, F., Vitale, K., Del Prete, J., Liguori, F., Liguori, G., & Gallè, F. (2024). Exposure to Pollutants and Vaccines’ Effectiveness: A Systematic Review. Vaccines, 12(11), 1252. https://doi.org/10.3390/vaccines12111252










