PRRSV-Vaccinated, Seronegative Sows and Maternally Derived Antibodies (II): Impact on PRRSV-1 Vaccine Effectiveness and Challenge Outcomes in Piglets
Abstract
:1. Introduction
2. Materials and Methods
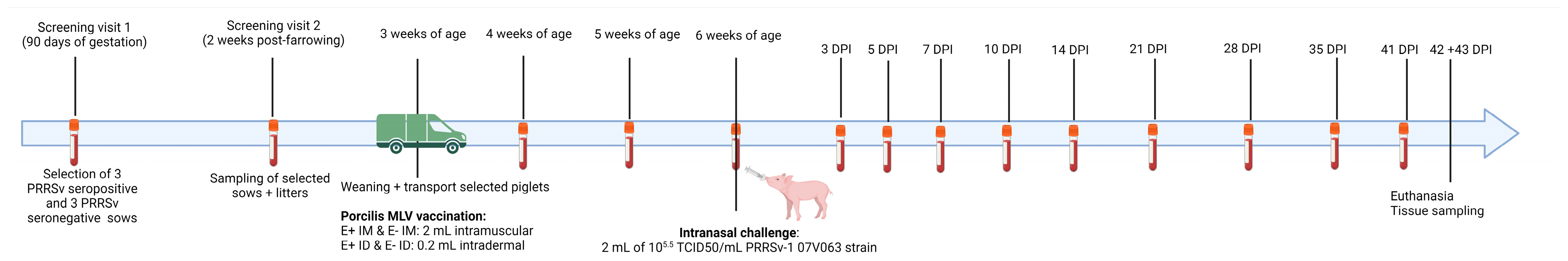
2.1. Study Design
2.2. Viruses and Viral Titration
2.3. Antibody Analysis
2.4. Cytokine Analysis
2.5. RT-qPCR
2.6. Interferon-Gamma ELISpot
2.7. Statistics
3. Results
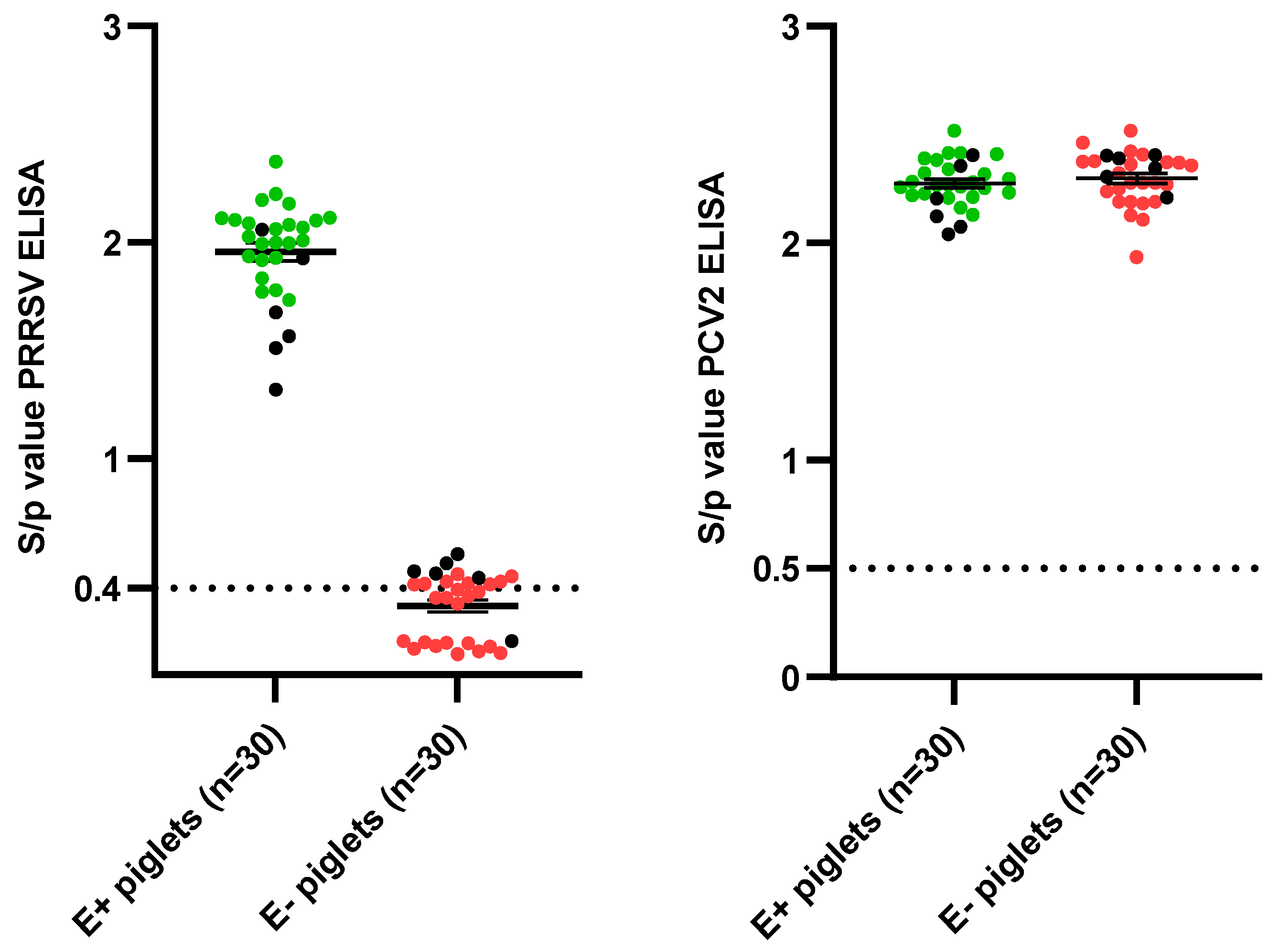
3.1. Sow and Piglet Selection
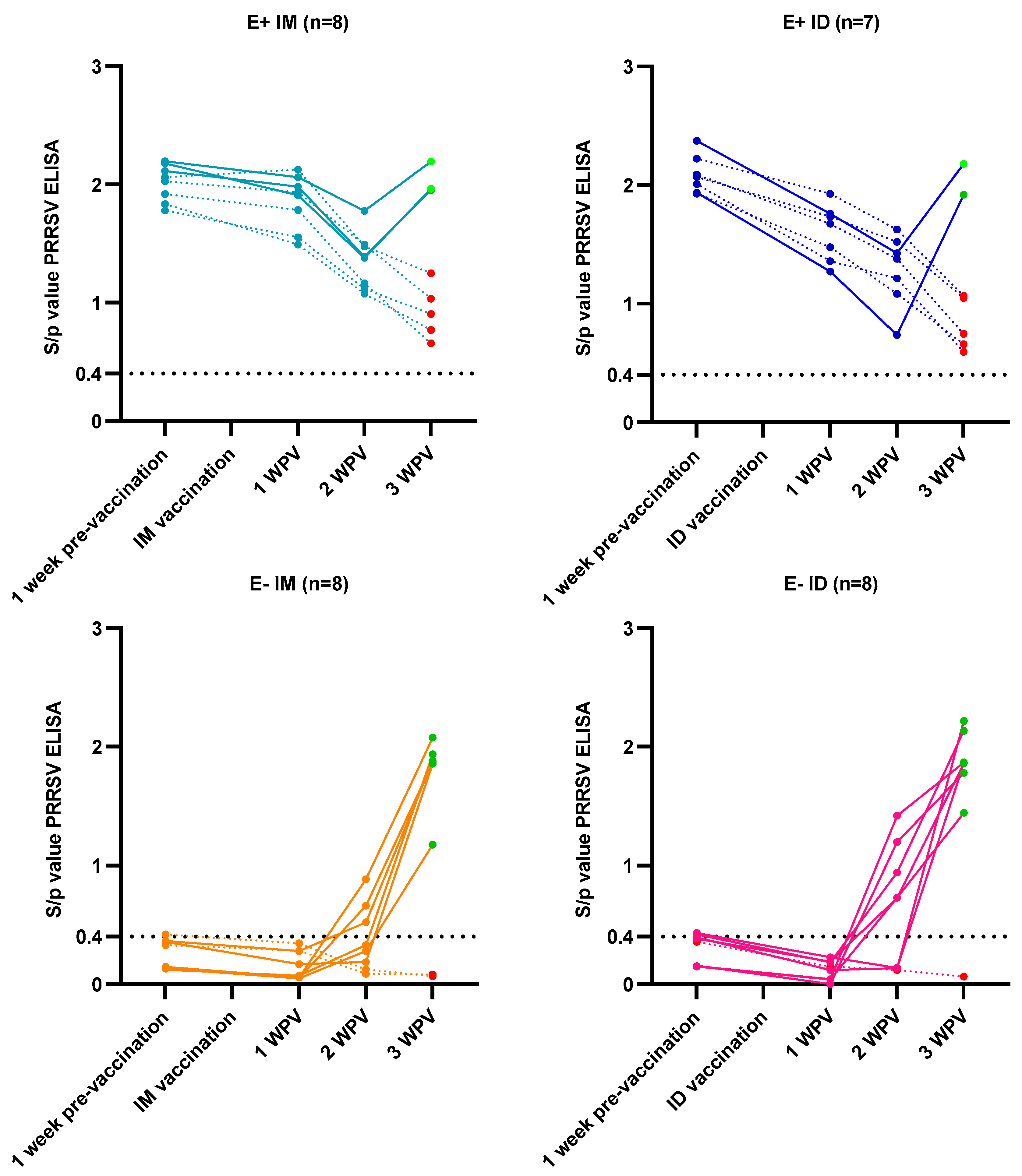
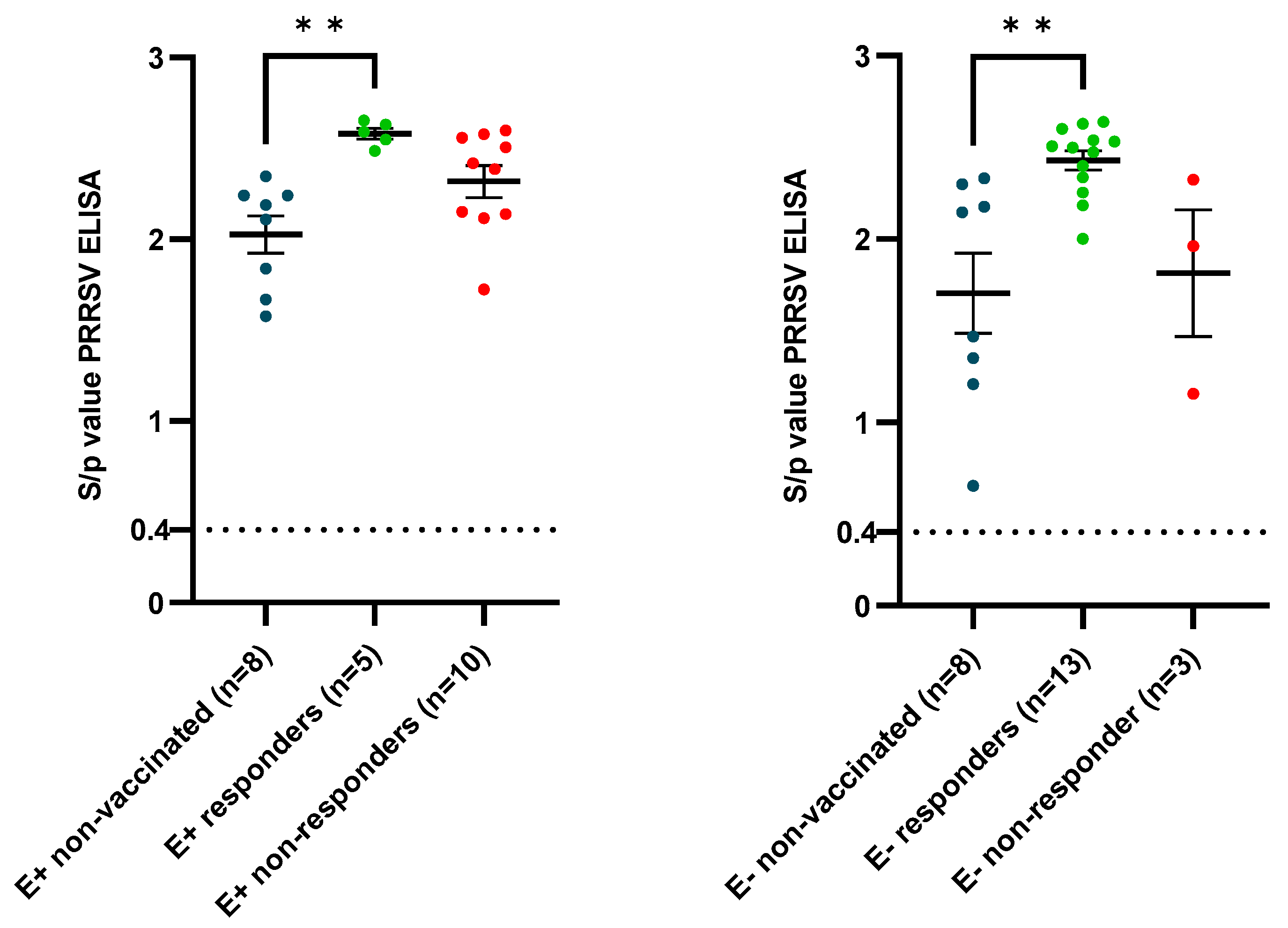
3.2. Vaccine Responses
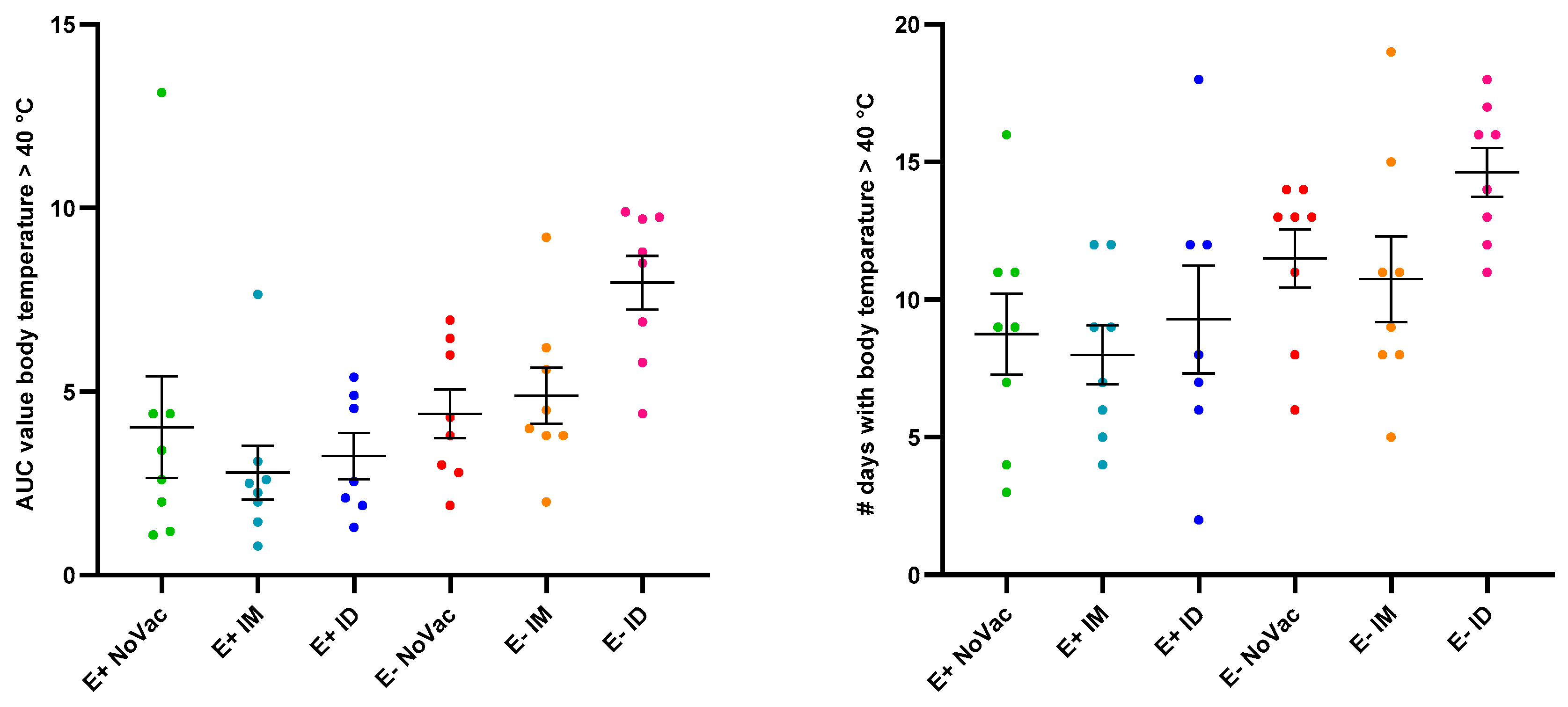
3.3. Fever Induction
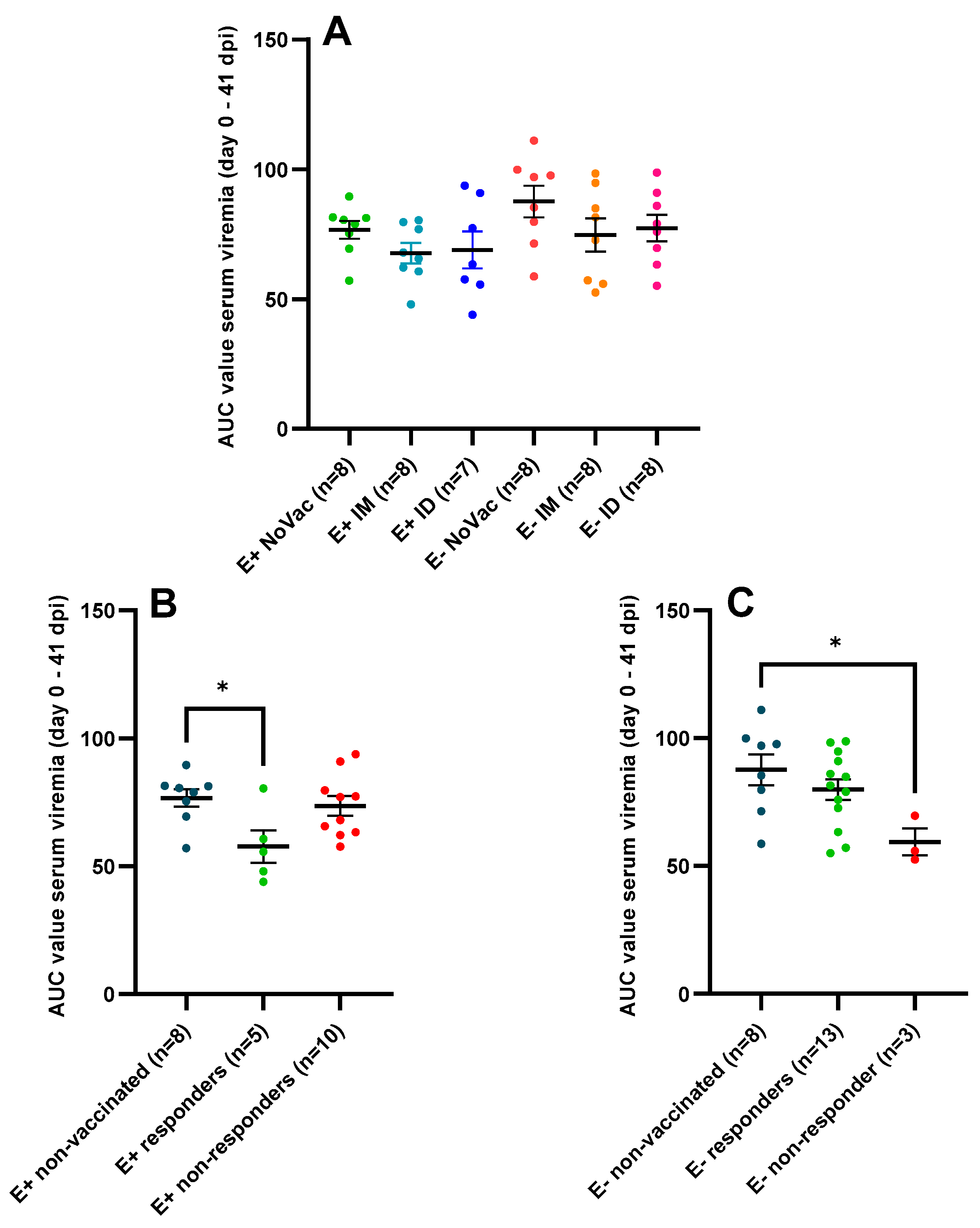
3.4. Serum Viral Load Quantified by RT-qPCR
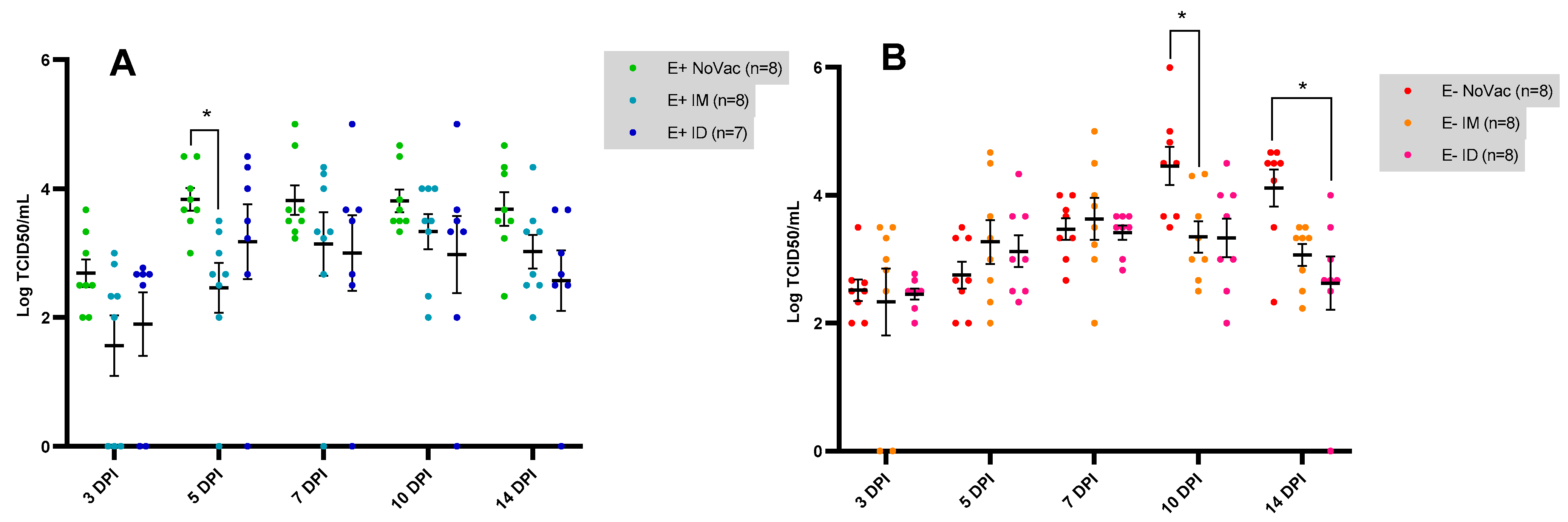
3.5. Serum Viral Load Quantified by Viral Titration
3.6. Antibody Kinetics Post-Challenge
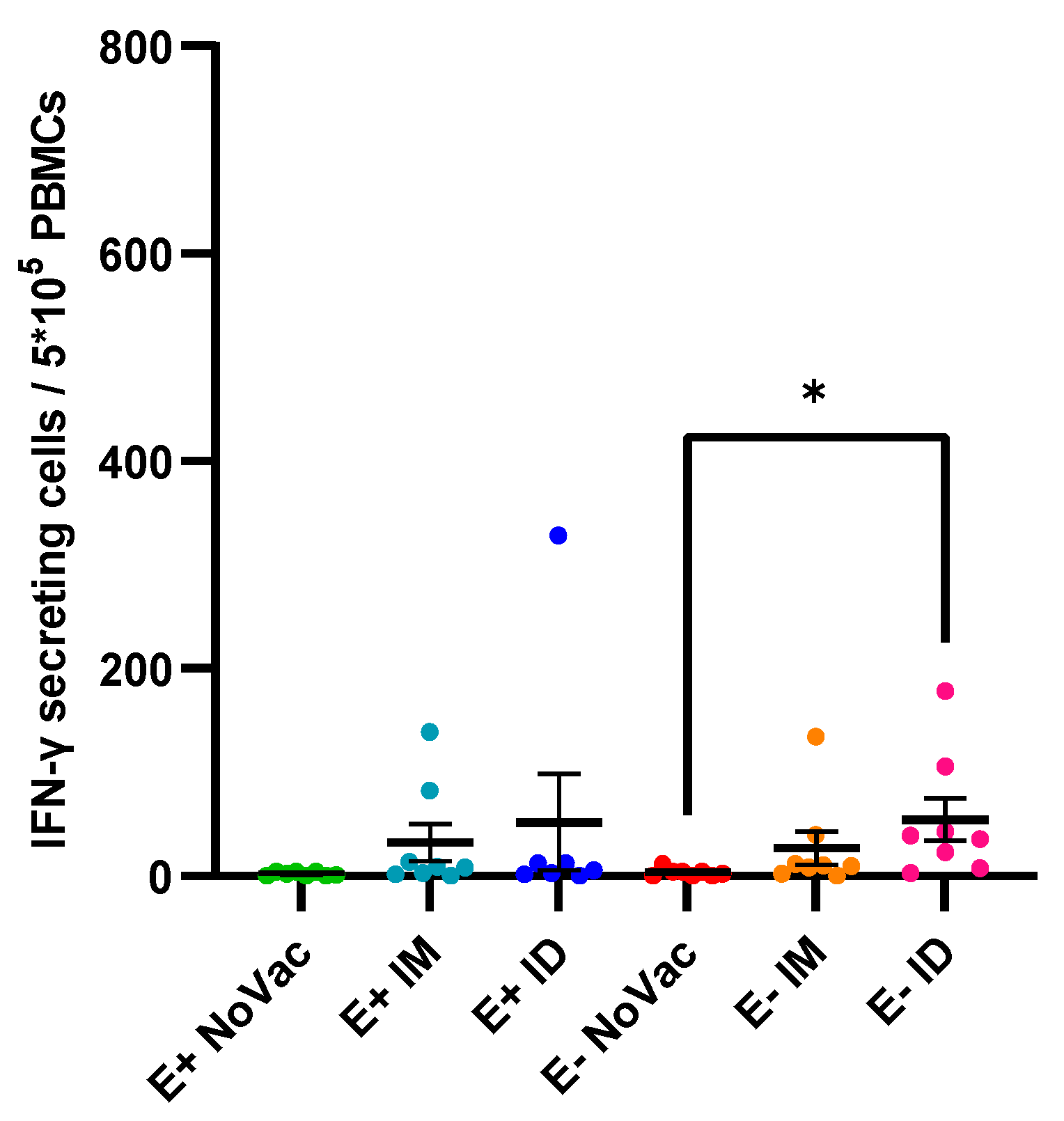
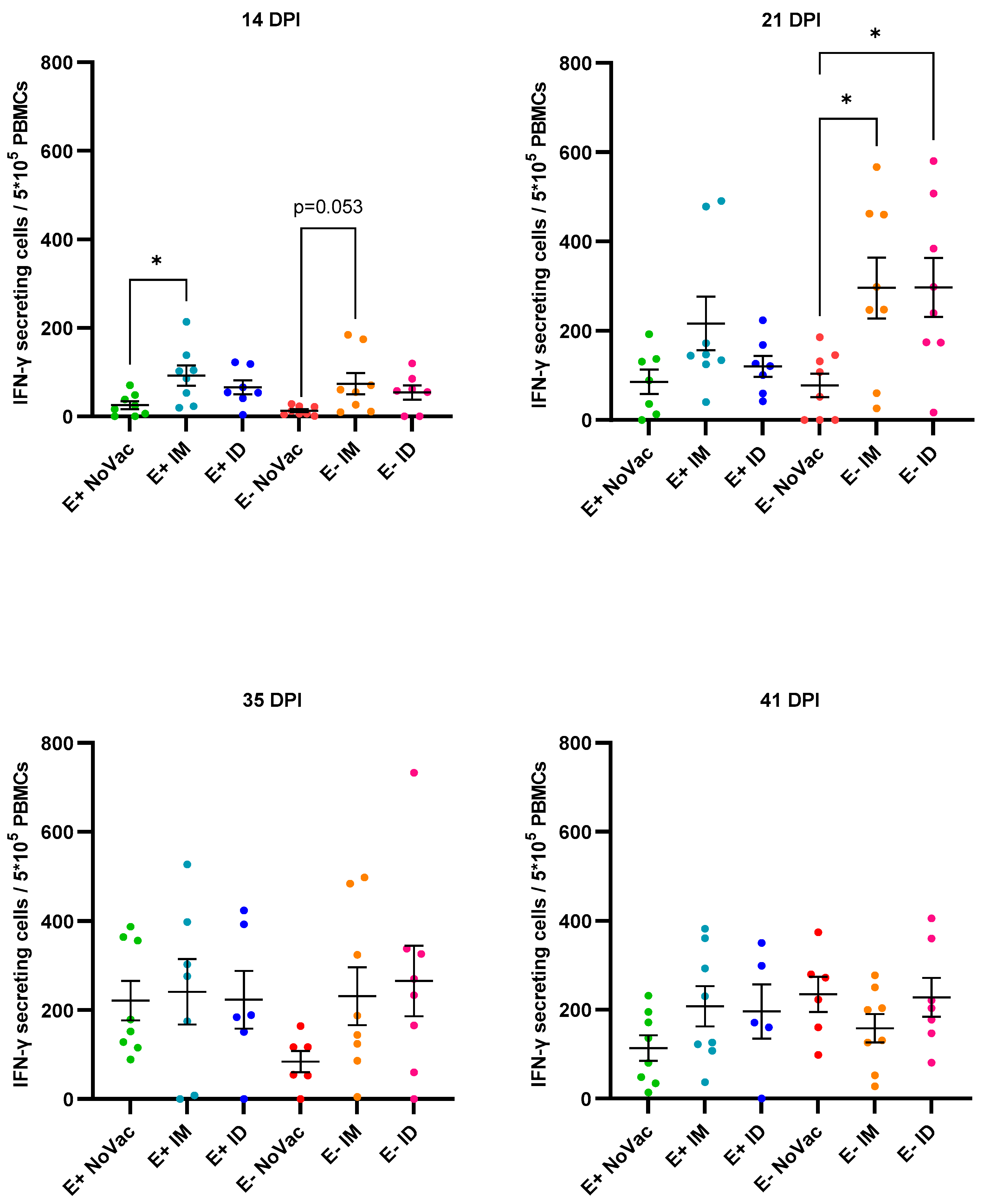
3.7. Induction of Interferon-γ Secreting Cells
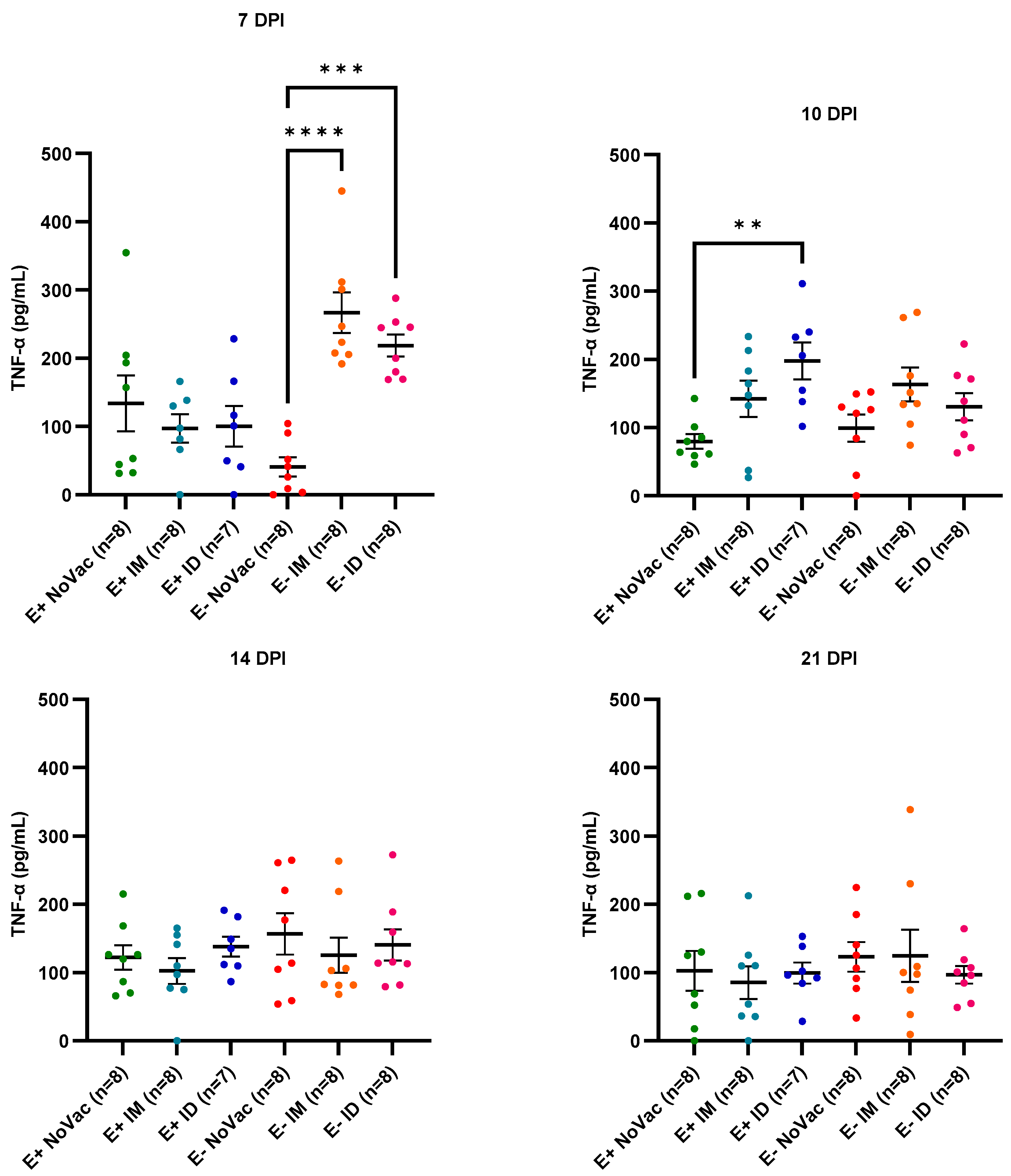
3.8. Cytokine Induction in Serum
4. Discussion
4.1. Prevalence and Consequences of Multiple Vaccinated, Seronegative Sows
4.2. Interference of MDAs on the PRRSV Piglet Vaccine Response
4.3. Vaccine Effectiveness after Challenge in Piglets Born from Responding and Non-Responding Sows
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, H. Overview and History of Mystery Swine Disease (Swine Infertility/Respiratory Syndrome). In Proceedings of the Mystery Swine Disease Committee Meeting, Livestock Conservation Institute, Denver, CO, USA, 6 October 1990. [Google Scholar]
- Keffaber, K.K. Reproductive Failure of Unknown Etiology. Am. Assoc. Swine Pract. Newsl. 1989, 1, 1–10. [Google Scholar]
- Goyal, S.M. Porcine Reproductive and Respiratory Syndrome. J. Vet. Diagn. Investig. 1993, 5, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, C.; Wensvoort, G.; Pol, J.M. Experimental Reproduction of Porcine Epidemic Abortion and Respiratory Syndrome (Mystery Swine Disease) by Infection with Lelystad Virus: Koch’s Postulates Fulfilled. Vet. Q. 1991, 13, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wensvoort, G.; Terpstra, C.; Pol, J.M.A.; Ter Laak, E.A.; Bloemraad, M.; De Kluyver, E.P.; Kragten, C.; Van Buiten, L.; den Besten, A.; Wagenaar, F.; et al. Mystery Swine Disease in The Netherlands: The Isolation of Lelystad Virus. Vet. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. ICTV Virus Taxonomy Profile: Arteriviridae. J. Gen. Virol. 2021, 102, 001632. [Google Scholar] [CrossRef] [PubMed]
- Dea, S.; Gagnon, C.A.; Mardassi, H.; Pirzadeh, B.; Rogan, D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) viru: Comparison of the North American and European isolates. Arch. Virol. 2000, 145, 659–688. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Nathues, H.; Alarcon, P.; Rushton, J.; Jolie, R.; Fiebig, K.; Jimenez, M.; Geurts, V.; Nathues, C. Cost of porcine reproductive and respiratory syndrome virus at individual farm level—An economic disease model. Prev. Vet. Med. 2017, 142, 16–29. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Dee, S.A.; Holtkamp, D.J.; Murtaugh, M.P.; Stadejek, T.; Stevenson, G.W.; Torremorell, M.; Yang, H.; Zhang, J. Porcine Reproductive and Respiratory Syndrome Viruses (Porcine Arteriviruses). In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 685–708. [Google Scholar]
- Thomann, B.; Rushton, J.; Schuepbach-Regula, G.; Nathues, H. Modeling Economic Effects of Vaccination Against Porcine Reproductive and Respiratory Syndrome: Impact of Vaccination Effectiveness, Vaccine Price, and Vaccination Coverage. Front. Vet. Sci. 2020, 7, 500. [Google Scholar] [CrossRef]
- Kroll, J.; Piontkowski, M.; Kraft, C.; Coll, T.; Gomez-Duran, O. Initial vaccination and revaccination with Type I PRRS 94881 MLV reduces viral load and infection with porcine reproductive and respiratory syndrome virus. Porcine Health Manag. 2018, 4, 23. [Google Scholar] [CrossRef]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Cornelissen, L.A.; Moormann, R.J.; Rebel, J.M.; Stockhofe-Zurwieden, N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine 2009, 27, 3704–3718. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar] [CrossRef] [PubMed]
- Bitsouni, V.; Lycett, S.; Opriessnig, T.; Doeschl-Wilson, A. Predicting vaccine effectiveness in livestock populations: A theoretical framework applied to PRRS virus infection in pigs. PLoS ONE 2019, 14, e0220738. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.P.; Stadejek, T.; Abrahante, J.E.; Lam, T.T.; Leung, F.C. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Holland, J.J. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 1997, 51, 154–178. [Google Scholar] [CrossRef] [PubMed]
- Marin-Vallis, G.E.; Kvisgaard, L.K.; Tello, M.; Darwich, L.; Cortey, M.; Burgara-Estrella, A.J.; Hernandez, J.; Larsen, L.E.; Mateu, E. Analysis of ORF5 and full-length genome sequences of porcine reproductive and respiratory syndrome virus isolates of genotypes 1 and 2 retrieved worldwide provides evidence that recombination is a common phenomenon and may produce mosaic isolates. J. Virol. 2014, 88, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Mathijs, E.; Tignon, M.; Vandersmissen, T.; Cay, A.B. WGS- versus ORF5-Based Typing of PRRSV: A Belgian Case Study. Viruses 2021, 13, 2419. [Google Scholar] [CrossRef]
- Eclercy, J.; Renson, P.; Lebret, A.; Hirchaud, E.; Normand, V.; Andraud, M.; Paboeuf, F.; Blanchard, Y.; Rose, N.; Bourry, O. A Field Recombinant Strain Derived from Two Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV-1) Modified Live Vaccines Shows Increased Viremia and Transmission in SPF Pigs. Viruses 2019, 11, 296. [Google Scholar] [CrossRef]
- Mateu, E.; Diaz, I. The challenge of PRRS immunology. Vet. J. 2008, 177, 345–351. [Google Scholar] [CrossRef]
- Butler, J.E.; Lager, K.M.; Golde, W.; Faaberg, K.S.; Sinkora, M.; Loving, C.; Zhang, Y.I. Porcine reproductive and respiratory syndrome (PRRS): An immune dysregulatory pandemic. Immunol. Res. 2014, 59, 81–108. [Google Scholar] [CrossRef]
- Wang, T.Y.; Sun, M.X.; Zhang, H.L.; Wang, G.; Zhan, G.; Tian, Z.J.; Cai, X.H.; Su, C.; Tang, Y.D. Evasion of antiviral innate immunity by porcine reproductive and respiratory syndrome virus. Front. Microbiol. 2021, 23, 12. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Fiers, J.; Tignon, M.; Cay, A.B.; Simons, X.; Maes, D. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): A Cross-Sectional Study on ELISA Seronegative, Multivaccinated Sows. Viruses 2022, 14, 1944. [Google Scholar] [CrossRef]
- Fiers, J.; Tignon, M.; Maes, D.; Cay, A.B. Follow-Up of PRRSV-Vaccinated Piglets Born from PRRSV-Vaccinated, ELISA-Seropositive and ELISA-Seronegative Sows. Viruses 2023, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Fablet, C.; Renson, P.; Eono, F.; Mahé, S.; Eveno, E.; Le Dimna, M.; Normand, V.; Lebret, A.; Rose, N.; Bourry, O. Maternally derived antibodies (MDAs) impair piglets’ humoral and cellular immune responses to vaccination against porcine reproductive and respiratory syndrome (PRRS). Vet. Microbiol. 2016, 192, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Renson, P.; Fablet, C.; Andraud, M.; Normand, V.; Lebret, A.; Paboeuf, F.; Rose, N.; Bourry, O. Maternally-derived neutralizing antibodies reduce vaccine efficacy against porcine reproductive and respiratory syndrome virus infection. Vaccine 2019, 37, 4318–4324. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Genís-Jorquera, B.; Martín-Valls, G.E.; Mateu, E. Using commercial ELISAs to assess humoral response in sows repeatedly vaccinated with modified live porcine reproductive and respiratory syndrome virus. Vet. Rec. 2020, 186, 123. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.; Thacker, E.; Thacker, B.; Vincent, A. A Preliminary Investigation into Possible PRRSV Anergy Induction from Repeated Immunization with A Modified Live Vaccine. In Proceedings of the 26th Allen, D. Leman Swine Conference, Minneapolis, MN, USA, 17 September 1999. [Google Scholar]
- Pedersen, K.; Kristensen, C.S.; Kvisgaard, L.K.; Larsen, L.E. Impact of Quarterly Sow Mass Vaccination with a Porcine Reproductive and Respiratory Syndrome Virus Type 1 (PRRSV-1) Modified Live Vaccine in Two Herds. Vaccines 2021, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Fiers, J.; Maes, D.; Cay, A.B.; Mostin, L.; Parys, A.; Tignon, M. PRRSV-Vaccinated, Seronegative Sows and Maternally Derived Antibodies (I): Impact on PRRSV-1 Challenge Outcomes in Piglets. Vaccines 2023, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Geldhof, M.F.; Vanhee, M.; Van Breedam, W.; Van Doorsselaere, J.; Karniychuk, U.U.; Nauwynck, H.J. Comparison of the efficacy of autogenous inactivated porcine reproductive and respiratory syndrome virus (PRRSV) vaccines against homologous and heterologous challenges. BMC Biotechnol. 2012, 8, 182. [Google Scholar] [CrossRef]
- Frydas, I.S.; Trus, I.; Kvisgaard, L.K.; Bonckaert, C.; Reddy, V.R.A.P.; Li, Y.; Larsen, L.E.; Nauwynck, H.J. Different clinical, Virological, serological and tissue tropism outcomes of two new and one old Belgian type 1 subtype 1 porcine reproductive and respiratory virus (PRRSV) isolates. Vet. Res. 2015, 46, 37. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Diaz, I.; Gimeno, M.; Callen, A.; Pujols, J.; Lopez, S.; Charreyre, C.; Joisel, F.; Mateu, E. Comparison of different vaccination schedules for sustaining the immune response against porcine reproductive and respiratory syndrome virus. Vet. J. 2013, 197, 2. [Google Scholar] [CrossRef]
- Martin-Valls, G.E.; Mortensen, P.; Clilvert, H.; Li, Y.; Cortey, M.; Sno, M.; Barna, T.; Terre, M.; Guerra, N.; Mateu, E. The use of a whole inactivated PRRS virus vaccine administered in sows and impact on maternally derived immunity and timing of PRRS virus infection in piglets. Vet. Rec. Open 2022, 9, e34. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Lager, K.M.; Splichal, I.; Francis, D.; Kacskovics, I.; Sinkora, M.; Wertz, N.; Sun, J.; Zhao, Y.; Brown, W.R.; et al. The piglet as a model for B cell and immune system development. Vet. Immunol. Immunopathol. 2009, 15, 128. [Google Scholar] [CrossRef]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ni, J.; Cao, Y.; Liu, X. Newcastle Disease Virus as a Vaccine Vector for 20 Years: A Focus on Maternally Derived Antibody Interference. Vaccines 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Kitikoon, P.; Nilubol, D.; Erickson, B.J.; Janke, B.H.; Hoover, T.C.; Sornsen, S.A.; Thacker, E.L. The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet. Immunol. Immunopathol. 2006, 112, 117–128. [Google Scholar] [CrossRef]
- Markowska-Daniel, I.; Pomorska-Mol, M.; Pejsak, Z. The influence of age and maternal antibodies on the postvaccinal response against swine influenza viruses in pigs. Vet. Immunol. Immunopathol. 2011, 142, 81–86. [Google Scholar] [CrossRef]
- Suradhat, S.; Damrongwatanapokin, S. The influence of maternal immunity on the efficacy of a classical swine fever vaccine against classical swine fever virus, genogroup 2.2, infection. Vet. Microbiol. 2003, 92, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.; Hennies, R.; Dreckmann, K.; Noguera, M.; Rathkjen, P.H.; Gassel, M.; Gereke, M. Evaluation of PRRSv specific, maternally derived and induced immune response in Ingelvac PRRSFLEX EU vaccinated piglets in the presence of maternally transferred immunity. PLoS ONE 2019, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Balasch, M.; Fort, M.; Taylor, L.P.; Calvert, J.G. Vaccination of 1-day-old pigs with a porcine reproductive and respiratory syndrome virus (PRRSV) modified live attenuated virus vaccine is able to overcome maternal immunity. Porc. Health Manag. 2018, 15, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Li, Y.; Baratelli, M.; Martín-Valls, G.; Cortey, M.; Miranda, J.; Martín, M.; Mateu, E. In the presence of non-neutralising maternally derived antibodies, intradermal and intramuscular vaccination with a modified live vaccine against porcine reproductive and respiratory syndrome virus 1 (PRRSV-1) induce similar levels of neutralising antibodies or interferon-gamma secreting cells. Porc. Health Manag. 2022, 8, 1–9. [Google Scholar]
- Brockmeier, S.L.; Loving, C.L.; Eberle, K.C.; Hau, S.J.; Buckley, A.; Van Geelen, A.; Montiel, N.A.; Nicholson, T.; Lager, K.M. Interferon alpha inhibits replication of a live-attenuated porcine reproductive and respiratory syndrome virus vaccine preventing development of an adaptive immune response in swine. Vet. Microbiol. 2017, 212, 48–51. [Google Scholar] [CrossRef]
- Renson, P.; Mahé, S.; Andraud, M.; Le Dimna, M.; Paboeuf, F.; Rose, N.; Bourry, O. Effect of vaccination route (intradermal vs. intramuscular) against porcine reproductive and respiratory syndrome using a modified live vaccine on systemic and mucosal immune response and virus transmission in pigs. BMC Vet. Res. 2024, 20, 5. [Google Scholar] [CrossRef]


| Experimental Group (n) | Vaccination (Age) | Compartment |
|---|---|---|
| E+ NoVac (8) | No vaccination | 1 |
| E+ IM (8) | Porcilis IM (3 woa) | 2 |
| E+ ID (8) * | Porcilis ID (3 woa) | 3 |
| E− NoVac (8) | No vaccination | 4 |
| E− IM (8) | Porcilis IM (3 woa) | 5 |
| E− ID (8) | Porcilis ID (3 woa) | 6 |
| Sow | Parity | S/p Value (90 Days Gestation) | VN Titer (90 Days Gestation) | S/p Value (14 dpf) | S/p Value Selected Piglets (14 dpf) (Mean ± SD) | VN Titer Selected Piglets (14 dpf) (Mean ± SD) |
|---|---|---|---|---|---|---|
| 1 | 3 | 2.08 | 0 | 1.76 | 1.86 ± 0.10 | 0 ± 0 |
| 2 | 3 | 2.16 | 2 | 1.95 | 2.04 ± 0.05 | 0 ± 0 |
| 3 | 8 | 2.79 | 0 | 2.57 | 2.17 ± 0.10 | 0 ± 0 |
| 4 | 3 | 0.25 | 0 | 0.18 | 0.13 ± 0.02 | 0 ± 0 |
| 5 | 3 | 0.43 | 0 | 0.52 | 0.37 ± 0.08 | 0 ± 0 |
| 6 | 7 | 0.51 | 0 | 0.38 | 0.41 ± 0.05 | 0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiers, J.; Maes, D.; Cay, A.-B.; Vandenbussche, F.; Mostin, L.; Parys, A.; Tignon, M. PRRSV-Vaccinated, Seronegative Sows and Maternally Derived Antibodies (II): Impact on PRRSV-1 Vaccine Effectiveness and Challenge Outcomes in Piglets. Vaccines 2024, 12, 257. https://doi.org/10.3390/vaccines12030257
Fiers J, Maes D, Cay A-B, Vandenbussche F, Mostin L, Parys A, Tignon M. PRRSV-Vaccinated, Seronegative Sows and Maternally Derived Antibodies (II): Impact on PRRSV-1 Vaccine Effectiveness and Challenge Outcomes in Piglets. Vaccines. 2024; 12(3):257. https://doi.org/10.3390/vaccines12030257
Chicago/Turabian StyleFiers, Jorian, Dominiek Maes, Ann-Brigitte Cay, Frank Vandenbussche, Laurent Mostin, Anna Parys, and Marylène Tignon. 2024. "PRRSV-Vaccinated, Seronegative Sows and Maternally Derived Antibodies (II): Impact on PRRSV-1 Vaccine Effectiveness and Challenge Outcomes in Piglets" Vaccines 12, no. 3: 257. https://doi.org/10.3390/vaccines12030257
APA StyleFiers, J., Maes, D., Cay, A.-B., Vandenbussche, F., Mostin, L., Parys, A., & Tignon, M. (2024). PRRSV-Vaccinated, Seronegative Sows and Maternally Derived Antibodies (II): Impact on PRRSV-1 Vaccine Effectiveness and Challenge Outcomes in Piglets. Vaccines, 12(3), 257. https://doi.org/10.3390/vaccines12030257









