Comparative Analysis of SARS-CoV-2 Antibody Responses across Global and Lesser-Studied Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Data Collection
2.3. Sample
2.4. Evaluated Vaccines and Age Groups
2.5. Laboratory Methods
2.6. Statistical Methods
3. Results
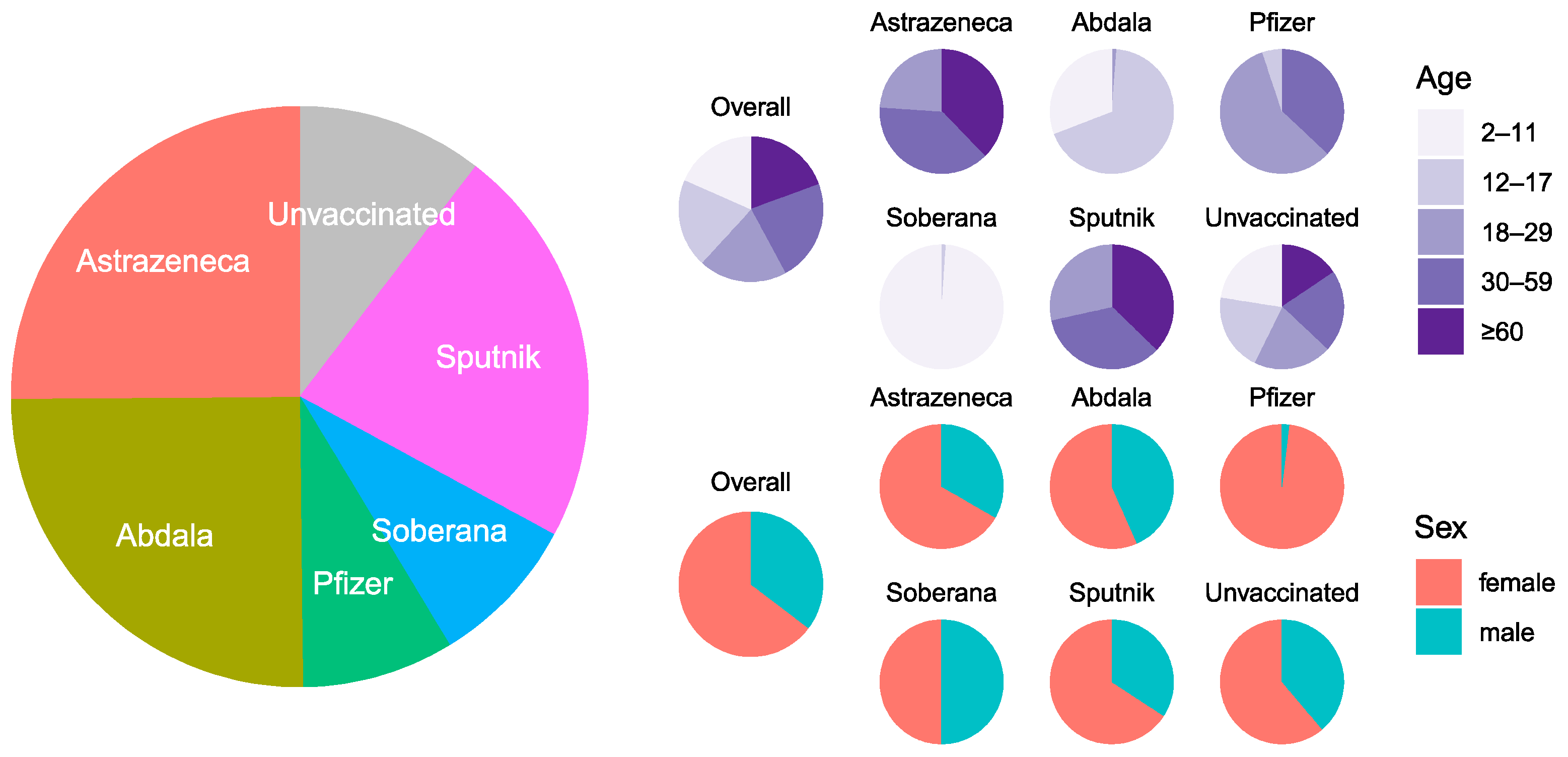
3.1. Seroprevalence and Antibody Titers in Unvaccinated and N Antibodies in Vaccinated
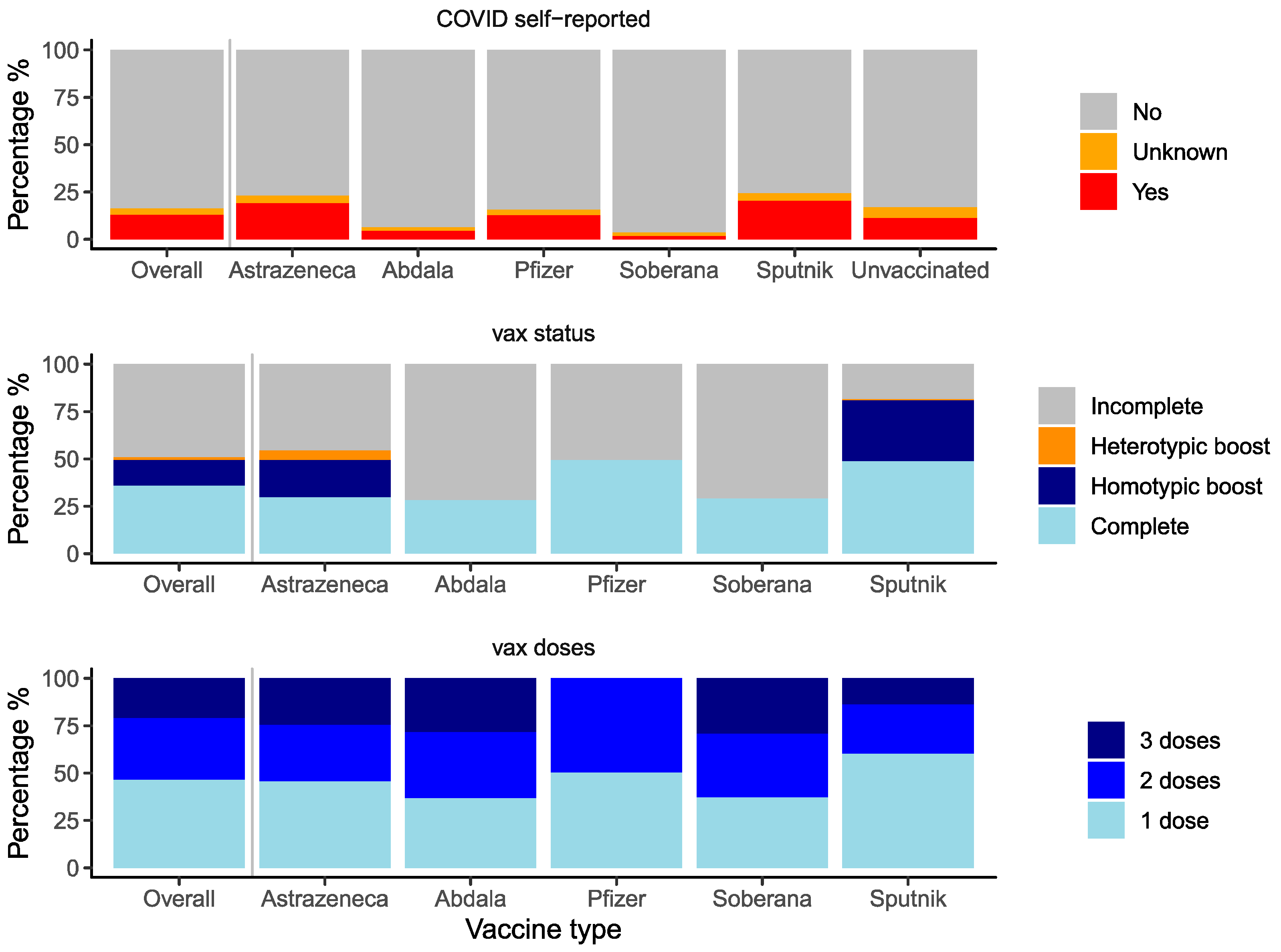
3.2. Antibody Titers
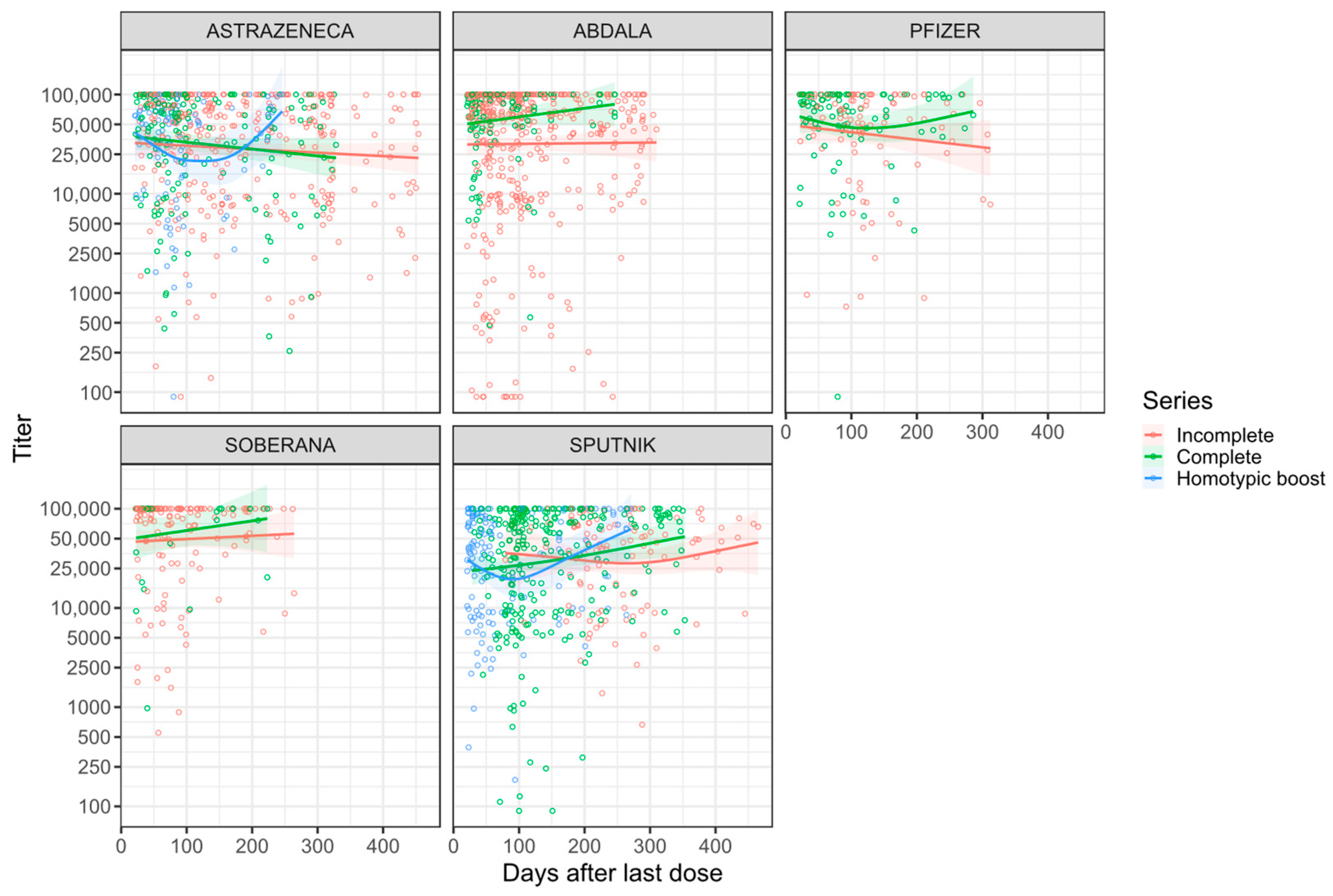
3.3. Antibody Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, N.P.; Stockwell, M.S.; Demarco, M.; Gaglani, M.; Kharbanda, A.B.; Irving, S.A.; Rao, S.; Grannis, S.J.; Dascomb, K.; Murthy, K.; et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA Vaccination in Preventing COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Nonimmunocompromised Children and Adolescents Aged 5-17 Years—VISION Network, 10 States, April 2021-January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Trajman, A.; Lachapelle-Chisholm, S.; Zikos, T.; Werneck, G.L.; Benedetti, A. Efficacy and effectiveness of SARS-CoV-2 vaccines for death prevention: A protocol for a systematic review and meta-analysis. PLoS ONE 2022, 17, e0265414. [Google Scholar] [CrossRef] [PubMed]
- CNN. Nicaragua Confirma el Primer caso de Coronavirus. Available online: https://www.cnn.com/videos/spanish/2020/03/19/nicaragua-primer-caso-coronavirus-pkg-mario-medrano.cnn (accessed on 8 March 2023).
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.E.; Kuan, G.; Saborio, S.; Carrillo, F.A.B.; Plazaola, M.; Barilla, C.; Sanchez, N.; Lopez, R.; Smith, M.; Kubale, J.; et al. Clinical Spectrum of Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Protection From Symptomatic Reinfection. Clin. Infect. Dis. 2022, 75, e257–e266. [Google Scholar] [CrossRef]
- Maier, H.E.; Balmaseda, A.; Saborio, S.; Ojeda, S.; Barilla, C.; Sanchez, N.; Lopez, R.; Plazaola, M.; Cerpas, C.; van Bakel, H.; et al. Protection Associated with Previous SARS-CoV-2 Infection in Nicaragua. N. Engl. J. Med. 2022, 387, 568–570. [Google Scholar] [CrossRef]
- Maier, H.E.; Balmaseda, A.; Ojeda, S.; Cerpas, C.; Sanchez, N.; Plazaola, M.; van Bakel, H.; Kubale, J.; Lopez, R.; Saborio, S.; et al. An immune correlate of SARS-CoV-2 infection and severity of reinfections. medRxiv 2021. [Google Scholar] [CrossRef]
- Swissinfo. La Variante Ómicron ya Circula en Nicaragua, Según la OPS. Available online: https://www.swissinfo.ch/spa/coronavirus-nicaragua_la-variante-%C3%B3micron-ya-circula-en-nicaragua--seg%C3%BAn-la-ops/47296182 (accessed on 1 March 2023).
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef]
- Mobarak, A.M.; Miguel, E.; Abaluck, J.; Ahuja, A.; Alsan, M.; Banerjee, A.; Breza, E.; Chandrasekhar, A.G.; Duflo, E.; Dzansi, J.; et al. End COVID-19 in low- and middle-income countries. Science 2022, 375, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- GAVI. COVAX Roll-Out—Nicaragua. Available online: https://www.gavi.org/covax-vaccine-roll-out/nicaragua?gclid=CjwKCAjwpKyYBhB7EiwAU2Hn2e0XNXalCzHFz9I9tMjGazsXGdJpp5qNKVyaatnSKqL_Z-X1UA5RbxoC5Z0QAvD_BwE (accessed on 29 April 2023).
- Afp. Nicaragua to Use Cuban COVID Vaccines. Available online: https://ticotimes.net/2021/10/04/nicaragua-to-use-cuban-covid-vaccines (accessed on 1 March 2023).
- TASS. Successive Batch of Russia’s Sputnik V Vaccine Delivered to Nicaragua. Available online: https://tass.com/society/1356929 (accessed on 29 April 2023).
- El 19 Digital. El 72% de Nicaragüenses Mayor de 30 Años ya Están Vacunados Contra la COVID-19. Available online: https://www.el19digital.com/coronavirus/articulo/titulo:122805-el-72-de-nicaraguenses-mayor-de-30-anos-ya-estan-vacunados-contra-la-covid-19 (accessed on 10 April 2023).
- Beaubien, J. A Small Island Nation Has Cooked Up Not 1, Not 2 but 5 COVID Vaccines. It’s Cuba! NPR 2022. Available online: https://www.npr.org/sections/goatsandsoda/2022/02/01/1056952488/a-small-island-nation-has-cooked-up-not-1-not-2-but-5-covid-vaccines-its-cuba (accessed on 10 April 2023).
- Fujigaki, H.; Yamamoto, Y.; Koseki, T.; Banno, S.; Ando, T.; Ito, H.; Fujita, T.; Naruse, H.; Hata, T.; Moriyama, S.; et al. Antibody Responses to BNT162b2 Vaccination in Japan: Monitoring Vaccine Efficacy by Measuring IgG Antibodies against the Receptor-Binding Domain of SARS-CoV-2. Microbiol. Spectr. 2022, 10, e0118121. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.J.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Thin plate regression splines. J. R. Stat. Soc. Ser. B-Stat. Methodol. 2003, 65, 95–114. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Dean, A.G.; Arner, T.G.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; et al. Epi Info™, a Database and Statistics Program for Public Health Professionals; CDC: Atlanta, GA, USA, 2011. [Google Scholar]
- Nam, M.; Seo, J.D.; Moon, H.W.; Kim, H.; Hur, M.; Yun, Y.M. Evaluation of Humoral Immune Response after SARS-CoV-2 Vaccination Using Two Binding Antibody Assays and a Neutralizing Antibody Assay. Microbiol. Spectr. 2021, 9, e0120221. [Google Scholar] [CrossRef] [PubMed]
- Demonbreun, A.R.; Sancilio, A.; Velez, M.P.; Ryan, D.T.; Saber, R.; Vaught, L.A.; Reiser, N.L.; Hsieh, R.R.; D’Aquila, R.T.; Mustanski, B.; et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. eClinicalMedicine 2021, 38, 101018. [Google Scholar] [CrossRef] [PubMed]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kümmerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streeck, H.; Saschenbrecker, S.; Steinhagen, K.; et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2021, 94, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, P.; Pasternack, A.; Maljanen, S.; Melen, K.; Kolehmainen, P.; Huttunen, M.; Lundberg, R.; Tripathi, L.; Khan, H.; Ritvos, M.A.; et al. A Combination of N and S Antigens With IgA and IgG Measurement Strengthens the Accuracy of SARS-CoV-2 Serodiagnostics. J. Infect. Dis. 2021, 224, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, Y.R.; Heo, S.T.; Oh, H.; Kim, M.; Lee, H.R.; Yoo, J.R. Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine. Front. Immunol. 2022, 13, 827306. [Google Scholar] [CrossRef]
- Kang, Y.M.; Minn, D.; Lim, J.; Lee, K.D.; Jo, D.H.; Choe, K.W.; Kim, M.J.; Kim, J.M.; Kim, K.N. Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine. J. Korean Med. Sci. 2021, 36, e311. [Google Scholar] [CrossRef]
- Lee, S.W.; Moon, J.Y.; Lee, S.K.; Lee, H.; Moon, S.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Kim, T.H.; et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021, 12, 779212. [Google Scholar] [CrossRef]
- Shapiro, L.C.; Thakkar, A.; Campbell, S.T.; Forest, S.K.; Pradhan, K.; Gonzalez-Lugo, J.D.; Quinn, R.; Bhagat, T.D.; Choudhary, G.S.; McCort, M.; et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 2022, 40, 3–5. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jurjenson, V.; Adamson, A.; Haljasmagi, L.; Rumm, A.P.; Maruste, R.; Karner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Chahla, R.E.; Tomas-Grau, R.H.; Cazorla, S.I.; Ploper, D.; Vera Pingitore, E.; Lopez, M.A.; Aznar, P.; Alcorta, M.E.; Velez, E.; Stagnetto, A.; et al. Long-term analysis of antibodies elicited by SPUTNIK V: A prospective cohort study in Tucuman, Argentina. Lancet Reg. Health Am. 2022, 6, 100123. [Google Scholar] [CrossRef] [PubMed]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Buckner, C.M.; Kardava, L.; El Merhebi, O.; Narpala, S.R.; Serebryannyy, L.; Lin, B.C.; Wang, W.; Zhang, X.; Lopes de Assis, F.; Kelly, S.E.M.; et al. Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses. Cell 2022, 185, 4333–4346 e4314. [Google Scholar] [CrossRef]
- Eugenia-Toledo-Romani, M.; Verdecia-Sanchez, L.; Rodriguez-Gonzalez, M.; Rodriguez-Noda, L.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sanchez-Ramirez, B.; Perez-Nicado, R.; Gonzalez-Mugica, R.; Hernandez-Garcia, T.; et al. Safety and immunogenicity of anti-SARS-CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: Open label phase I and phase IIa clinical trials. Vaccine 2022, 40, 4220–4230. [Google Scholar] [CrossRef]
- Hernandez-Bernal, F.; Ricardo-Cobas, M.C.; Martin-Bauta, Y.; Navarro-Rodriguez, Z.; Pinera-Martinez, M.; Quintana-Guerra, J.; Urrutia-Perez, K.; Urrutia-Perez, K.; Chavez-Chong, C.O.; Azor-Hernandez, J.L.; et al. Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: A randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA Study). eClinicalMedicine 2022, 46, 101383. [Google Scholar] [CrossRef]
- Lemos-Pérez, G.; Chávez-Valdés, S.; González-Formental, H.; Freyre-Corrales, G.; Vázquez-Arteaga, A.; Álvarez-Acevedo, B.; Ávila-Díaz, L.; Martínez-Rosales, R.U.; Chacón-Quintero, Y.; Coizeau-Rodríguez, E.; et al. Elevated antibody titers in Abdala vaccinees evaluated by Elecsys® anti-SARS-CoV-2 S highly correlate with UMELISA SARS-CoV-2 ANTI RBD, ACE-2 binding inhibition and viral neutralization assays. medRxiv 2021. [Google Scholar] [CrossRef]
- Ashrafian, F.; Bagheri Amiri, F.; Bavand, A.; Zali, M.; Sadat Larijani, M.; Ramezani, A. A Comparative Study of Immunogenicity, Antibody Persistence, and Safety of Three Different COVID-19 Boosters between Individuals with Comorbidities and the Normal Population. Vaccines 2023, 11, 1376. [Google Scholar] [CrossRef]
- Muller, L.; Andree, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef] [PubMed]
- WHO. Nicaragua: WHO and UNICEF Estimates of Immunization Coverage: 2022 Revision. Available online: https://data.unicef.org/wp-content/uploads/cp/immunisation/nic.pdf (accessed on 2 May 2023).
- Adjobimey, T.; Meyer, J.; Sollberg, L.; Bawolt, M.; Berens, C.; Kovacevic, P.; Trudic, A.; Parcina, M.; Hoerauf, A. Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization With Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm’s COVID-19 Vaccines. Front. Immunol. 2022, 13, 917905. [Google Scholar] [CrossRef] [PubMed]

| Dosage for Series Completion 1 | Optional Boost | ||||
|---|---|---|---|---|---|
| Age Groups | Vaccine | Doses | Dosage Interval (Days) | Homotypic | Heterotypic |
| 2–11 y/o | Soberana (FINLAY-FR-2) 2 | 3 | 28 | - | - |
| 2–17 y/o | Abdala (CIGB-66) 3 | 3 | 28 | - | - |
| ≥12 y/o | Pfizer-BioNTech (BNT162b2) | 2 | 21 | - | - |
| ≥18 y/o | Sputnik V (Gam-COVID-Vac) | 2 | 21 | Sputnik Light | Covishield or Vaxzevria |
| ≥18 y/o | Sputnik light | 1 | - | Sputnik Light | Covishield or Vaxzevria |
| ≥18 | Covishield-AstraZeneca (ChAdOx1-S)/Vaxzevria (ChAdOx1-S) 4 | 2 | 120 | Covishield or Vaxzevria | Sputnik Light |
| Variable | Categories | N | Seroprevalence (%) | Fisher Test * | Median Titer (IQR) | Anova * |
|---|---|---|---|---|---|---|
| Overall | - | 333 | 95.2 (92.2–97.4) | - | 10,867 (3330–70,641) | - |
| Age group | 2–11 y/o | 75 | 90.7 (81.1–95.8) | 0.2562 | 9054 (1286–57,433) | 0.42 |
| 12–17 y/o | 67 | 97.0 (88.7–99.5) | 8284 (2837–78,859) | |||
| 18–29 y/o | 68 | 98.5 (91.0–99.9) | 11,912 (4982–75,010) | |||
| 30–59 y/o | 71 | 95.8 (87.3–98.9) | 14,352 (4039–68,012) | |||
| >60 y/o | 52 | 94.2 (83.1–98.5) | 22,280 (6250–89,087) | |||
| Sex | Female | 204 | 96.1 (92.1–98.2) | 0.46884 | 9821 (3903–71,550) | 0.883 |
| Male | 129 | 93.8 (87.8–97.1) | 11,627 (2723–67,046) | |||
| Health Center | Roger Osorio | 108 | 96.3 (90.2–98.8) | 0.08052 | 10,662 (4448–77,328) | 0.088 |
| Silvia Ferrufino | 85 | 90.6 (81.8–95.6) | 6233 (1268–51,066) | |||
| Villa Libertad | 140 | 97.1 (92.4–99.1) | 19,448 (5040–76,761) |
| Univariate Analysis | Multivariate Analysis 1 | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Category | n | Median Titer | Model Estimate | p-Value | Model Estimate | p-Value |
| Age groups | 2–11 y/o | 515 | 80,940 | 14,300.9 [10,013.3, 18,588.6] | <0.001 | −15,335.4 [−31,112.9, 442.0] | 0.057 |
| 12–17 y/o | 560 | 67,498 | 10,670.0 [6473.8, 14,866.2] | <0.001 | −13,642.1 [−28,749, 1464.9] | 0.077 | |
| 18–29 y/o | 563 | 55,200 | 2851.8 [−1338.7, 7042.4] | 0.18 | −2323.6 [−6821.7, 2174.4] | 0.311 | |
| 30–59 y/o | 654 | 50,514 | 89.9 [−3951.3, 4131.1] | 0.97 | −2885.9 [−7053.5, 1281.6] | 0.175 | |
| ≥60 y/o | 570 | 51,712 | Ref. | - | Ref. | - | |
| Sex | Male | 999 | 58,195 | Ref. | - | Ref. | - |
| Female | 1863 | 60,555 | 1638.6 [−1160.2, 4437.4] | 0.25 | 2783.1 [−88.1, 5654.3] | 0.058 | |
| Type of vaccine | AstraZeneca | 802 | 47,914 | Ref. | - | Ref. | |
| Abdala | 801 | 67,501 | 12,033.5 [8534.9, 15,532.1] | <0.001 | 25,889.9 [10,884.1, 40,895.7] | <0.001 | |
| Pfizer | 272 | 67,368 | 12,928.7 [8014.4, 17,843.0] | <0.001 | 12,967.2 [7543.7, 18,390.8] | <0.001 | |
| Soberana | 270 | 94,062 | 21,211.7 [16,283.8, 26,139.6] | <0.001 | 36,498.8 [20,312.2, 52,685.5] | <0.001 | |
| Sputnik | 717 | 49,048 | 1426.3 [−2173.3, 5026.0] | 0.44 | 100.5 [−3714.4, 3915.3] | 0.959 | |
| Vaccination status | Incomplete | 1405 | 59,933 | Ref. | - | Ref. | |
| Complete | 1027 | 66,362 | 3144.5 [223.7, 6065.2] | 0.0349 | 7181.5 [4122.8, 10,240.2] | <0.001 | |
| Homotypic boost | 387 | 47,700 | −6585.3 [−10,669.6, −2501] | 0.0016 | 2105.7 [−2537.3, 6748.6] | 0.374 | |
| Heterotypic boost | 43 | 60,413 | −6108.3 [−17,122.5, 4905.8] | 0.2771 | 956.8 [−10,600.0, 12,513.5] | 0.871 | |
| Days after last dose | Titer change by day | - | - | −0.6 [−1.5, 0.3] | 0.19 | −0.5 [−1.4, 0.3] | 0.222 |
| Number of doses | 1 | 1334 | 54,096 | Ref. | - | - | - |
| 2 | 932 | 65,250 | 7160.9 [4127.3, 10,194.6] | <0.001 | - | - | |
| 3 | 596 | 64,768 | 7062.2 [3561.2, 10,563.3] | <0.001 | - | - | |
| Self-reported SARS-CoV-2 infection | Yes | 378 | 60,392 | Ref. | - | Ref. | - |
| Unsure | 90 | 53,623 | −5592.7 [−13,964.7, 2779.4] | 0.19 | −5602.9 [−13,984.4, 2778.7] | 0.19 | |
| No | 2393 | 59,861 | 614.9 [−3335.9, 4565.6] | 0.76 | −2176.4 [−6512.7, 2159.8] | 0.325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrana, J.V.; Saenz, C.; Maier, H.E.; Brenes, M.; Nuñez, A.; Matamoros, A.; Hernández, M.; Dumas, K.; Toledo, C.; Peralta, L.; et al. Comparative Analysis of SARS-CoV-2 Antibody Responses across Global and Lesser-Studied Vaccines. Vaccines 2024, 12, 326. https://doi.org/10.3390/vaccines12030326
Zambrana JV, Saenz C, Maier HE, Brenes M, Nuñez A, Matamoros A, Hernández M, Dumas K, Toledo C, Peralta L, et al. Comparative Analysis of SARS-CoV-2 Antibody Responses across Global and Lesser-Studied Vaccines. Vaccines. 2024; 12(3):326. https://doi.org/10.3390/vaccines12030326
Chicago/Turabian StyleZambrana, José Victor, Carlos Saenz, Hannah E. Maier, Mayling Brenes, Andrea Nuñez, Anita Matamoros, Mabel Hernández, Keyla Dumas, Cristhian Toledo, Leonardo Peralta, and et al. 2024. "Comparative Analysis of SARS-CoV-2 Antibody Responses across Global and Lesser-Studied Vaccines" Vaccines 12, no. 3: 326. https://doi.org/10.3390/vaccines12030326
APA StyleZambrana, J. V., Saenz, C., Maier, H. E., Brenes, M., Nuñez, A., Matamoros, A., Hernández, M., Dumas, K., Toledo, C., Peralta, L., Gordon, A., & Balmaseda, A. (2024). Comparative Analysis of SARS-CoV-2 Antibody Responses across Global and Lesser-Studied Vaccines. Vaccines, 12(3), 326. https://doi.org/10.3390/vaccines12030326






