Treatment and Prevention of HPV-Associated Skin Tumors by HPV Vaccination
Abstract
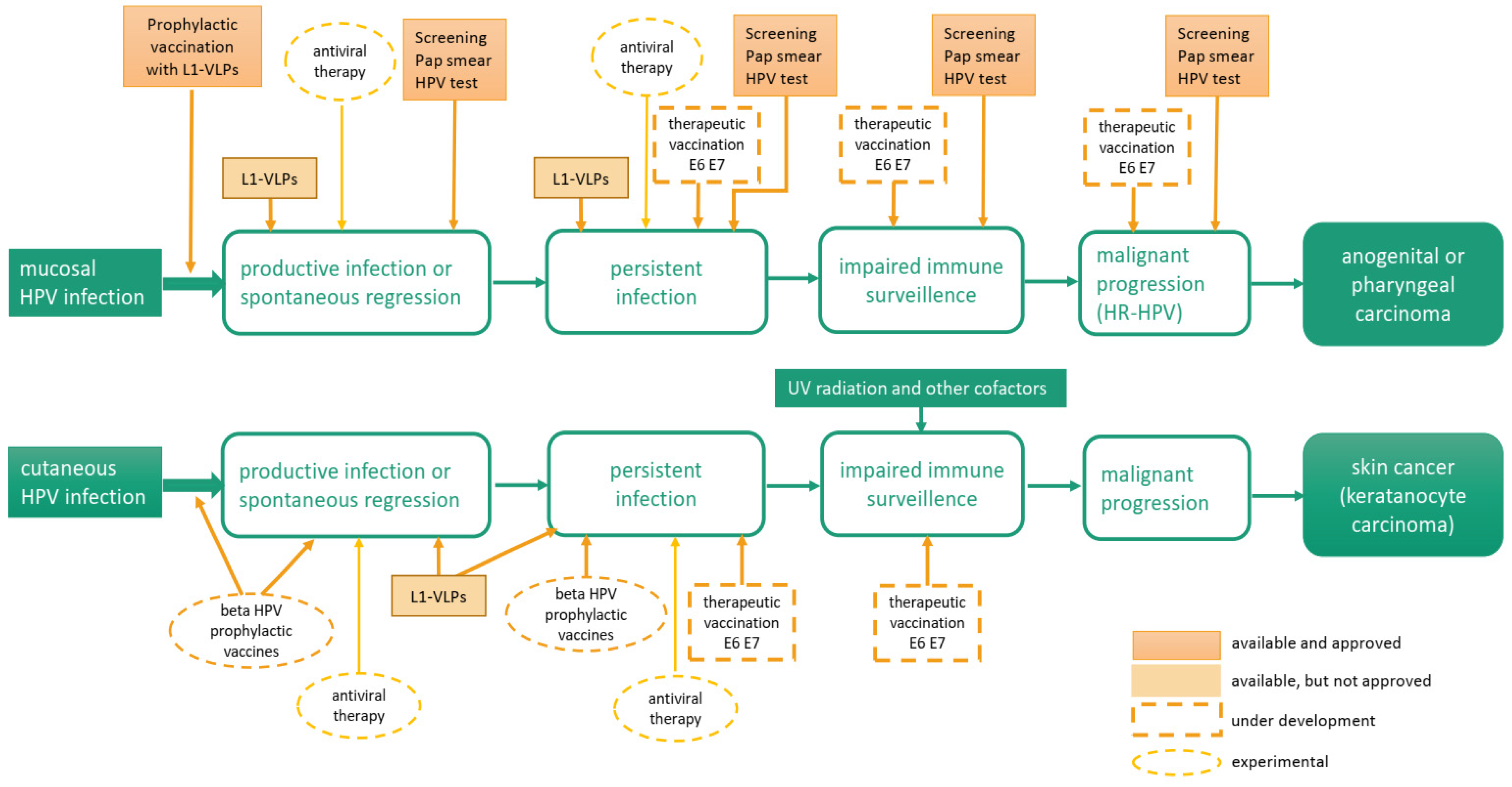
:1. Introduction
2. Human Papillomaviruses (HPV)
3. Mechanisms of HPV-Induced Cancers
3.1. Alpha HPV
3.2. Beta HPV
4. Therapeutic Interventions to Inhibit HPV-Associated Cutaneous Diseases
4.1. Management of HPV-Associated Cutaneous Lesions with Licensed HPV Vaccines
4.2. Next Generation VLP-Based Vaccines for Beta HPV-Associated Skin Cancer
4.3. Therapeutic Vaccination Against Viral Oncoproteins
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Grce, M.; Mravak-Stipetic, M. Human papillomavirus-associated diseases. Clin. Dermatol. 2014, 32, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Herrero, R.; Dai, M.; Snijders, P.J.; Meijer, C.J.; Thomas, J.O.; Hoang Anh, P.T.; Ferreccio, C.; Matos, E.; Posso, H.; et al. Reproductive factors, oral contraceptive use, and human papillomavirus infection: Pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens–Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef]
- Hancock, G.; Hellner, K.; Dorrell, L. Therapeutic HPV vaccines. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 59–72. [Google Scholar] [CrossRef]
- Schiller, J.T.; Lowy, D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012, 10, 681–692. [Google Scholar] [CrossRef]
- Antonsson, A.; Karanfilovska, S.; Lindqvist, P.G.; Hansson, B.G. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 2003, 41, 2509–2514. [Google Scholar] [CrossRef]
- Nanz, L.; Keim, U.; Katalinic, A.; Meyer, T.; Garbe, C.; Leiter, U. Epidemiology of Keratinocyte Skin Cancer with a Focus on Cutaneous Squamous Cell Carcinoma. Cancers 2024, 16, 606. [Google Scholar] [CrossRef]
- Keim, U.; Katalinic, A.; Holleczek, B.; Wakkee, M.; Garbe, C.; Leiter, U. Incidence, mortality and trends of cutaneous squamous cell carcinoma in Germany, the Netherlands, and Scotland. Eur. J. Cancer 2023, 183, 60–68. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Black, J.O. Xeroderma pigmentosum. Head Neck Pathol. 2016, 10, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bresler, S.C.; Padwa, B.L.; Granter, S.R. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016, 10, 119–124. [Google Scholar] [CrossRef]
- Lazarczyk, M.; Cassonnet, P.; Pons, C.; Jacob, Y.; Favre, M. The EVER proteins as a natural barrier against papillomaviruses: A new insight into the pathogenesis of human papillomavirus infections. Microbiol. Mol. Biol. Rev. 2009, 73, 348–370. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. 2001, 63, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Bajdik, C.D.; Willemze, R.; De Gruijl, F.R.; Bavinck, J.N.B. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J. Investig. Dermatol. 2003, 120, 1087–1093. [Google Scholar] [CrossRef]
- Nindl, I.; Rösl, F. Molecular concepts of virus infections causing skin cancer in organ transplant recipients. Am. J. Transplant. 2008, 8, 2199–2204. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Harwood, C.A.; Toland, A.E.; Proby, C.M.; Euvrard, S.; Hofbauer, G.F.L.; Tommasino, M.; Bavinck, J.N.B.; KeraCon Consortium. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br. J. Dermatol. 2017, 177, 1217–1224. [Google Scholar] [CrossRef]
- Proby, C.M.; Harwood, C.A.; Neale, R.E.; Green, A.C.; Euvrard, S.; Naldi, L.; Tessari, G.; Feltkamp, M.C.; de Koning, M.N.; Quint, W.G.; et al. A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am. J. Transplant. 2011, 11, 1498–1508. [Google Scholar] [CrossRef]
- Ulrich, C.; Kanitakis, J.; Stockfleth, E.; Euvrard, S. Skin cancer in organ transplant recipients—Where do we stand today? Am. J. Transplant. 2008, 8, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, K.; Chen, Z.; Bernard, H.U.; Chan, P.K.S.; De Salle, R.; Dillner, J.; Forslund, O.; Haga, T.; McBride, A.A.; Villa, L.L.; et al. ICTV Virus Taxonomy Profile: Papillomaviridae. J. Gen. Virol. 2018, 99, 989–990. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Franceschi, S.; Diaz, M.; Muñoz, N.; Villa, L.L. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006, 24 (Suppl. S3), 26–34. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Howley, P.M.; Pfister, H.J. Beta genus papillomaviruses and skin cancer. Virology 2015, 479–480, 290–296. [Google Scholar] [CrossRef]
- Hasche, D.; Vinzón, S.E.; Rösl, F. Cutaneous Papillomaviruses and Non-melanoma Skin Cancer: Causal Agents or Innocent Bystanders? Front. Microbiol. 2018, 9, 874. [Google Scholar] [CrossRef]
- Johansson, H.; Bzhalava, D.; Ekstrom, J.; Hultin, E.; Dillner, J.; Forslund, O. Metagenomic sequencing of “HPV-negative” condylomas detects novel putative HPV types. Virology 2013, 440, 1–7. [Google Scholar] [CrossRef]
- Ma, Y.; Madupu, R.; Karaoz, U.; Nossa, C.W.; Yang, L.; Yooseph, S.; Yachimski, P.S.; Brodie, E.L.; Nelson, K.E.; Pei, Z. Human papillomavirus community in healthy persons, defined by metagenomics analysis of human microbiome project shotgun sequencing data sets. J. Virol. 2014, 88, 4786–4797. [Google Scholar] [CrossRef]
- Mejlhede, N.; Pedersen, B.V.; Frisch, M.; Fomsgaard, A. Multiple human papilloma virus types in cervical infections: Competition or synergy? APMIS 2010, 118, 346–352. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25 (Suppl. S1), 2–23. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30 (Suppl. S5), F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Freese, U.K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; zur Hausen, H. Structure and transcription of human papillomavirus type 18 and 16 sequences in cervical carcinoma cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.I.; Constandinou-Williams, C.; Wen, K.; Young, L.S.; Roberts, S.; Murray, P.G.; Woodman, C.B. Disruption of the E2 gene is a common and early event in the natural history of cervical human papillomavirus infection: A longitudinal cohort study. Cancer Res. 2009, 69, 3828–3832. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Prabhakar, A.T.; Morgan, I.M. A new role for human papillomavirus 16 E2: Mitotic activation of the DNA damage response to promote viral genome segregation. Tumour Virus Res. 2024, 18, 200291. [Google Scholar] [CrossRef]
- Skelin, J.; Tomaic, V. Comparative analysis of alpha and beta HPV E6 oncoproteins: Insights into functional distinctions and divergent mechanisms of pathogenesis. Viruses 2023, 15, 2253. [Google Scholar] [CrossRef]
- Venuti, A.; Paolini, F.; Nasir, L.; Corteggio, A.; Roperto, S.; Campo, M.S.; Borzacchiello, G. Papillomavirus E5: The smallest oncoprotein with many functions. Mol. Cancer 2011, 10, 140. [Google Scholar] [CrossRef]
- Raffa, S.; Mancini, V.; French, D.; Rollo, F.; Benevolo, M.; Giuliani, E.; Donà, M.G.; Ranieri, D.; Belleudi, F. The Expression of HPV-16 E5 Oncoprotein Impacts the Transcript Profiles of FGFR2 and EMT-Related Genes in Preneoplastic Anal Epithelium Lesions. Int. J. Mol. Sci. 2024, 25, 12085. [Google Scholar] [CrossRef]
- Antonsson, A.; Erfurt, C.; Hazard, K.; Holmgren, V.; Simon, M.; Kataoka, A.; Hossain, S.; Hakangard, C.; Hansson, B.G. Prevalence and type spectrum of human papillomaviruses in healthy skin samples collected in three continents. J. Gen. Virol. 2003, 84, 1881–1886. [Google Scholar] [CrossRef]
- Viarisio, D.; Gissmann, L.; Tommasino, M. Human papillomaviruses and carcinogenesis: Well-established and novel models. Curr. Opin. Virol. 2017, 26, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wendel, S.O.; Wallace, N.A. Loss of genome fidelity: Beta HPVs and the DNA damage response. Front. Microbiol. 2017, 8, 2250. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S.J.; Crequer, A.; Matos, I.; Hum, D.; Gunasekharan, V.; Lorenzo, L.; Jabot-Hanin, F.; Imahorn, E.; Arias, A.A.; Vahidnezhad, H.; et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to beta-papillomaviruses. J. Exp. Med. 2018, 215, 2289–2310. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, S.J.; Nindl, I.; Purdie, K.; Harwood, C.; Proby, C.; Breuer, J.; Majewski, S.; Pfister, H.; Wieland, U. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J. Investig. Dermatol. 2005, 125, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Arron, S.T.; Ruby, J.G.; Dybbro, E.; Ganem, D.; Derisi, J.L. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2011, 131, 1745–1753. [Google Scholar] [CrossRef]
- Chockalingam, R.; Downing, C.; Tyring, S.K. Cutaneous squamous cell carcinomas in organ transplant recipients. J. Clin. Med. 2015, 4, 1229–1239. [Google Scholar] [CrossRef]
- Bavinck, J.N.B.; Feltkamp, M.C.W.; Green, A.C.; Fiocco, M.; Euvrard, S.; Harwood, C.A.; Nasir, S.; Thomson, J.; Proby, C.M.; Naldi, L.; et al. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: A multicenter, prospective cohort study. Am. J. Transplant. 2018, 18, 1220–1230. [Google Scholar] [CrossRef]
- Hasche, D.; Akgül, B. Prevention and Treatment of HPV-Induced Skin Tumors. Cancers 2023, 15, 1709. [Google Scholar] [CrossRef]
- Hasche, D.; Stephan, S.; Braspenning-Wesch, I.; Mikulec, J.; Niebler, M.; Gröne, H.J.; Flechtenmacher, C.; Akgül, B.; Rösl, F.; Vinzón, S.E. The interplay of UV and cutaneous papillomavirus infection in skin cancer development. PLoS Pathog. 2017, 13, e1006723. [Google Scholar] [CrossRef]
- Schaper, I.D.; Marcuzzi, G.P.; Weissenborn, S.J.; Kasper, H.U.; Dries, V.; Smyth, N.; Fuchs, P.; Pfister, H. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005, 65, 1394–1400. [Google Scholar] [CrossRef]
- Viarisio, D.; Mueller-Decker, K.; Kloz, U.; Aengeneyndt, B.; Kopp-Schneider, A.; Gröne, H.J.; Gheit, T.; Flechtenmacher, C.; Gissmann, L.; Tommasino, M. E6 and E7 from Beta Hpv38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog. 2011, 7, e1002125. [Google Scholar] [CrossRef] [PubMed]
- Hufbauer, M.; Lazic, D.; Akgül, B.; Brandsma, J.L.; Pfister, H.; Weissenborn, S.J. Enhanced human papillomavirus type 8 oncogene expression levels are crucial for skin tumorigenesis in transgenic mice. Virology 2010, 403, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Viarisio, D.; Müller-Decker, K.; Accardi, R.; Robitaille, A.; Dürst, M.; Beer, K.; Jansen, L.; Flechtenmacher, C.; Bozza, M.; Harbottle, R.; et al. Beta HPV38 oncoproteins act with a hit-and-run mechanism in ultraviolet radiation-induced skin carcinogenesis in mice. PLoS Pathog. 2018, 14, e1006783. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Kramer, R.E.; Tan, M.J.; Hayes, S.D.; Harper, J.W.; Howley, P.M. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J. Virol. 2012, 86, 13174–131786. [Google Scholar] [CrossRef]
- Wallace, N.A.; Robinson, K.; Howie, H.L.; Galloway, D.A. β-HPV 5 and 8 E6 disrupt homology dependent double strand break repair by attenuating BRCA1 and BRCA2 expression and foci formation. PLoS Pathog. 2015, 11, e1004687. [Google Scholar] [CrossRef]
- Akgül, B.; Ghali, L.; Davies, D.; Pfister, H.; Leigh, I.M.; Storey, A. HPV8 early genes modulate differentiation and cell cycle of primary human adult keratinocytes. Exp. Dermatol. 2007, 16, 590–599. [Google Scholar] [CrossRef]
- Bandolin, L.; Borsetto, D.; Fussey, J.; Da Mosto, M.C.; Nicolai, P.; Menegaldo, A.; Calabrese, L.; Tommasino, M.; Boscolo-Rizzo, P. Beta human papillomaviruses infection and skin carcinogenesis. Rev. Med. Virol. 2020, 30, e2104. [Google Scholar] [CrossRef]
- White, E.A.; Howley, P.M. Proteomic approaches to the study of papillomavirus–host interactions. Virology 2013, 435, 57–69. [Google Scholar] [CrossRef]
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Li, T.; Hasegawa, T.; Ha, D.T.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 575, 519–522. [Google Scholar] [CrossRef]
- Reschner, A.; Bontems, S.; Le Gac, S.; Lambermont, J.; Marcelis, L.; Defrancq, E.; Hubert, P.; Moucheron, C.; Mesmaeker, K.-D.; Raes, M. Ruthenium oligonucleotides, targeting HPV16 E6 oncogene, inhibit the growth of cervical cancer cells under illumination by a mechanism involving p53. Gene Ther. 2013, 20, 435–443. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Rao, Z. Ribozyme targeting HPV16 E6E7 transcripts in cervical cancer cells suppresses cell growth and sensitizes cells to chemotherapy and radiotherapy. Cancer Biol. Ther. 2004, 3, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.M.; Kornepati, A.V.; Goldstein, M.; Bogerd, H.P.; Poling, B.C.; Whisnant, A.W.; Kastan, M.B.; Cullen, B.R. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J. Virol. 2014, 88, 11965–11972. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Liu, J.; Fan, W.; Li, R.; Cui, Z.; Jin, Z.; Huang, Z.; Xie, H.; Li, L.; Huang, Z.; et al. Gene knock-out chain reaction enables high disruption efficiency of HPV18 E6/E7 genes in cervical cancer cells. Mol. Ther. Oncol. 2022, 24, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, C.S.; Roden, R.B.; Garcea, R.L. Immunizing against Anogenital Cancer: HPV Vaccines. PLoS Pathog. 2016, 12, e1005587. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, S.E.; Giuliano, A.R.; Palefsky, J.M.; Lazcano-Ponce, E.; Penny, M.E.; Cabello, R.E.; Moreira, E.D.; Baraldi, E.; Jessen, H.; Ferenczy, A.; et al. Efficacy, Immunogenicity, and Safety of a Quadrivalent HPV Vaccine in Men: Results of an Open-Label, Long-Term Extension of a Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Infect. Dis. 2022, 22, 413–425. [Google Scholar] [CrossRef]
- Bissett, S.L.; Draper, E.; Myers, R.E.; Godi, A.; Beddows, S. Cross-neutralizing antibodies elicited by the Cervarix(R) human papillomavirus vaccine display a range of Alpha-9 inter-type specificities. Vaccine 2014, 32, 1139–1146. [Google Scholar] [CrossRef]
- Hildesheim, A.; Herrero, R.; Wacholder, S.; Rodriguez, A.C.; Solomon, D.; Bratti, M.C.; Schiller, J.T.; Gonzalez, P.; Dubin, G.; Porras, C.; et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: A randomized trial. JAMA 2007, 298, 743–753. [Google Scholar] [CrossRef]
- Gay, J.; Johnson, N.; Kavuru, V.; Phillips, M. Utility of the Human Papillomavirus Vaccination in Management of HPV-associated Cutaneous Lesions. Skin Ther. Lett. 2021, 26, 6–8. [Google Scholar]
- Șandru, F.; Radu, A.M.; Petca, A.; Dumitrașcu, M.C.; Petca, R.C.; Roman, A.M. Unveiling the Therapeutic Horizon: HPV Vaccines and Their Impact on Cutaneous Diseases-A Comprehensive Review. Vaccines 2024, 12, 228. [Google Scholar] [CrossRef]
- Shin, J.O.; Son, J.H.; Lee, J.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B.; Shin, K. Nonavalent Human Papilloma Virus Vaccine for the Treatment of Multiple Recalcitrant Warts: An Open-Label Study. J. Am. Acad. Dermatol. 2022, 86, 940–941. [Google Scholar] [CrossRef]
- Kost, Y.; Deutsch, A.; Zhu, T.H.; Hulur, I.; Blasiak, R.C. Vaccination against Human Papillomavirus Is Not Associated with Resolution of Verruca Vulgaris in Immunocompetent 9- to 21-Year Olds. J. Am. Acad. Dermatol. 2022, 87, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, G.; Herzum, A.; Serviddio, G.; Occella, C.; Parodi, A.; Drago, F. Efficacy of Human Papillomavirus Vaccines for Recalcitrant Anogenital and Oral Warts. J. Clin. Med. 2023, 12, 7317. [Google Scholar] [CrossRef] [PubMed]
- Nofal, E.; Emam, S.; Aldesoky, F.; Ghonemy, S.; Adelshafy, A. Intralesional Bivalent Human Papilloma Virus Vaccine as a Treatment for Anogenital Warts versus Topical Podophyllin Resin 25%: A Pilot Study. Dermatol. Ther. 2022, 35, e15384. [Google Scholar] [CrossRef]
- Fawzy, M.; Nofal, E.; Abdelkhalek, N.; Ehab, R. Intralesional Bivalent and Quadrivalent Human Papillomavirus Vaccines Didn’t Significantly Enhance the Response of Multiple Anogenital Warts When Co-Administered with Intralesional Candida Antigen Immunotherapy. A Randomized Controlled Trial. Arch. Dermatol. Res. 2023, 315, 2813–2823. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.K.; Kim, D.H.; Yoon, M.S. Condyloma accuminatum treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, 18). J. Am. Acad. Dermatol. 2011, 64, e130–e132. [Google Scholar] [CrossRef]
- Bossart, S.; Gabutti, M.P.; Seyed Jafari, S.M.; Hunger, R.E. Nonavalent human papillomavirus vaccination as alternative treatment for genital warts. Dermatol. Ther. 2020, 33, e13771. [Google Scholar] [CrossRef]
- Kreuter, A.; Wieland, U. Lack of efficacy in treating condyloma acuminata and preventing recurrences with the recombinant quadrivalent human papillomavirus vaccine in a case series of immunocompetent patients. J. Am. Acad. Dermatol. 2013, 68, 179–180. [Google Scholar] [CrossRef]
- Gilson, R.; Nugent, D.; Bennett, K.; Doré, C.J.; Murray, M.L.; Meadows, J.; Haddow, L.J.; Lacey, C.; Sandmann, F.; Jit, M.; et al. Imiquimod versus podophyllotoxin, with and without human papillomavirus vaccine, for anogenital warts: The HIPvac factorial RCT. Health Technol. Assess. 2020, 24, 1–86. [Google Scholar] [CrossRef]
- Nofal, A.; Nofal, H.; Alwirshiffani, E.; El Ghareeb, M.I. Treatment Response and Tolerability of Intralesional Quadrivalent versus Bivalent Human Papillomavirus Vaccine for Recalcitrant Warts: A Randomized Controlled Trial. J. Am. Acad. Dermatol. 2023, 89, 1051–1052. [Google Scholar] [CrossRef]
- Nygård, S.; Nygård, M.; Orumaa, M.; Hansen, B.T. Quadrivalent HPV vaccine effectiveness against anogenital warts: A registry-based study of 2,2 million individuals. Vaccine 2023, 41, 5469–5476. [Google Scholar] [CrossRef]
- Nichols, A.J.; Allen, A.H.; Shareef, S.; Badiavas, E.V.; Kirsner, R.S.; Ioannides, T. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017, 153, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.J.; Gonzalez, A.; Clark, E.S.; Han, W.N.; Rosen, A.C.; Guzman, W.; Rabinovitz, H.; Badiavas, E.V.; Kirsner, R.S.; Ioannides, T. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018, 154, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Bossart, S.; Daneluzzi, C.; Moor, M.B.; Hirzel, C.; Heidemeyer, K.; Seyed Jafari, S.M.; Hunger, R.E.; Sidler, D. HPV Vaccination in Immunosuppressed Patients with Established Skin Warts and Non-Melanoma Skin Cancer: A Single-Institutional Cohort Study. Vaccines 2023, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Wenande, E.; Bech-Thomsen, N.; Togsverd-Bo, K.; Haedersdal, M. Off-Label 9-Valent Human Papillomavirus Vaccination for Actinic Keratosis: A Case Series. Case Rep. Dermatol. 2021, 13, 457–463. [Google Scholar] [CrossRef]
- Kreuter, A.; Waterboer, T.; Wieland, U. Regression of cutaneous warts in a patient with WILD syndrome following recombinant quadrivalent human papillomavirus vaccination. Arch. Dermatol. 2010, 146, 1196–1197. [Google Scholar] [CrossRef]
- Vinzón, S.E.; Braspenning-Wesch, I.; Müller, M.; Geissler, E.K.; Nindl, I.; Grone, H.J.; Schäfer, K.; Rösl, F. Protective vaccination against papillomavirus-induced skin tumors under immunocompetent and immunosuppressive conditions: A preclinical study using a natural outbred animal model. PLoS Pathog. 2014, 10, e1003924. [Google Scholar] [CrossRef]
- Gambhira, R.; Karanam, B.; Jagu, S.; Roberts, J.N.; Buck, C.B.; Bossis, I.; Alphs, H.; Culp, T.; Christensen, N.D.; Roden, R.B. S A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J. Virol. 2007, 81, 13927–13931. [Google Scholar] [CrossRef]
- Kwak, K.; Jiang, R.; Wang, J.W.; Jagu, S.; Kirnbauer, R.; Roden, R.B. Impact of inhibitors and L2 antibodies upon the infectivity of diverse alpha and beta human papillomavirus types. PLoS ONE 2014, 9, e97232. [Google Scholar] [CrossRef]
- Tsakogiannis, D.; Nikolaidis, M.; Zagouri, F.; Zografos, E.; Kottaridi, C.; Kyriakopoulou, Z.; Tzioga, L.; Markoulatos, P.; Amoutzias, G.D.; Bletsa, G. Mutation Profile of HPV16 L1 and L2 Genes in Different Geographic Areas. Viruses 2022, 15, 141. [Google Scholar] [CrossRef]
- Kondo, K.; Ishii, Y.; Ochi, H.; Matsumoto, T.; Yoshikawa, H.; Kanda, T. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology 2007, 358, 266–272. [Google Scholar] [CrossRef]
- Wang, J.W.; Jagu, S.; Wu, W.H.; Viscidi, R.P.; Macgregor-Das, A.; Fogel, J.M.; Kwak, K.; Daayana, S.; Kitchener, H.; Stern, P.L.; et al. Seroepidemiology of Human Papillomavirus 16 (HPV16) L2 and Generation of L2-Specific Human Chimeric Monoclonal Antibodies. Clin. Vaccine Immunol. 2015, 22, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Jagu, S.; Karanam, B.; Gambhira, R.; Chivukula, S.V.; Chaganti, R.J.; Lowy, D.R.; Schiller, J.T.; Roden, R.B. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J. Natl. Cancer Inst. 2009, 101, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Kalnin, K.; Tibbitts, T.; Yan, Y.; Stegalkina, S.; Shen, L.; Costa, V.; Sabharwal, R.; Anderson, S.F.; Day, P.M.; Christensen, N.; et al. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine 2014, 32, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Chen, X.; Wang, Z.; Wang, S.; Qu, C.; Zhang, J.; Xu, X. Lipidated L2 epitope repeats fused with a single-chain antibody fragment targeting human FcgammaRI elicited cross-neutralizing antibodies against a broad spectrum of human papillomavirus types. Vaccine 2016, 34, 5531–5539. [Google Scholar] [CrossRef]
- Spagnoli, G.; Bolchi, A.; Cavazzini, D.; Pouyanfard, S.; Muller, M.; Ottonello, S. Secretory production of designed multipeptides displayed on a thermostable bacterial thioredoxin scaffold in Pichia pastoris. Protein Expr. Purif. 2017, 129, 150–157. [Google Scholar] [CrossRef]
- Nieto, K.; Stahl-Hennig, C.; Leuchs, B.; Muller, M.; Gissmann, L.; Kleinschmidt, J.A. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Hum. Gene Ther. 2012, 23, 733–741. [Google Scholar] [CrossRef]
- Wu, W.H.; Alkutkar, T.; Karanam, B.; Roden, R.B.; Ketner, G.; Ibeanu, O.A. Capsid display of a conserved human papillomavirus L2 peptide in the adenovirus 5 hexon protein: A candidate prophylactic hpv vaccine approach. Virol. J. 2015, 12, 140. [Google Scholar] [CrossRef]
- Vujadinovic, M.; Khan, S.; Oosterhuis, K.; Uil, T.G.; Wunderlich, K.; Damman, S.; Boedhoe, S.; Verwilligen, A.; Knibbe, J.; Serroyen, J.; et al. Adenovirus based HPV L2 vaccine induces broad cross-reactive humoral immune responses. Vaccine 2018, 36, 4462–4470. [Google Scholar] [CrossRef]
- Tumban, E.; Peabody, J.; Peabody, D.S.; Chackerian, B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS ONE 2011, 6, e23310. [Google Scholar] [CrossRef]
- Yoon, S.W.; Lee, T.Y.; Kim, S.J.; Lee, I.H.; Sung, M.H.; Park, J.S.; Poo, H. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine 2012, 30, 3286–3294. [Google Scholar] [CrossRef]
- Huber, B.; Schellenbacher, C.; Shafti-Keramat, S.; Jindra, C.; Christensen, N.; Kirnbauer, R. Chimeric L2-Based Virus-Like Particle (VLP) Vaccines Targeting Cutaneous Human Papillomaviruses (HPV). PLoS ONE 2017, 12, e0169533. [Google Scholar] [CrossRef] [PubMed]
- Schellenbacher, C.; Kwak, K.; Fink, D.; Shafti-Keramat, S.; Huber, B.; Jindra, C.; Faust, H.; Dillner, J.; Roden, R.B.S.; Kirnbauer, R. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J. Investig. Dermatol. 2013, 133, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Schellenbacher, C.; Roden, R.; Kirnbauer, R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J. Virol. 2009, 83, 10085–10095. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Wang, J.W.; Roden, R.B.S.; Kirnbauer, R. RG1-VLP and Other L2-Based, Broad-Spectrum HPV Vaccine Candidates. J. Clin. Med. 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Ahmels, M.; Mariz, F.C.; Braspenning-Wesch, I.; Stephan, S.; Huber, B.; Schmidt, G.; Cao, R.; Müller, M.; Kirnbauer, R.; Rösl, F.; et al. Next generation L2-based HPV vaccines cross-protect against cutaneous papillomavirus infection and tumor development. Front. Immunol. 2022, 13, 1010790. [Google Scholar] [CrossRef]
- Mariz, F.C.; Balz, K.; Dittrich, M.; Zhang, Y.; Yang, F.; Zhao, X.; Bolchi, A.; Ottonello, S.; Müller, M. A broadly protective vaccine against cutaneous human papillomaviruses. NPJ Vaccines 2022, 7, 116. [Google Scholar] [CrossRef]
- Olczak, P.; Matsui, K.; Wong, M.; Alvarez, J.; Lambert, P.; Christensen, N.D.; Hu, J.; Huber, B.; Kirnbauer, R.; Wang, J.W.; et al. RG2-VLP: A vaccine designed to broadly protect against anogenital and skin human papillomaviruses causing human cancer. J. Virol. 2022, 96, e0056622. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Zhang, H.; Shen, J.; Chen, J.; Hong, J.; Xu, Y.; Qian, C. Prophylactic and Therapeutic HPV Vaccines: Current Scenario and Perspectives. Front. Cell. Infect. Microbiol. 2022, 12, 909223. [Google Scholar] [CrossRef]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic Vaccines for High-Risk HPV-Associated Diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef]
- Smith, J.A.; Haberstroh, F.S.; White, E.A.; Livingston, D.M.; DeCaprio, J.A.; Howley, P.M. SMCX and Components of the TIP60 Complex Contribute to E2 Regulation of the HPV E6/E7 Promoter. Virology 2014, 468–470, 311–321. [Google Scholar] [CrossRef]
- Hufbauer, M.; Rattay, S.; Hagen, C.; Quaas, A.; Pfister, H.; Hartmann, G.; Coch, C.; Akgül, B. Poly(I:C) Treatment Prevents Skin Tumor Formation in the Preclinical HPV8 Transgenic Mouse Model. J. Investig. Dermatol. 2023, 143, 1197–1207.e3. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Bellone, S.; Palmieri, M.; Zanolini, A.; Ravaggi, A.; Siegel, E.R.; Roman, J.J.; Pecorelli, S.; Cannon, M.J. Human Papillomavirus Type 16 and 18 E7-Pulsed Dendritic Cell Vaccination of Stage IB or IIA Cervical Cancer Patients: A Phase I Escalating Dose Trial. J. Virol. 2008, 82, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Mehta, A.; Jain, M.; Gupta, S.; Nagarkar, R.V.; John, S.; Petit, R. A Randomized Phase 2 Study of ADXS11-001 Listeria MonocytogenesListeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M.; Feng, L.; Lee, J.J.; Tran, H.; Kim, Y.U.; et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef]
- van Poelgeest, M.I.; Welters, M.J.; van Esch, E.M.; Stynenbosch, L.F.; Kerpershoek, G.; van Persijn van Meerten, E.L.; van den Hende, M.; Löwik, M.J.; Berends-van der Meer, D.M.; Fathers, L.M.; et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef]
- Ren, F.; Xu, Y.; Mao, L.; Ou, R.; Ding, Z.; Zhang, X.; Tang, J.; Li, B.; Jia, Z.; Tian, Z.; et al. Heat Shock Protein 110 Improves the Antitumor Effects of the Cytotoxic T Lymphocyte Epitope E7(49-57) in Mice. Cancer Biol. Ther. 2010, 9, 134–141. [Google Scholar] [CrossRef]
- Arribillaga, L.; Echeverria, I.; Belsue, V.; Gomez, T.; Lozano, T.; Casares, N.; Villanueva, L.; Domingos-Pereira, S.; Romero, P.J.; Nardelli-Haefliger, D.; et al. Bivalent Therapeutic Vaccine Against HPV16/18 Genotypes Consisting of a Fusion Protein Between the Extra Domain A From Human Fibronectin and HPV16/18 E7 Viral Antigens. J. Immunother. Cancer 2020, 8, e000704. [Google Scholar] [CrossRef]
- Weissenborn, S.J.; De Koning, M.N.; Wieland, U.; Quint, W.G.; Pfister, H.J. Intrafamilial transmission and family-specific spectra of cutaneous betapapillomaviruses. J. Virol. 2009, 83, 811–816. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Chen, A.C.; Keleher, A.; McMillan, N.A.; Antonsson, A. Shared and persistent asymptomatic cutaneous human papillomavirus infections in healthy skin. J. Med. Virol. 2009, 81, 1444–1449. [Google Scholar] [CrossRef]
- Van der Burg, S.; Kwappenberg, K.; O’Neill, T.; Brandt, R.; Melief, C.; Hickling, J.; Offringa, R. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine 2001, 19, 3652–3660. [Google Scholar] [CrossRef]
- De Jong, A.; O’Neill, T.; Khan, A.; Kwappenberg, K.; Chisholm, S.; Whittle, N.; Dobson, J.; Jack, L.; Roberts, J.S.C.; Offringa, R.; et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 2002, 20, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Daayana, S.; Elkord, E.; Winters, U.; Pawlita, M.; Roden, R.B.S.; Stern, P.L.; Kitchener, H.C. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Cancer 2010, 102, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; Davies, M.; Holding, F.; Fallon, R.; Mann, A.; O’Neill, T.; Roberts, J.S.C. Phase I safety and antigenicity of TA-GW: A recombinant HPV6 L2E7 vaccine for the treatment of genital warts. Vaccine 1999, 17, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.J.N.; Thompson, H.S.G.; Monteiro, E.F.; O’Neill, T.; Davies, M.L.; Holding, F.P.; Fallon, R.E.; Roberts, J.S.C. Phase IIa safety and immunogenicity of a therapeutic vaccine, TA-GW: A recombinant HPV6 L2E7 vaccine for the treatment of genital warts. J. Infect. Dis. 1999, 179, 612–618. [Google Scholar] [CrossRef]
- Vandepapelière, P.; Barrasso, R.; Meijer, C.J.L.M.; Walboomers, J.M.M.; Wettendorff, M.; Stanberry, L.R.; Lacey, C.J.N. Randomized Controlled Trial of an Adjuvanted Human Papillomavirus (HPV) Type 6 L2E7 Vaccine: Infection of External Anogenital Warts with Multiple HPV Types and Failure of Therapeutic Vaccination. J. Infect. Dis. 2005, 192, 2099–2107. [Google Scholar] [CrossRef]
- Kim, D.; Gambhira, R.; Karanam, B.; Monie, A.; Hung, C.-F.; Roden, R.; Wu, T.-C. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine 2008, 26, 351–360. [Google Scholar] [CrossRef]
- Jiang, R.T.; Wang, J.W.; Peng, S.; Huang, T.C.; Wang, C.; Cannella, F.; Chang, Y.N.; Viscidi, R.; Best, S.R.A.; Hung, C.-F.; et al. Spontaneous and vaccine-induced clearance of Mus musculus papillomavirus 1 infection. J. Virol. 2017, 91, e00699-17. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef]
- Brito, L.A.; Kommareddy, S.; Maione, D.; Uematsu, Y.; Giovani, C.; Scorza, F.B.; Otten, G.R.; Yu, D.; Mandl, C.W.; Mason, P.W.; et al. Self-Amplifying mRNA Vaccines. Adv. Genet. 2015, 89, 179–233. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Thomson, J.; Harwood, C.; Leigh, I. The Role of the Immune System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2009. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Naumann, R.W.; Leath, C.A., 3rd. Advances in Immunotherapy for Cervical Cancer. Curr. Opin. Oncol. 2020, 32, 481–487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, T.; Stockfleth, E. Treatment and Prevention of HPV-Associated Skin Tumors by HPV Vaccination. Vaccines 2024, 12, 1439. https://doi.org/10.3390/vaccines12121439
Meyer T, Stockfleth E. Treatment and Prevention of HPV-Associated Skin Tumors by HPV Vaccination. Vaccines. 2024; 12(12):1439. https://doi.org/10.3390/vaccines12121439
Chicago/Turabian StyleMeyer, Thomas, and Eggert Stockfleth. 2024. "Treatment and Prevention of HPV-Associated Skin Tumors by HPV Vaccination" Vaccines 12, no. 12: 1439. https://doi.org/10.3390/vaccines12121439
APA StyleMeyer, T., & Stockfleth, E. (2024). Treatment and Prevention of HPV-Associated Skin Tumors by HPV Vaccination. Vaccines, 12(12), 1439. https://doi.org/10.3390/vaccines12121439






