EMSIG: Uncovering Factors Influencing COVID-19 Vaccination Across Different Subgroups Characterized by Embedding-Based Spatial Information Gain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. The Data-Driven Correlation-Based Factor Extraction and Factor Annotation Based on the Large Language Model
2.3. The Participant’s Latent Projection Based on the 85-Item Questionnaire with a Five-Point Likert Scale
2.4. The Regions of Interest (ROI) Discovery to Form the Participant’s Subgroup in Latent Projection
2.5. The Correlation Analysis Between the Factor and COVID-19 Doses in Each ROI Subgroup
2.6. The Statistical Analysis of the Patient’s Characteristics in ROI Subgroups
2.7. The Regression-Based Machine Learning Model for COVID-19 Vaccine Dose Prediction
3. Results
3.1. Sample Descriptives
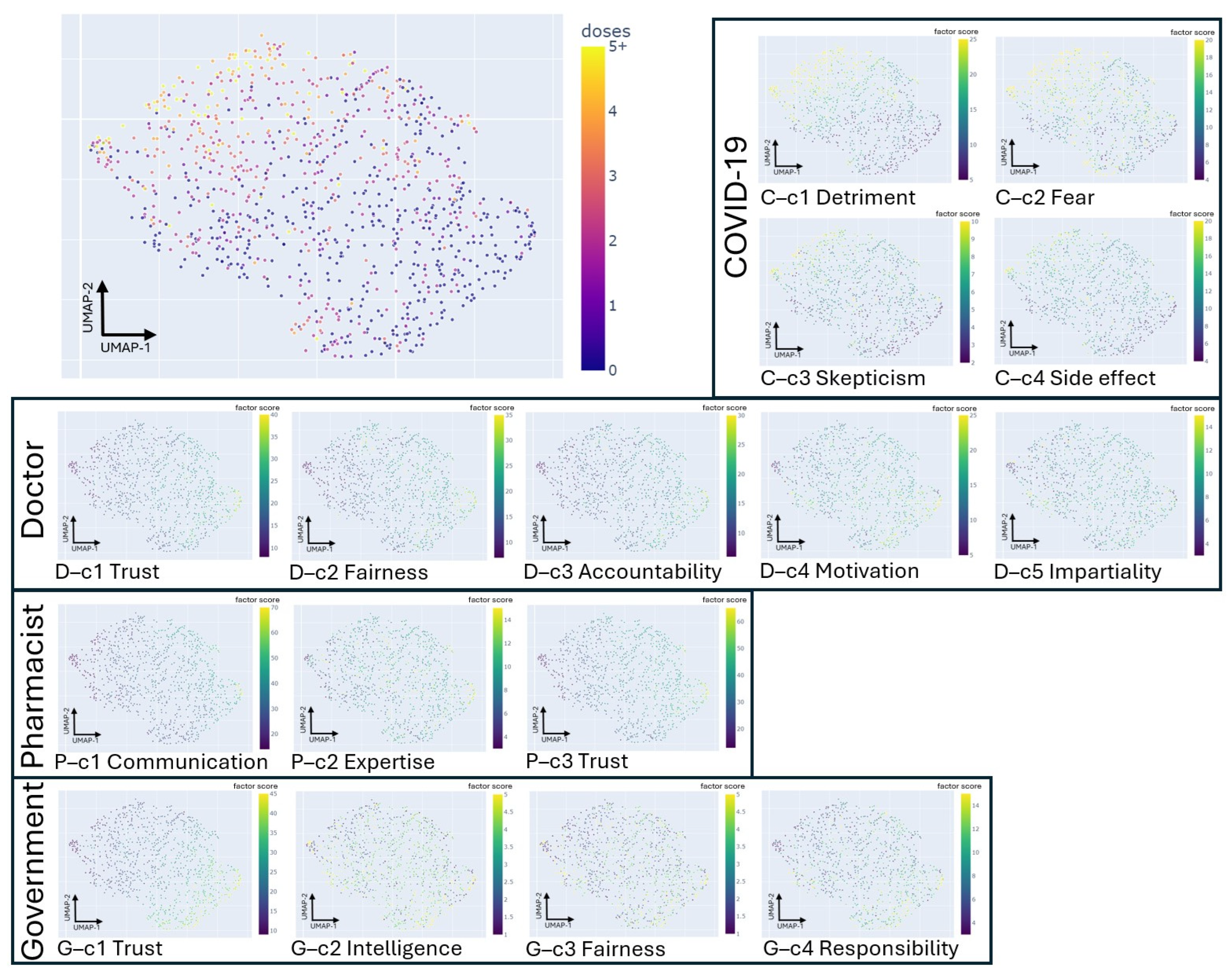
3.2. The 16 Factors Extracted from the 85-Item Questionnaire with a Five-Point Likert Scale
3.3. The Random Forest Regressor Performed the Best for the COVID-19 Vaccine Dose Regression Using the 16 Factors
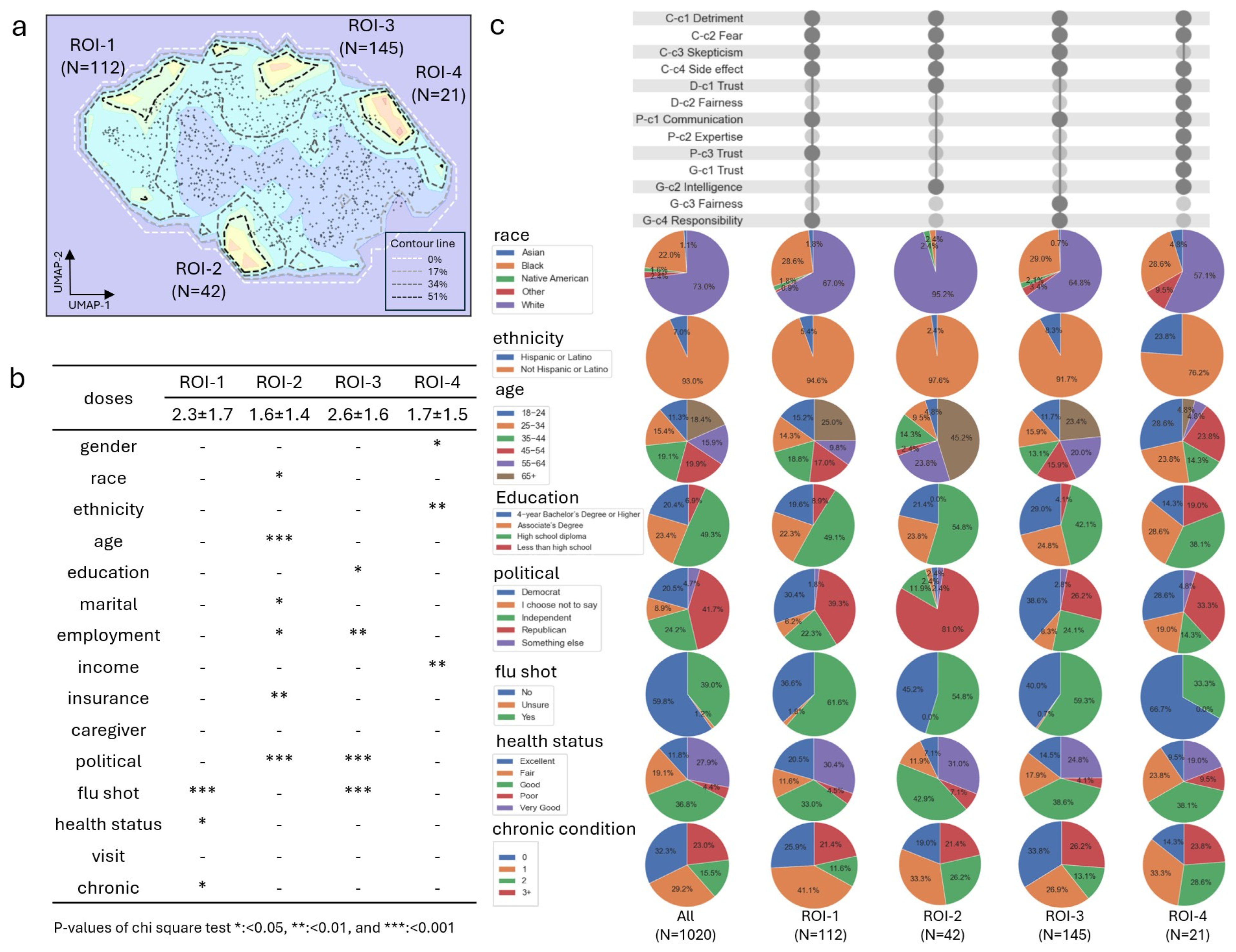
3.4. The Four ROI Subgroups Extracted Based on the SIG Signal in Latent Projection and the Demographic’s Disparity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World in Data: 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 12 October 2024).
- Omer, S.B.; Malani, P.N. Booster Vaccination to Prevent COVID-19 in the Era of Omicron: An Effective Part of a Layered Public Health Approach. JAMA 2022, 327, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Zhang, J.; Li, Z.; Sun, X.; Ning, H.; Yang, X.; Weissman, S.; Olatosi, B.; Li, X. Residential Segregation and County-Level COVID-19 Booster Coverage in the Deep South: Surveillance Report and Ecological Study. JMIR Public Health Surveill. 2023, 9, e44257. [Google Scholar] [CrossRef] [PubMed]
- Motta, M. Republicans, Not Democrats, Are More Likely to Endorse Anti-Vaccine Misinformation. Am. Politics Res. 2021, 49, 428–438. [Google Scholar] [CrossRef]
- Albrecht, D. Vaccination, politics and COVID-19 impacts. BMC Public Health 2022, 22, 96. [Google Scholar] [CrossRef]
- Albrecht, D. Vaccination, politics and COVID-19 impacts: Update. J. Cell. Mol. Immunol. 2022, 1, 17–19. [Google Scholar]
- Alemi, F.; Lee, K.H. Impact of Political Leaning on COVID-19 Vaccine Hesitancy: A Network-Based Multiple Mediation Analysis. Cureus 2023, 15, e43232. [Google Scholar] [CrossRef]
- Bolsen, T.; Palm, R. Politicization and COVID-19 vaccine resistance in the U.S. Prog. Mol. Biol. Transl. Sci. 2022, 188, 81–100. [Google Scholar] [CrossRef]
- Gadarian, S.K.; Goodman, S.W.; Pepinsky, T.B. Partisanship, health behavior, and policy attitudes in the early stages of the COVID-19 pandemic. PLoS ONE 2021, 16, e0249596. [Google Scholar] [CrossRef]
- Facciani, M.; Lazic, A.; Viggiano, G.; McKay, T. Political network composition predicts vaccination attitudes. Soc. Sci. Med. 2023, 328, 116004. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Callaghan, T.; Sylvester, S. Knowing less but presuming more: Dunning-Kruger effects and the endorsement of anti-vaccine policy attitudes. Soc. Sci. Med. 2018, 211, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Loomba, S.; de Figueiredo, A.; Piatek, S.J.; de Graaf, K.; Larson, H.J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 2021, 5, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Faheem, M.; Nawaz, B.; Khan, M.; Khan, N.H.; Ullah, N.; Khan, T.A.; Khan, R.U.; Haleem, K.S.; Ren, Z.G.; et al. Knowledge, Attitude, and Perception of Cancer Patients towards COVID-19 in Pakistan: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 7926. [Google Scholar] [CrossRef] [PubMed]
- Guarducci, G.; Mereu, G.; Golinelli, D.; Galletti, G.; Gemmi, F.; Cartocci, A.; Holczer, N.; Bacci, L.; Sergi, A.; Messina, G.; et al. Factors Influencing the Healthcare Workers’ Willingness to Receive the COVID-19 Booster Dose in Tuscany (Italy). Vaccines 2023, 11, 1751. [Google Scholar] [CrossRef]
- Kotta, I.; Kalcza-Janosi, K.; Szabo, K.; Marschalko, E.E. Development and Validation of the Multidimensional COVID-19 Vaccine Hesitancy Scale. Hum. Vaccin. Immunother. 2022, 18, 1–10. [Google Scholar] [CrossRef]
- Ngorsuraches, S.; Lerkiatbundit, S.; Li, S.C.; Treesak, C.; Sirithorn, R.; Korwiwattanakarn, M. Development and validation of the patient trust in community pharmacists (TRUST-Ph) scale: Results from a study conducted in Thailand. Res. Soc. Adm. Pharm. 2008, 4, 272–283. [Google Scholar] [CrossRef]
- Holroyd, T.A.; Limaye, R.J.; Gerber, J.E.; Rimal, R.N.; Musci, R.J.; Brewer, J.; Sutherland, A.; Blunt, M.; Geller, G.; Salmon, D.A. Development of a Scale to Measure Trust in Public Health Authorities: Prevalence of Trust and Association with Vaccination. J. Health Commun. 2021, 26, 272–280. [Google Scholar] [CrossRef]
- Richmond, J.; Boynton, M.H.; Ozawa, S.; Muessig, K.E.; Cykert, S.; Ribisl, K.M. Development and Validation of the Trust in My Doctor, Trust in Doctors in General, and Trust in the Health Care Team Scales. Soc. Sci. Med. 2022, 298, 114827. [Google Scholar] [CrossRef]
- McAbee, L.; Tapera, O.; Kanyangarara, M. Factors Associated with COVID-19 Vaccine Intentions in Eastern Zimbabwe: A Cross-Sectional Study. Vaccines 2021, 9, 1109. [Google Scholar] [CrossRef]
- Terry, E.; Cartledge, S.; Damery, S.; Greenfield, S. Factors associated with COVID-19 vaccine intentions during the COVID-19 pandemic; a systematic review and meta-analysis of cross-sectional studies. BMC Public Health 2022, 22, 1667. [Google Scholar] [CrossRef] [PubMed]
- Lun, P.; Ning, K.; Wang, Y.; Ma, T.S.W.; Flores, F.P.; Xiao, X.; Subramaniam, M.; Abdin, E.; Tian, L.; Tsang, T.K.; et al. COVID-19 Vaccination Willingness and Reasons for Vaccine Refusal. JAMA Netw. Open 2023, 6, e2337909. [Google Scholar] [CrossRef] [PubMed]
- Rajshekhar, N.; Pinchoff, J.; Boyer, C.B.; Barasa, E.; Abuya, T.; Muluve, E.; Mwanga, D.; Mbushi, F.; Austrian, K. Exploring COVID-19 vaccine hesitancy and uptake in Nairobi’s urban informal settlements: An unsupervised machine learning analysis of a longitudinal prospective cohort study from 2021 to 2022. BMJ Open 2023, 13, e071032. [Google Scholar] [CrossRef] [PubMed]
- Razai, M.S.; Chaudhry, U.A.R.; Doerholt, K.; Bauld, L.; Majeed, A. COVID-19 vaccination hesitancy. BMJ 2021, 373, n1138. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Masters, N.B.; Lu, P.J.; Singleton, J.A.; Kriss, J.L.; Zhou, T.; Weiss, D.; Black, C.L. Cluster analysis of adults unvaccinated for COVID-19 based on behavioral and social factors, National Immunization Survey-Adult COVID Module, United States. Prev. Med. 2023, 167, 107415. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, B.; Abakoumkin, G.; Leander, N.P.; Stroebe, W. Why some people do not get vaccinated against COVID-19: Social-cognitive determinants of vaccination behavior. Appl. Psychol. Health Well-Being 2023, 15, 825–845. [Google Scholar] [CrossRef]
- Dudek, A. Silhouette Index as Clustering Evaluation Tool. In Classification and Data Analysis; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- McInnes, L.; Healy, J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv 2018, arXiv:abs/1802.03426. [Google Scholar]
- Jiang, Z.; Shekhar, S.; Mohan, P.; Knight, J.; Corcoran, J. Learning spatial decision tree for geographical classification: A summary of results. In Proceedings of the 20th International Conference on Advances in Geographic Information Systems, Redondo Beach, CA, USA, 6 November 2012; pp. 390–393. [Google Scholar]
- Yan, X.; Su, X.G. Linear Regression Analysis: Theory and Computing; World Scientific Publishing Co., Inc.: Hackensack, NJ, USA, 2009. [Google Scholar]
- Efron, B.; Hastie, T.; Johnstone, I.; Tibshirani, R. Least angle regression. Ann. Stat. 2004, 32, 407–499. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, Q. A statistical learning assessment of Huber regression. J. Approx. Theory 2022, 273, 105660. [Google Scholar] [CrossRef]
- Wang, J.; Kwon, S.; Shim, B. Generalized Orthogonal Matching Pursuit. IEEE Trans. Signal Process. 2012, 60, 6202–6216. [Google Scholar] [CrossRef]
- Crammer, K.; Dekel, O.; Keshet, J.; Shalev-Shwartz, S.; Singer, Y. Online Passive-Aggressive Algorithms. J. Mach. Learn. Res. 2006, 7, 551–585. [Google Scholar]
- Calle, M.L.; Sanchez-Espigares, J.A. Classification trees and regression in biomedical research. Med. Clin. 2007, 129, 702–706. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Classification and regression trees. Nat. Methods 2017, 14, 757–758. [Google Scholar] [CrossRef]
- Grüning, M.; Kropf, S. A Ridge Classification Method for High-dimensional Observations. In From Data and Information Analysis to Knowledge Engineering, Proceedings of the 29th Annual Conference of the Gesellschaft für Klassifikation eV University of Magdeburg, 9–11 March 2005; Springer: Berlin/Heidelberg, Germany, 2006; pp. 684–691. [Google Scholar]
- Jerome, H.F. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.-Y. LightGBM: A highly efficient gradient boosting decision tree. In Proceedings of the 31st International Conference on Neural Information Processing Systems; Curran Associates Inc.: Long Beach, CA, USA, 2017; pp. 3149–3157. [Google Scholar]
- Sheridan, R.P.; Wang, W.M.; Liaw, A.; Ma, J.; Gifford, E.M. Extreme Gradient Boosting as a Method for Quantitative Structure–Activity Relationships. J. Chem. Inf. Model. 2016, 56, 2353–2360. [Google Scholar] [CrossRef]
- Vens, C. Random Forest. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 1812–1813. [Google Scholar] [CrossRef]
- Scalzo, F.; Hamilton, R.; Asgari, S.; Kim, S.; Hu, X. Intracranial hypertension prediction using extremely randomized decision trees. Med. Eng. Phys. 2012, 34, 1058–1065. [Google Scholar] [CrossRef]
- Freund, Y.; Schapire, R.E. A Desicion-Theoretic Generalization of On-Line Learning and an Application to Boosting. In Computational Learning Theory; Springer: Berlin/Heidelberg, Germany, 1995; pp. 23–37. [Google Scholar]
- Pudjihartono, N.; Fadason, T.; Kempa-Liehr, A.W.; O’Sullivan, J.M. A Review of Feature Selection Methods for Machine Learning-Based Disease Risk Prediction. Front. Bioinform. 2022, 2, 927312. [Google Scholar] [CrossRef]
- Saarela, M.; Jauhiainen, S. Comparison of feature importance measures as explanations for classification models. SN Appl. Sci. 2021, 3, 272. [Google Scholar] [CrossRef]
- Roth, A.E. The Shapley Value: Essays in Honor of Lloyd S. Shapley; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Patil, R.; Gudivada, V. A Review of Current Trends, Techniques, and Challenges in Large Language Models (LLMs). Appl. Sci. 2024, 14, 2074. [Google Scholar] [CrossRef]
- Wubishet, B.L.; Tesfaye, W.H.; Khan, M.N.; Thomas, J.; Tuffaha, H.; Comans, T.A.; Scuffham, P.; Erku, D.A. Public hesitancy to COVID-19 vaccine and the role of pharmacists in addressing the problem and improving uptake. J. Pharm. Pract. Res. Off. J. Soc. Hosp. Pharm. Aust. 2021, 51, 494–500. [Google Scholar] [CrossRef]
- Perlis, R.H.; Ognyanova, K.; Uslu, A.; Lunz Trujillo, K.; Santillana, M.; Druckman, J.N.; Baum, M.A.; Lazer, D. Trust in Physicians and Hospitals During the COVID-19 Pandemic in a 50-State Survey of US Adults. JAMA Netw. Open 2024, 7, e2424984. [Google Scholar] [CrossRef] [PubMed]
- Viskupic, F.; Wiltse, D.L. Political Partisanship and Trust in Government Predict Popular Support for COVID-19 Vaccine Mandates for Various Professions and Demographic Groups: A Research Note. Am. Polit. Res. 2023, 51, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rellosa, N. COVID-19 Vaccine Hesitancy and Refusal: The Same But Different? Del. J. Public Health 2022, 8, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Disease 2019 (COVID-19) Vaccine Safety. Vaccine Safety from the Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/vaccine-safety/vaccines/covid-19.html (accessed on 12 October 2024).
- Garza, N.; Leibensperger, M.; Bonnevie, E. The Association Between Receiving the Flu and COVID-19 Vaccines and Related Factors, Data from the StopFlu Campaign in Eight States and the District of Columbia, 2022. J. Community Health 2023, 48, 731–739. [Google Scholar] [CrossRef]
- Qassim, S.H.; Chemaitelly, H.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al Thani, A.A.; Al-Khatib, H.A.; Hasan, M.R.; Al-Kanaani, Z.; et al. Population immunity of natural infection, primary-series vaccination, and booster vaccination in Qatar during the COVID-19 pandemic: An observational study. EClinicalMedicine 2023, 62, 102102. [Google Scholar] [CrossRef]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef]
- Choukou, M.A.; Sanchez-Ramirez, D.C.; Pol, M.; Uddin, M.; Monnin, C.; Syed-Abdul, S. COVID-19 infodemic and digital health literacy in vulnerable populations: A scoping review. Digit Health 2022, 8, 20552076221076927. [Google Scholar] [CrossRef]
- Okan, O.; Bollweg, T.M.; Berens, E.M.; Hurrelmann, K.; Bauer, U.; Schaeffer, D. Coronavirus-Related Health Literacy: A Cross-Sectional Study in Adults during the COVID-19 Infodemic in Germany. Int. J. Environ. Res. Public Health 2020, 17, 5503. [Google Scholar] [CrossRef]
- Moustafa, H.A.M.; Kassem, A.B. COVID-19-related health literacy and preparedness to what may come: A cross-sectional study. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 114. [Google Scholar] [CrossRef]

| Variable | N (%) |
|---|---|
| Sex | |
| Male | 305 (29.9) |
| Female | 715 (70.1) |
| Race | |
| White | 710 (69.6) |
| Black | 216 (21.8) |
| Asian | 11 (1.1) |
| Other | 40 (3.9) |
| Multi-racial | 43 (4.2) |
| Ethnicity | |
| Hispanic or Latino | 71 (7.0) |
| Not Hispanic or Latino | 949 (93.0) |
| Age | |
| 18–24 | 115 (11.3) |
| 25–34 | 157 (15.4) |
| 35–44 | 195 (19.1) |
| 45–54 | 203 (19.9) |
| 55–64 | 162 (15.9) |
| 65+ | 188 (18.4) |
| Highest Degree Obtained | |
| Less than high school | 70 (6.9) |
| High school diploma or GED | 503 (49.3) |
| Associate’s degree or Vocational Certificate | 239 (23.4) |
| 4-year Bachelor’s Degree or Higher | 208 (20.4) |
| Marital Status | |
| Married | 423 (41.5) |
| Not Married | 597 (58.5) |
| Employment Status | |
| Employed | 449 (44.0) |
| Not Employed | 199 (19.5) |
| Retired | 211 (20.7) |
| Disabled, not able to work | 161 (15.8) |
| Household Income Level | |
| $0–$30,000 | 388 (38.0) |
| $30,001–$60,000 | 313 (30.7) |
| $60,001–$90,000 | 142 (13.9) |
| $90,001–$120,000 | 60 (5.9) |
| $120,000+ | 62 (6.1) |
| I choose not to say | 55 (5.4) |
| Caregiver Status | |
| Caregiver | 150 (14.7) |
| Not Caregiver | 870 (85.3) |
| Political Affiliation | |
| Republican | 425 (41.7) |
| Democrat | 209 (20.5) |
| Independent | 247 (24.2) |
| Something Else | 48 (4.7) |
| I choose not to say | 72 (7.3) |
| Insurance Status | |
| Insured | 913 (92.7) |
| Not Insured | 72 (7.3) |
| Health Status ^ | |
| Excellent | 120 (11.8) |
| Very Good | 285 (27.9) |
| Good | 375 (36.8) |
| Fair | 195 (19.1) |
| Poor | 45 (4.4) |
| Total Number of Chronic Conditions Endorsed | |
| 0 | 329 (32.3) |
| 1 | 298 (29.2) |
| 2 | 158 (15.5) |
| 3 | 125 (12.3) |
| 4 or more | 108 (10.6) |
| Missing | 2 (0.2) |
| Ever Received COVID-19 Vaccination | |
| Yes, Received | 573 (56.2) |
| No, Did Not Ever Receive | 447 (43.8) |
| Total Number of COVID-19 Doses Received | |
| 0 | 447 (43.8) |
| 1 | 80 (7.8) |
| 2 | 248 (24.3) |
| 3 | 123 (12.1) |
| 4 | 65 (6.4) |
| 5 or more | 57 (5.6) |
| Received Influenza Vaccination in 2023–2024 | |
| Yes, Received | 398 (39.5) |
| No, Did Not Receive | 610 (60.5) |
| Model | R2 | MAE | MSE | RMSE | RMSLE | MAPE |
|---|---|---|---|---|---|---|
| Random Forest Regressor | 0.45 | 0.90 | 1.35 | 1.16 | 0.52 | 0.43 |
| Bayesian Ridge | 0.43 | 0.94 | 1.39 | 1.18 | 0.53 | 0.39 |
| Linear Regression | 0.42 | 0.94 | 1.40 | 1.18 | 0.53 | 0.40 |
| Ridge Regression | 0.42 | 0.94 | 1.40 | 1.18 | 0.53 | 0.40 |
| Extra Trees Regressor | 0.42 | 0.92 | 1.41 | 1.18 | 0.52 | 0.44 |
| Elastic Net | 0.42 | 0.94 | 1.40 | 1.18 | 0.53 | 0.39 |
| Least Angle Regression | 0.42 | 0.94 | 1.41 | 1.19 | 0.53 | 0.40 |
| Huber Regressor | 0.41 | 0.94 | 1.43 | 1.19 | 0.53 | 0.41 |
| Lasso Least Angle Regression | 0.41 | 0.95 | 1.44 | 1.20 | 0.54 | 0.38 |
| Lasso Regression | 0.41 | 0.95 | 1.44 | 1.20 | 0.54 | 0.38 |
| Gradient Boosting Regressor | 0.41 | 0.92 | 1.44 | 1.20 | 0.51 | 0.45 |
| K Neighbors Regressor | 0.40 | 0.92 | 1.47 | 1.21 | 0.54 | 0.46 |
| Orthogonal Matching Pursuit | 0.40 | 0.95 | 1.45 | 1.21 | 0.54 | 0.40 |
| Light Gradient Boosting Machine | 0.39 | 0.93 | 1.49 | 1.22 | 0.53 | 0.47 |
| AdaBoost Regressor | 0.36 | 1.05 | 1.55 | 1.24 | 0.59 | 0.37 |
| Extreme Gradient Boosting | 0.29 | 1.00 | 1.74 | 1.31 | 0.56 | 0.50 |
| Dummy Regressor | −0.02 | 1.38 | 2.51 | 1.58 | 0.70 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Z.; McCormick, N.P.; Ezeala, O.M.; Durham, S.H.; Westrick, S.C. EMSIG: Uncovering Factors Influencing COVID-19 Vaccination Across Different Subgroups Characterized by Embedding-Based Spatial Information Gain. Vaccines 2024, 12, 1253. https://doi.org/10.3390/vaccines12111253
Yue Z, McCormick NP, Ezeala OM, Durham SH, Westrick SC. EMSIG: Uncovering Factors Influencing COVID-19 Vaccination Across Different Subgroups Characterized by Embedding-Based Spatial Information Gain. Vaccines. 2024; 12(11):1253. https://doi.org/10.3390/vaccines12111253
Chicago/Turabian StyleYue, Zongliang, Nicholas P. McCormick, Oluchukwu M. Ezeala, Spencer H. Durham, and Salisa C. Westrick. 2024. "EMSIG: Uncovering Factors Influencing COVID-19 Vaccination Across Different Subgroups Characterized by Embedding-Based Spatial Information Gain" Vaccines 12, no. 11: 1253. https://doi.org/10.3390/vaccines12111253
APA StyleYue, Z., McCormick, N. P., Ezeala, O. M., Durham, S. H., & Westrick, S. C. (2024). EMSIG: Uncovering Factors Influencing COVID-19 Vaccination Across Different Subgroups Characterized by Embedding-Based Spatial Information Gain. Vaccines, 12(11), 1253. https://doi.org/10.3390/vaccines12111253








