Specific and Nonspecific Effects of Influenza Vaccines
Abstract
:1. Introduction
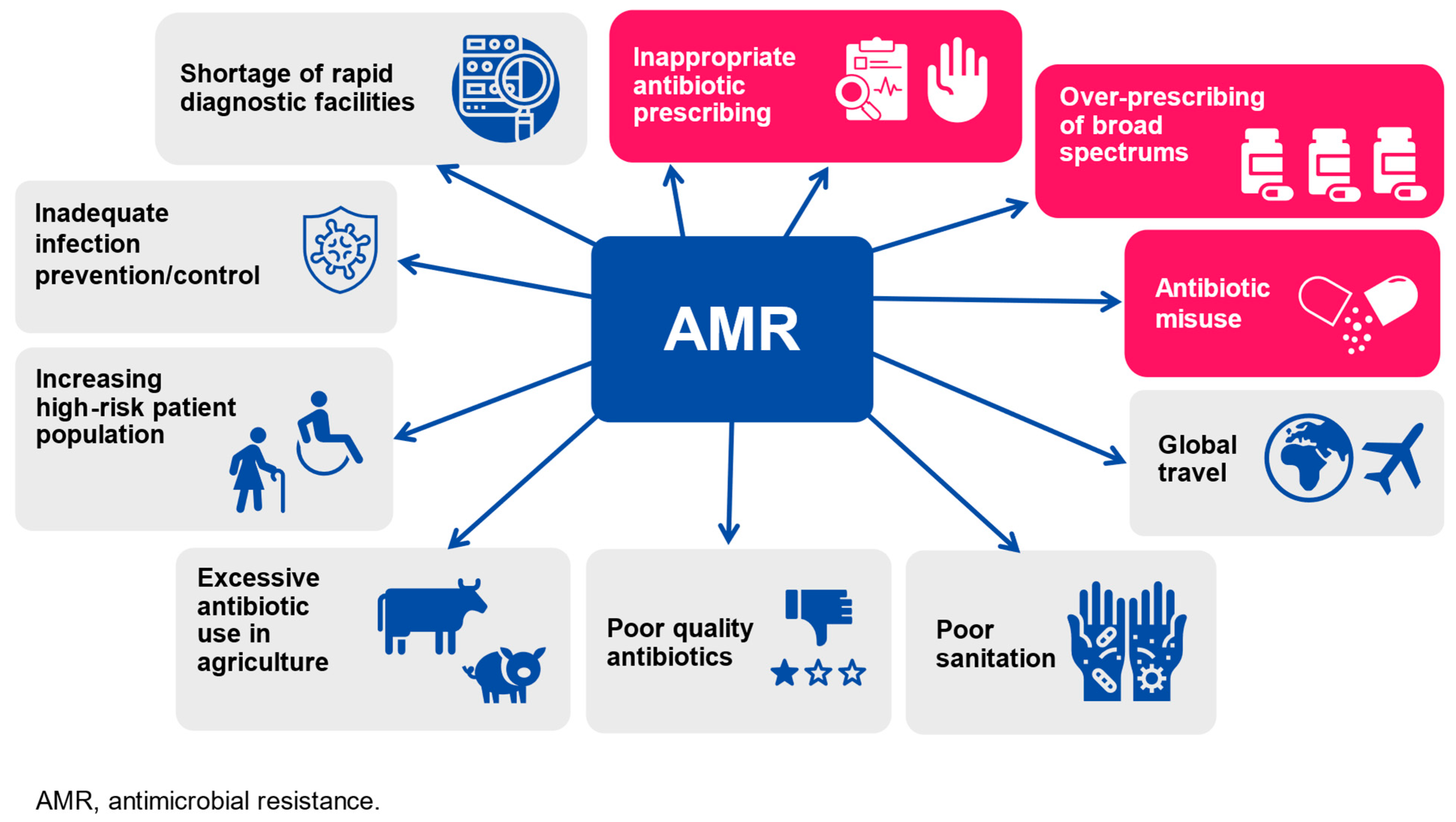
2. Influenza Vaccine Administration as a Measure to Contain Antimicrobial Resistance Development
3. Mechanisms and Examples of Nonspecific Effects of Vaccines
4. Influenza Immunization and Nonspecific Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, S. 100 years since the 1918 influenza pandemic. Hum. Vaccines Immunother. 2018, 14, 504–507. [Google Scholar] [CrossRef]
- Esposito, S.; Molteni, C.G.; Daleno, C.; Valzano, A.; Fossali, E.; Da Dalt, L.; Cecinati, V.; Bruzzese, E.; Giacchino, R.; Giaquinto, C.; et al. Clinical and socioeconomic impact of different types and subtypes of seasonal influenza viruses in children during influenza seasons 2007/2008 and 2008/2009. BMC Infect. Dis. 2011, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Ip, D.K.M.; Lau, L.L.H.; Leung, N.H.L.; Fang, V.J.; Chan, K.-H.; Chu, D.K.W.; Leung, G.M.; Peiris, J.S.M.; Uyeki, T.M.; Cowling, B.J. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infections in the community. Clin. Infect. Dis. 2017, 64, 736–742. [Google Scholar] [PubMed]
- Cohen, C.; Kleynhans, J.; Moyes, J.; McMorrow, M.L.; Treurnicht, F.K.; Hellferscee, O.; Mathunjwa, A.; von Gottberg, A.; Wolter, N.; Martinson, N.A.; et al. Asymptomatic transmission and high community burden of seasonal influenza in an urban anda rural community in South Africa, 2017–2018 (PHIRST): A population cohort study. Lancet Glob. Health 2021, 9, e863–e874. [Google Scholar] [CrossRef]
- Hayward, A.C.; Fragaszy, E.B.; Bermingham, A.; Wang, L.; Copas, A.; Edmunds, W.J.; Ferguson, N.; Goonetilleke, N.; Harvey, G.; Kovar, J.; et al. Comparative community burden and severity of seasonal and pandemic influenza: Results of the Flu Watch cohort study. Lancet Respir. Med. 2014, 2, 445–454. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 10 February 2024).
- Centers for Disease Control and Prevention. People at Higher Risk for Flu Complication. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 10 February 2024).
- Federici, C.; Cavazza, M.; Costa, F.; Jommi, C. Health care costs of influenza-related episodes in high income countries: A systematic review. PLoS ONE 2018, 13, e0202787. [Google Scholar] [CrossRef] [PubMed]
- Putri, W.; Muscatello, D.J.; Stockwell, M.S.; Newall, A.T. Economic burden of seasonal influenza in the United States. Vaccine 2018, 36, 3960–3966. [Google Scholar] [CrossRef]
- Bianchini, S.; Argentiero, A.; Camilloni, B.; Silvestri, E.; Alunno, A.; Esposito, S. Vaccination against Paediatric Respiratory Pathogens. Vaccines 2019, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hong, K.J.; Kim, H.; Nam, J.H. Influenza vaccines: Past, present, and future. Rev. Med. Virol. 2022, 32, e2243. [Google Scholar] [CrossRef]
- World Health Organization. Global Influenza Program. Available online: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/influenza-surveillance-outputs#:~:text=The%20WHO%20Global%20Influenza%20Programme,GISRS)%20and%20national%20epidemiological%20institutions (accessed on 10 February 2024).
- University of Minnesota. WHO Advisers Recommend Switch Back to Trivalent Flu Vaccines. Available online: https://www.cidrap.umn.edu/influenza-vaccines/who-advisers-recommend-switch-back-trivalent-flu-vaccines (accessed on 10 February 2024).
- Rizzo, C.; Rezza, G.; Ricciardi, W. Strategies in recommending influenza vaccination in Europe and US. Hum. Vaccin. Immunother. 2018, 14, 693–698. [Google Scholar] [CrossRef]
- Morales, K.F.; Brown, D.W.; Dumolard, L.; Steulet, C.; Vilajeliu, A.; Ropero Alvarez, A.M.; Moen, A.; Friede, M.; Lambach, P. Seasonal influenza vaccination policies in the 194 WHO Member States: The evolution of global influenza pandemic preparedness and the challenge of sustaining equitable vaccine access. Vaccine 2021, 8, 100097. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Gruber, W.C.; Simon, R.; Wassil, J.; Anderson, A.S. The impact of human vaccines on bacterial antimicrobial resistance: A review. Environ. Chem. Lett. 2021, 19, 4031–4062. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N.; European Society of Clinical Microbiology Infectious Diseases (ESCMID) Vaccine Study Group (EVASG). Influenza vaccination and prevention of antimicrobial resistance. Expert. Rev. Vaccines 2018, 17, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Casillas, A.; Rodriguez-Quintero, C.M.; Redwan, E.M.; Gupta, M.N.; Uversky, V.N.; Raszek, M. Do vaccines increase or decrease susceptibility to diseases other than those they protect against? Vaccine 2023, 42, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Munkwase, G. Implications of vaccine non-specific effects on licensure of new vaccines. Vaccine 2024, 42, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Paget, J.; Caini, S.; Cowling, B.; Esposito, S.; Falsey, A.R.; Gentile, A.; Kyncl, J.; MacIntyre, C.; Pitman, R.; Lina, B. The impact of influenza vaccination on the COVID-19 pandemic? Evidence and lessons for public health policies. Vaccine 2020, 38, 6485–6486. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, U.; Pappalardo, M.; Chinè, V.; Gismondi, P.; Neglia, C.; Argentiero, A.; Calderaro, A.; Prati, A.; Esposito, S. Role of Artificial Intelligence in Fighting Antimicrobial Resistance in Pediatrics. Antibiotics 2020, 9, 767. [Google Scholar] [CrossRef]
- Papan, C.; Argentiero, A.; Porwoll, M.; Hakim, U.; Farinelli, E.; Testa, I.; Pasticci, M.B.; Mezzetti, D.; Perruccio, K.; Etshtein, L.; et al. A host signature based on TRAIL, IP-10, and CRP for reducing antibiotic overuse in children by differentiating bacterial from viral infections: A prospective, multicentre cohort study. Clin. Microbiol. Infect. 2022, 28, 723–730. [Google Scholar] [CrossRef]
- European Commission. A European One Health Action Plan against Antimicrobial Resistance (AMR). Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf (accessed on 10 February 2024).
- ASPE. National. Action Plan for Combating Antibiotic-Resistant Bacteria, 2020–2025. Available online: https://aspe.hhs.gov/reports/national-action-plan-combating-antibiotic-resistant-bacteria-2020-2025 (accessed on 10 February 2024).
- World Health Organization. Antimicrobial Resistance. Global Action Plan on Antimicrobial Resistance. Available online: https://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html (accessed on 10 February 2024).
- Linder, J.A. Breaking the Ambulatory Antibiotic Prescribing Cycle with All-Antibiotic Stewardship, Patient Stewardship, and Visit Stewardship. Clin. Infect. Dis. 2021, 73, e1680–e1683. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Antimicrobial stewardship in paediatrics. BMC Infect. Dis. 2016, 16, 424. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- European Center for Disease Prevention and Control. Antimicrobial Surveillance in Europe 2023. 2021 Data. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial%20resistance%20surveillance%20in%20Europe%202023%20-%202021%20data.pdf (accessed on 10 February 2024).
- van Heuvel, L.; Caini, S.; Dückers, M.L.A.; Paget, J. Assessment of the inclusion of vaccination as an intervention to reduce antimicrobial resistance in AMR national action plans: A global review. Glob. Health 2022, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- van Houten, C.B.; Cohen, A.; Engelhard, D.; Hays, J.P.; Karlsson, R.; Moore, E.; Fernández, D.; Kreisberg, R.; Collins, L.V.; de Waal, W.; et al. Antibiotic misuse in respiratory tract infections in children and adults-a prospective, multicentre study (TAILORED Treatment). Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef]
- Minozzi, S.; Lytras, T.; Gianola, S.; Gonzalez-Lorenzo, M.; Castellini, G.; Galli, C.; Cereda, D.; Bonovas, S.; Pariani, E.; Moja, L. Comparative efficacy and safety of vaccines to prevent seasonal influenza: A systematic review and network meta-analysis. EClinicalMedicine 2022, 46, 101331. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Hausdorff, W.P.; Kristinsson, K.G. Effect of vaccination on the use of antimicrobial agents: A systematic literature review. Ann. Med. 2020, 52, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, P.; Esposito, S.; Bianchini, S.; Dusi, E.; Fusi, M.; Nazzari, E.; Picchi, R.; Galeone, C.; Principi, N. Efficacy of injectable trivalent virosomal-adjuvanted inactivated influenza vaccine in preventing acute otitis media in children with recurrent complicated or noncomplicated acute otitis media. Pediatr. Infect. Dis. J. 2009, 28, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.G.; Dewez, J.E.; Nijman, R.G.; Yeung, S. Clinical practice guidelines for acute otitis media in children: A systematic review and appraisal of European national guidelines. BMJ Open 2020, 10, e035343. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Unsolved problems and new medical approaches to otitis media. Expert. Opin. Biol. Ther. 2020, 20, 741–749. [Google Scholar] [CrossRef]
- Principi, N.; Marchisio, P.; Rosazza, C.; Sciarrabba, C.S.; Esposito, S. Acute otitis media with spontaneous tympanic membrane perforation. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 11–18. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccines for Antimicrobial Resistance (AMR). Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/product-and-delivery-research/anti-microbial-resistance (accessed on 10 February 2024).
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef]
- Curtis, N. BCG Vaccination and all-cause neonatal mortality. Pediatr. Infect. Dis. J. 2019, 38, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Andersen, A.; Martins, C.L.; Fisker, A.B.; Rodrigues, A.; Whittle, H.C.; Benn, C.S. Does oral polio vaccine have non-specific effects on all-cause mortality? Natural experiments within a randomised controlled trial of early measles vaccine. BMJ Open 2016, 6, e013335. [Google Scholar] [CrossRef]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity—Basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Balz, K.; Trassl, L.; Härtel, V.; Nelson, P.P.; Skevaki, C. Virus-Induced T Cell-Mediated Heterologous Immunity and Vaccine Development. Front. Immunol. 2020, 11, 513. [Google Scholar] [CrossRef]
- van Leent, M.M.T.; Meerwaldt, A.E.; Berchouchi, A.; Toner, Y.C.; Burnett, M.E.; Klein, E.D.; Verschuur, A.V.D.; Nauta, S.A.; Munitz, J.; Prévot, G.; et al. A modular approach toward producing nanotherapeutics targeting the innate immune system. Sci. Adv. 2021, 7, eabe7853. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Li, X.; Mitroulis, I.; Chavakis, T. Trained Innate Immunity and Its Implications for Mucosal Immunity and Inflammation. Adv. Exp. Med. Biol. 2019, 1197, 11–26. [Google Scholar] [PubMed]
- Hu, S.; Xiang, D.; Zhang, X.; Zhang, L.; Wang, S.; Jin, K.; You, L.; Huang, J. The mechanisms and cross-protection of trained innate immunity. Virol. J. 2022, 19, 210. [Google Scholar] [CrossRef]
- Bindu, S.; Dandapat, S.; Manikandan, R.; Dinesh, M.; Subbaiyan, A.; Mani, P.; Dhawan, M.; Tiwari, R.; Bilal, M.; Bin Emran, T.; et al. Prophylactic and therapeutic insights into trained immunity: A renewed concept of innate immune memory. Hum. Vaccines Immunother. 2022, 18, 2040238. [Google Scholar] [CrossRef]
- De Bree, L.C.J.; Koeken, V.A.; Joosten, L.A.; Aaby, P.; Benn, C.S.; van Crevel, R.; Netea, M.G. Non-specific effects of vaccines: Current evidence and potential implications. Semin. Immunol. 2018, 39, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, S.; Aaby, P.; Lund, N.; Monteiro, I.; Jensen, K.J.; Eriksen, H.B.; Schaltz Buchholzer, F.; Jørgensen, A.S.P.; Rodrigues, A.; Fisker, A.B.; et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin. Infect. Dis. 2017, 65, 1183–1190. [Google Scholar] [PubMed]
- Kowalewicz-Kulbat, M.; Locht, C. BCG and protection against inflammatory and auto-immune diseases. Expert. Rev. Vaccines 2017, 16, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Bhuiya, A.; Nahar, L.; Knudsen, K.; de Francisco, A.; Strong, M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: A community study from rural Bangladesh. Int. J. Epidemiol. 2003, 32, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P. Assumptions and contradictions in measles and measles immunization research: Is measles good for something? Soc. Sci. Med. 1995, 41, 673–686. [Google Scholar] [CrossRef] [PubMed]
- du Loû, A.D.; Pison, G.; Aaby, P. Role of immunizations in the recent decline in childhood mortality and the changes in the female/male mortality ratio in rural Senegal. Am. J. Epidemiol. 1995, 142, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G. Sabin’s vaccine used for nonspecific prevention of infant diarrhea of viral etiology. Bull. Pan Am. Health Organ. 1974, 8, 1974. [Google Scholar]
- Chumakov, M.; Voroshilova, M.; Antsupova, A.; Boiko, V.; Blinova, M.; Rodin, V. Live enterovirus vaccines for emergency non-specific prevention of mass respiratory diseases during autumn-winter outbreaks of influenza and other acute respiratory diseases. Zh Mikrobiol Epidemiol Immunobiol 1992, 11, 37–40. [Google Scholar]
- Nielsen, S.; Khalek, M.A.; Benn, C.S.; Aaby, P.; Hanifi, S.M.A. National immunization campaigns with oral polio vaccine may reduce all-cause mortality: Analysis of 2004–2019 demographic surveillance data in rural Bangladesh. EClinicalMedicine 2021, 36, 100886. [Google Scholar] [CrossRef] [PubMed]
- Welaga, P.; Oduro, A.; Debpuur, C.; Aaby, P.; Ravn, H.; Andersen, A.; Binka, F.; Hodgson, A. Fewer out-ofsequence vaccinations and reduction of child mortality in Northern Ghana. Vaccine 2017, 35, 2496–2503. [Google Scholar] [CrossRef]
- Andersen, A.; Fisker, A.B.; Rodrigues, A.; Martins, C.; Ravn, H.; Lund, N.; Biering-Sørensen, S.; Benn, C.S.; Aaby, P. National immunization campaigns with oral polio vaccine reduce all-cause mortality: A natural experiment within seven randomized trials. Front. Public Health 2018, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Clipet-Jensen, C.; Andersen, A.; Jensen, A.K.G.; Aaby, P.; Zaman, K. Out-of-sequence vaccinations with measles vaccine and diphtheria-tetanus-pertussis vaccine: A reanalysis of demographic surveillance data from rural Bangladesh. Clin. Infect. Dis. 2021, 72, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Soares-Weiser, K.; A López-López, J.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.A.C.; Reingold, A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 2016, 355, 5170. [Google Scholar] [CrossRef] [PubMed]
- Hanifi SM, A.; Fisker, A.B.; Welaga, P.; Rieckmann, A.; Jensen, A.G.; Benn, C.S.; Aaby, P. Diphtheria-tetanus-pertussis (DTP) vaccine is associated with increased female-male mortality. Studies of DTP administered before and after measles vaccine. J. Infect. Dis. 2021, 223, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Andersen, A.; Ravn, H.; Zaman, K. Co-administration of BCG and Diphtheria-tetanus-pertussis (DTP) Vaccinations May Reduce Infant Mortality More Than the WHO-schedule of BCG First and Then DTP. A Re-analysis of Demographic Surveillance Data From Rural Bangladesh. EBioMedicine 2017, 22, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Garly, M.L.; Nielsen, J.; Ravn, H.; Martins, C.; Balé, C.; Rodrigues, A.; Benn, C.S.; Lisse, I.M. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus- pertussis vaccines: Observations from vaccination trials in Guinea-Bissau. Pediatr. Infect. Dis. J. 2007, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.N.; Flanagan, K.L.; Furman, D.; Klein, S.L.; Kollmann, T.R.; Jeppesen, D.L.; Levy, O.; Marchant, A.; Namachivayam, S.; Netea, M.G.; et al. Changing oral vaccine to inactivated polio vaccine might increase mortality. Lancet 2016, 387, 1054–1055. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Shann, F.; Moss, W.J.; Benn, C.S.; Aaby, P. RTS,S Malaria Vaccine and Increased Mortality in Girls. mBio 2016, 7, e00514-16. [Google Scholar] [CrossRef] [PubMed]
- Garly, M.L.; Jensen, H.; Martins, C.L.; Balé, C.; Baldé, M.A.; Lisse, I.M.; Aaby, P. Hepatitis B vaccination associated with higher female than male mortality in Guinea-Bissau: An observational study. Pediatr. Infect. Dis. J. 2004, 23, 1086–1092. [Google Scholar] [CrossRef]
- Esposito, S.; Montinaro, V.; Groppali, E.; Tenconi, R.; Semino, M.; Principi, N. Live attenuated intranasal influenza vaccine. Hum. Vaccin. Immunother. 2012, 8, 76–80. [Google Scholar] [CrossRef]
- Piedra, P.A.; Gaglani, M.J.; Kozinetz, C.A.; Herschler, G.B.; Fewlass, C.; Harvey, D.; Zimmerman, N.; Glezen, W.P. Trivalent live attenuated intranasal influenza vaccine administered during the 2003–2004 influenza type A (H3N2) outbreak provided immediate, direct, and indirect protection in children. Pediatrics 2007, 120, e553–e564. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, J.Y.; Jang, Y.H.; Seo, S.U.; Chang, J.; Seong, B.L. Non-specific Effect of Vaccines: Immediate Protection against Respiratory Syncytial Virus Infection by a Live Attenuated Influenza Vaccine. Front. Microbiol. 2018, 9, 83. [Google Scholar] [CrossRef]
- Sundaram, M.E.; McClure, D.L.; VanWormer, J.J.; Friedrich, T.C.; Meece, J.K.; Belongia, E.A. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin. Infect. Dis. 2013, 57, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Fowlkes, A.L.; Steffens, A.; Finelli, A.; Cowling, B.J. Assessment of virus interference in a test-negative study of influenza vaccine effectiveness. Epidemiology 2017, 28, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Sumitani, M.; Tochino, Y.; Kamimori, T.; Fujiwara, H.; Fujikawa, T. Additive inoculation of influenza vaccine and 23-valent pneumococcal polysaccharide vaccine to prevent lower respiratory tract infections in chronic respiratory disease patients. Int. Med. 2008, 47, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Daleno, C.; Prunotto, G.; Scala, A.; Tagliabue, C.; Borzani, I.; Fossali, E.; Pelucchi, C.; Principi, N. Impact of viral infections in children with community-acquired pneumonia: Results of a study of 17 respiratory viruses. Influenza Other Respir Viruses 2013, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, S.; Sadeghzadeh-Bazargan, A.; Ebrahimi, S.; Yassin, Z.; Faiz, S.H.R.; Kabir, A.; Baghestani, A.; Mashayekhi, F.; Bokharaei-Salim, F.; Goodarzi, A. The effect of influenza vaccine on severity of COVID-19 infection: An original study from Iran. Med. J. Islam. Repub. Iran. 2021, 35, 114. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Zou, M.; Clarke, Q.; Chambers, C.; A Dickinson, J.; Sabaiduc, S.; Olsha, R.; Gubbay, J.B.; Drews, S.J.; Charest, H.; et al. Influenza Vaccine Does Not Increase the Risk of Coronavirus or Other Noninfluenza Respiratory Viruses: Retrospective Analysis From Canada, 2010–2011 to 2016–2017. Clin. Infect. Dis. 2020, 71, 2285–2288. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef]
- Rikin, S.; Jia, H.; Vargas, C.Y.; de Belliard, Y.C.; Reed, C.; LaRussa, P.; Larson, E.L.; Saiman, L.; Stockwell, M.S. Assessment of temporally-related acute respiratory illness following influenza vaccination. Vaccine 2018, 36, 1958–1964. [Google Scholar] [CrossRef]
- Cowling, B.J.; Fang, V.J.; Nishiura, H.; Chan, K.H.; Ng, S.; Ip, D.K.; Chiu, S.S.; Leung, G.M.; Malik Peiris, J.S. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin. Infect. Dis. 2012, 54, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.J.; et al. Induction of trained immunity by influenza vaccination—Impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef] [PubMed]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am. J. Infect. Control 2021, 49, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Orsi, A.; Sticchi, L.; Panatto, D.; Dini, G.; Ferrari, A.; Ogliastro, M.; Boccotti, S.; De Pace, V.; Ricucci, V.; et al. Effect of the 2020/21 season influenza vaccine on SARS-CoV-2 infection in a cohort of Italian healthcare workers. Vaccine 2022, 40, 1755–1760. [Google Scholar] [CrossRef]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O’Horo, J.C.; Gores, G.J.; Williams, A.M.; Halamka, J.; et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid. Based Med. 2021, 26, 192–193. [Google Scholar] [CrossRef]
- Andersen, A.; Fisker, A.B.; Nielsen, S.; Rodrigues, A.; Benn, C.S.; Aaby, P. National immunization campaigns with oral polio vaccine may reduce all-cause mortality: An analysis of 13 years of demographic surveillance data from an urban African area. Clin. Infect. Dis. 2021, 72, e596–e603. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, H.; Ahmed, J.; Oria, P.A.; Arunga, G.; Williamson, J.; Kosgey, A.; Muthoka, P.; Mott, J.A.; Breiman, R.F.; Katz, M.A. Uptake and effectiveness of monovalent influenza A (H1N1) pandemic 2009 vaccine among healthcare personnel in Kenya, 2010. Vaccine 2010, 31, 4662–4667. [Google Scholar] [CrossRef]
- Le, H.; de Klerk, N.; Blyth, C.C.; Gidding, H.; Fathima, P.; Moore, H.C. Non-specific benefit of seasonal influenza vaccine on respiratory syncytial virus-hospitalisations in children: An instrumental variable approach using population-based data. Vaccine 2023, 41, 5029–5036. [Google Scholar] [CrossRef]
- Hjelholt, A.J.; Bergh, C.; Bhatt, D.L.; Fröbert, O.; Kjolby, M.F. Pleiotropic Effects of Influenza Vaccination. Vaccines 2023, 11, 1419. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Banerjee, A.; Douglas, I.; Leyrat, C.; Pebody, R.; I McDonald, H.; Herrett, E.; Forbes, H.; Smeeth, L.; Warren-Gash, C. Primary prevention of acute cardiovascular events by influenza vaccination: An observational study. Eur. Heart J. 2023, 44, 610–620. [Google Scholar] [CrossRef]
- Larsson, H.E.; for the TEDDY Study Group; Lynch, K.F.; Lönnrot, M.; Haller, M.J.; Lernmark, Å.; Hagopian, W.A.; She, J.-X.; Simell, O.; Toppari, J.; et al. Pandemrix® vaccination is not associated with increased risk of islet autoimmunity or type 1 diabetes in the TEDDY study children. Diabetologia 2018, 61, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Mariotti Zani, E.; Torelli, L.; Scavone, S.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Principi, N. Childhood Vaccinations and Type 1 Diabetes. Front. Immunol. 2021, 12, 667889. [Google Scholar] [CrossRef] [PubMed]
- Jong, H.C.; Zheng, J.Q.; Zheng, C.M.; Lin, C.H.; Chiu, C.C.; Hsu, M.H.; Fang, Y.A.; Hao, W.R.; Chen, C.C.; Yang, T.Y.; et al. Effect of Annual Influenza Vaccination on the Risk of Lung Cancer Among Patients With Hypertension: A Population-Based Cohort Study in Taiwan. Int. J. Public Health 2023, 68, 1605370. [Google Scholar] [CrossRef]
- Chen, K.Y.; Wu, S.M.; Liu, J.C.; Lee, K.Y. Effect of annual influenza vaccination on reducing lung cancer in patients with chronic obstructive pulmonary disease from a population-based cohort study. Medicine 2019, 98, e18035. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Wu, C.-H.; Lin, C.-H.; Chiu, C.-C.; Yang, T.-Y.; Lei, M.-H.; Yeh, H.-T.; Jian, W.; Fang, Y.-A.; Hao, W.-R.; et al. Influenza Vaccination and Risk of Lung Cancer in Patients with Chronic Kidney Disease: A Nationwide, Population-Based Cohort Study. Cancers 2022, 14, 2926. [Google Scholar] [CrossRef] [PubMed]
- Gogenur, M.; Fransgard, T.; Krause, T.G.; Thygesen, L.C.; Gogenur, I. Association of postoperative influenza vaccine on overall mortality in patients undergoing curative surgery for solid tumors. Int. J. Cancer 2021, 148, 1821–1827. [Google Scholar] [CrossRef]
- Esposito, S.; Cecinati, V.; Scicchitano, B.; Delvecchio, G.C.; Santoro, N.; Amato, D.; Pelucchi, C.; Jankovic, M.; De Mattia, D.; Principi, N. Impact of influenza-like illness and effectiveness of influenza vaccination in oncohematological children who have completed cancer therapy. Vaccine 2010, 28, 1558–1565. [Google Scholar] [CrossRef]
- Bukhbinder, A.S.; Ling, Y.; Hasan, O.; Jiang, X.; Kim, Y.; Phelps, K.N.; Schmandt, R.E.; Amran, A.; Coburn, R.; Ramesh, S.; et al. Risk of Alzheimer’s Disease Following Influenza Vaccination: A Claims-Based Cohort Study Using Propensity Score Matching. J. Alzheimers Dis. 2022, 88, 1061–1074. [Google Scholar] [CrossRef]
- Verreault, R.; Laurin, D.; Lindsay, J.; De Serres, G. Past exposure to vaccines and subsequent risk of Alzheimer’s disease. CMAJ 2001, 165, 1495–1498. [Google Scholar] [PubMed]
- Wimmers, F.; Donato, M.; Kuo, A.; Ashuach, T.; Gupta, S.; Li, C.; Dvorak, M.; Foecke, M.H.; Chang, S.E.; Hagan, T.; et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell 2021, 184, 3915–3935.e21. [Google Scholar] [CrossRef] [PubMed]
| Type of Effect and Vaccine | Nonspecific Effects |
|---|---|
| Positive | |
| Bacillus Calmette-Guerin (BCG) vaccine | Reduced mortality within the neonatal period or by age of 12 months; protection against malignancies, allergy, and autoimmune diseases, including type 1 diabetes |
| Measles vaccine | Reduction in global pediatric mortality, with girls showing the greatest benefit |
| Smallpox | Reduction in global pediatric mortality |
| Live poliovirus vaccine (OPV) | Reduction in gastrointestinal infections in Latin America, of respiratory infections in Russia, and of global child mortality in several underdeveloped countries |
| Negative | |
| Diphtheria-tetanus-pertussis (DTP) vaccine | Increased deaths from other diseases than it prevents from the target infections when is given after live vaccines |
| Inactivated polio vaccine (IPV) | Increase all-cause mortality by 10% |
| Malaria vaccine RTS,S/AS01 | Increase in all-cause mortality in girls |
| Hepatitis B vaccine (HBV) | Increase in mortality with the difference being particularly strong for girls |
| Type of Vaccine | Nonspecific Effects |
|---|---|
| Live attenuated influenza vaccine (LAIV) | Some data on the reduction in the total number of non-influenza medical attended respiratory infections in both children and adults |
| Nonspecific cross-protection against respiratory syncytial virus, suggesting a remodulation of innate immune activity | |
| Inactivated influenza vaccine (IIV) | Conflicting results on the incidence of non-influenza respiratory infections |
| Significant protective effect of 4IIV against COVID-19 | |
| Reduction in RSV hospitalizations in children, especially in those <2 years, with 4IIVs | |
| Reduced risk of developing or worsening coronary heart disease and heart failure; no effect on stroke | |
| Potential protective effect of influenza vaccine in development and progression of type 1 diabetes (T1D), cancer, and Alzheimer’s disease | |
| Conflicting results on epigenetic and transcriptional reprogramming as well as cytokine responses of immune cells after administration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Principi, N.; Esposito, S. Specific and Nonspecific Effects of Influenza Vaccines. Vaccines 2024, 12, 384. https://doi.org/10.3390/vaccines12040384
Principi N, Esposito S. Specific and Nonspecific Effects of Influenza Vaccines. Vaccines. 2024; 12(4):384. https://doi.org/10.3390/vaccines12040384
Chicago/Turabian StylePrincipi, Nicola, and Susanna Esposito. 2024. "Specific and Nonspecific Effects of Influenza Vaccines" Vaccines 12, no. 4: 384. https://doi.org/10.3390/vaccines12040384






