A Quadrivalent mRNA Immunization Elicits Potent Immune Responses against Multiple Orthopoxviral Antigens and Neutralization of Monkeypox Virus in Rodent Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell and Virus
2.2. Animal Study
2.3. Construction of Recombinant Plasmid
2.4. ALAB mRNA Preparation
2.5. mRNA-LNP Preparation
2.6. Detection of Antigen Expression Using Flow Cytometry
2.7. Antigen-Specific Binding Antibody Detection Assay
2.8. Neutralizing Assay
2.9. ELISPOT Assay for Cellular Immune Response Detection
2.10. Safety Evaluation of ALAB-LNP Vaccine in Rodent Model
2.11. Statistical Analysis
3. Results
3.1. Design, Preparation, and Characterization of the mRNA Candidates
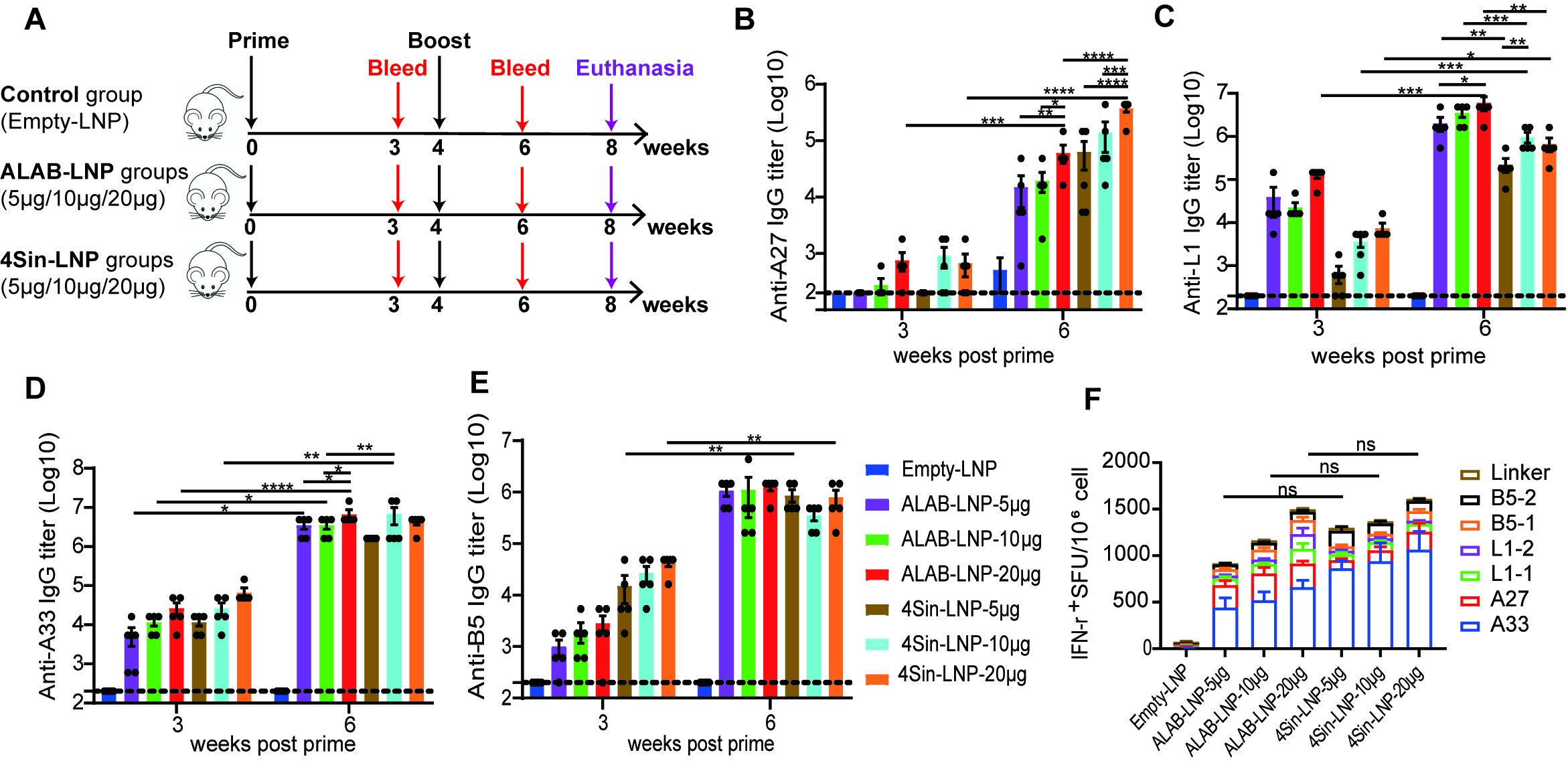
3.2. mRNA Vaccine Candidates Induced Robust Antibody Responses and Cellular Immune Responses in Mice
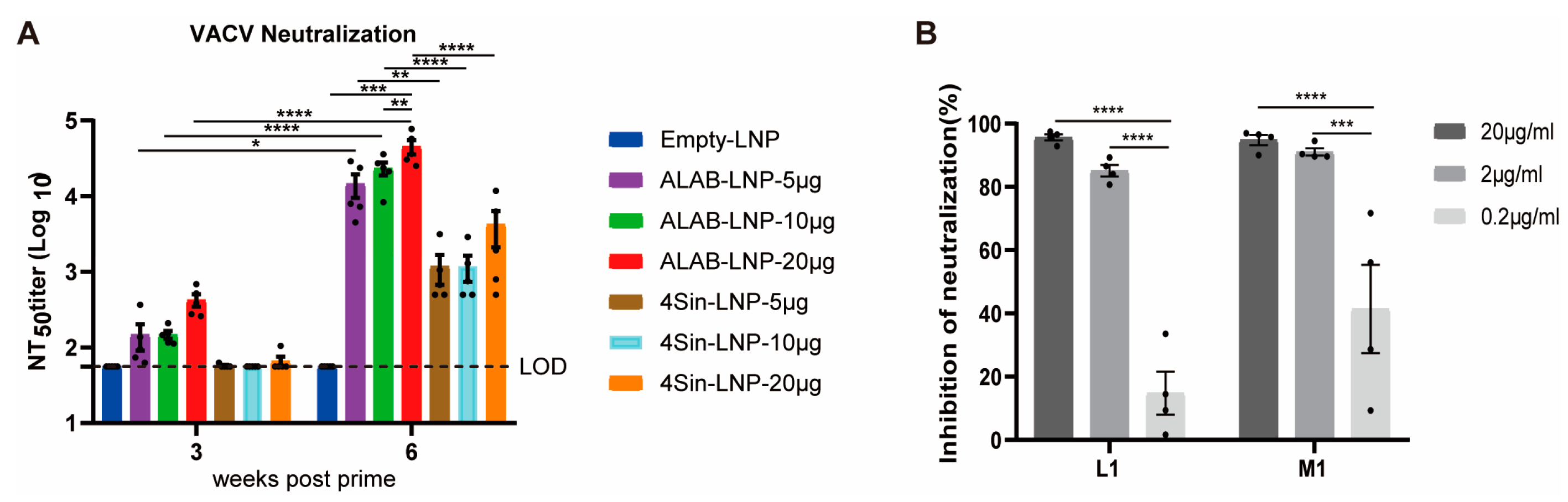
3.3. mRNA Vaccine Candidates ALAB-LNP Induced Potent Neutralizing Antibody against VACV
3.4. mRNA Vaccine Candidates ALAB-LNP Induced Potent Neutralizing Antibody against MPXV
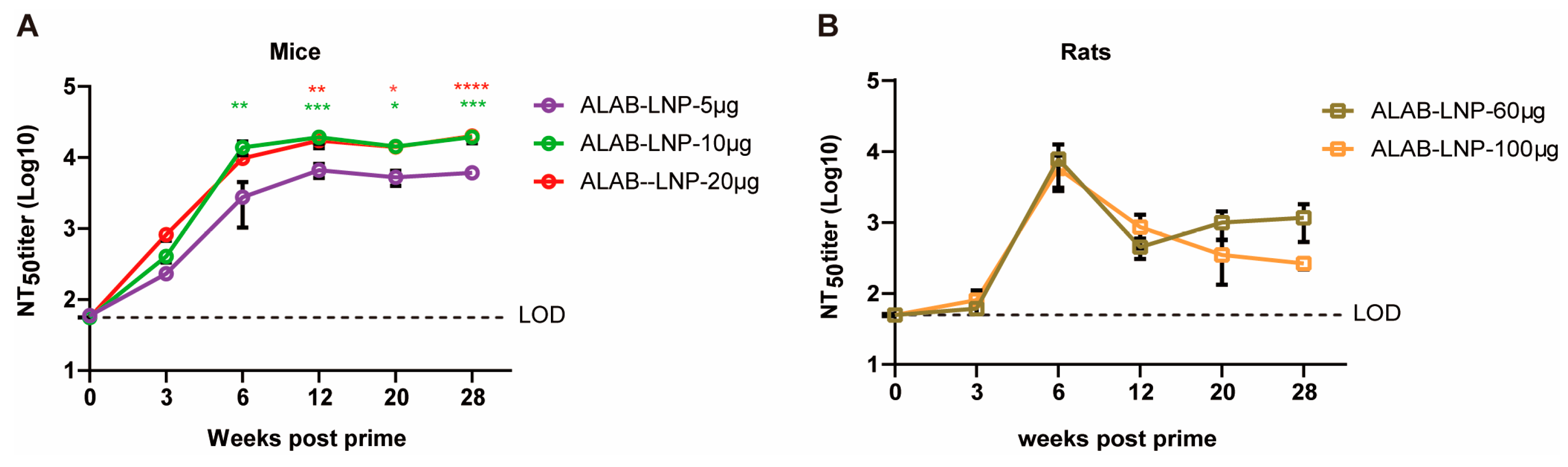
3.5. ALAB-LNP Was Immunogenic in Rats
3.6. ALAB-LNP Induced Long-Lasting Immune Responses in Mice and Rats
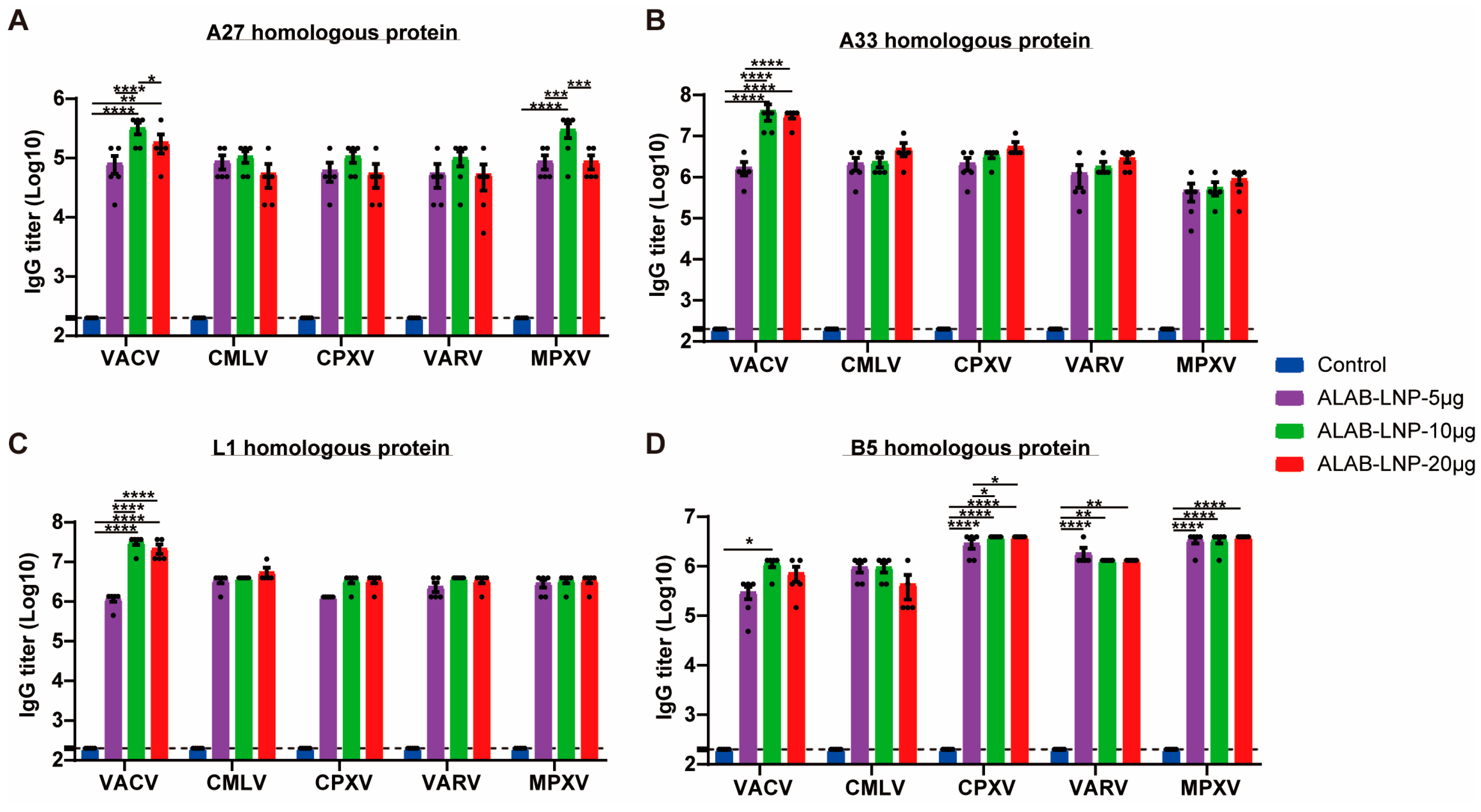
3.7. ALAB-LNP Induced Cross Binding Antibody Responses to Multiple Orthopoxviral Antigens
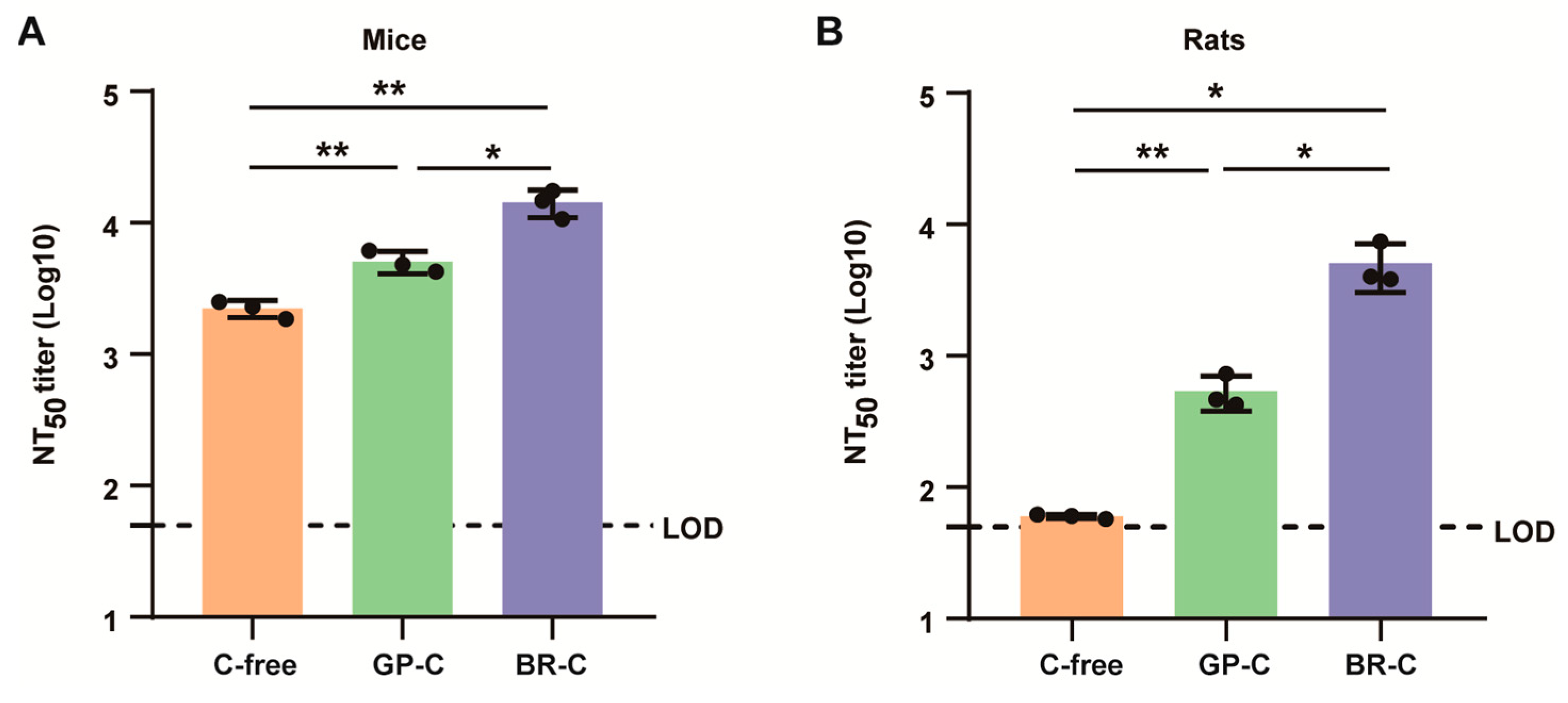
3.8. ALAB-LNP Induced Complement-Dependent Enhancement of Neutralizing Activity
3.9. Preliminary Safety Evaluation of ALAB-LNP in Mice and Rats
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baxby, D. Human poxvirus infection after the eradication of smallpox. Epidemiol. Infect. 1988, 100, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Nakoune, E.; Yazdanpanah, Y. Monkeypox. N. Engl. J. Med. 2022, 387, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.-J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Mpox Caused by Human-to-Human Transmission of Monkeypox Virus with Geographic Spread in the Democratic Republic of the Congo. 2023. Available online: https://emergency.cdc.gov/han/2023/han00501.asp (accessed on 30 March 2024).
- Smallpox Eradication: Destruction of Variola Virus Stocks. 2024. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA72/A72_28-en.pdf (accessed on 30 March 2024).
- Bennett, M.; Gaskell, C.J.; Baxbyt, D.; Gaskell, R.M.; Kelly, D.F.; Naidoot, J. Feline cowpox virus infection. J. Small Anim. Pract. 1990, 31, 167–173. [Google Scholar] [CrossRef]
- Kurth, A.; Wibbelt, G.; Gerber, H.P.; Petschaelis, A.; Pauli, G.; Nitsche, A. Rat-to-elephant-to-human transmission of cowpox virus. Emerg. Infect. Dis. 2008, 14, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Bera, B.C.; Shanmugasundaram, K.; Barua, S.; Venkatesan, G.; Virmani, N.; Riyesh, T.; Gulati, B.R.; Bhanuprakash, V.; Vaid, R.K.; Kakker, N.K.; et al. Zoonotic cases of camelpox infection in India. Vet. Microbiol. 2011, 152, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.M.; Li, Y.; Geleishvili, M.; Emerson, G.L.; Khmaladze, E.; Maghlakelidze, G.; Navdarashvili, A.; Zakhashvili, K.; Kokhreidze, M.; Endeladze, M.; et al. Human infection with a zoonotic orthopoxvirus in the country of Georgia. N. Engl. J. Med. 2015, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Gigante, C.M.; Gao, J.; Tang, S.; McCollum, A.M.; Wilkins, K.; Reynolds, M.G.; Davidson, W.; McLaughlin, J.; Olson, V.A.; Li, Y. Genome of Alaskapox Virus, A Novel Orthopoxvirus Isolated from Alaska. Viruses 2019, 11, 708. [Google Scholar] [CrossRef]
- Qin, L.; Upton, C.; Hazes, B.; Evans David, H. Genomic Analysis of the Vaccinia Virus Strain Variants Found in Dryvax Vaccine. J. Virol. 2011, 85, 13049–13060. [Google Scholar] [CrossRef]
- Fenner, F.J. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Monath, T.P.; Caldwell, J.R.; Mundt, W.; Fusco, J.; Johnson, C.S.; Buller, M.; Liu, J.; Gardner, B.; Downing, G.; Blum, P.S.; et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) —A second-generation smallpox vaccine for biological defense. Int. J. Infect. Dis. 2004, 8, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; Di Pilato, M.; Esteban, M. The evolution of poxvirus vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef]
- Nalca, A.; Zumbrun, E.E. ACAM2000: The new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010, 4, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Greenberg, R.N. IMVAMUNE®: Modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev. Vaccines 2009, 8, 13–24. [Google Scholar] [CrossRef]
- Kenner, J.; Cameron, F.; Empig, C.; Jobes, D.V.; Gurwith, M. LC16m8: An attenuated smallpox vaccine. Vaccine 2006, 24, 7009–7022. [Google Scholar] [CrossRef] [PubMed]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A.; et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef]
- Von Sonnenburg, F.; Perona, P.; Darsow, U.; Ring, J.; von Krempelhuber, A.; Vollmar, J.; Roesch, S.; Baedeker, N.; Kollaritsch, H.; Chaplin, P. Safety and immunogenicity of modified vaccinia Ankara as a smallpox vaccine in people with atopic dermatitis. Vaccine 2014, 32, 5696–5702. [Google Scholar] [CrossRef]
- Overton, E.T.; Stapleton, J.; Frank, I.; Hassler, S.; Goepfert, P.A.; Barker, D.; Wagner, E.; von Krempelhuber, A.; Virgin, G.; Meyer, T.P.; et al. Safety and Immunogenicity of Modified Vaccinia Ankara-Bavarian Nordic Smallpox Vaccine in Vaccinia-Naive and Experienced Human Immunodeficiency Virus-Infected Individuals: An Open-Label, Controlled Clinical Phase II Trial. Open Forum Infect. Dis. 2015, 2, ofv040. [Google Scholar] [CrossRef]
- Greenberg, R.N.; Hay, C.M.; Stapleton, J.T.; Marbury, T.C.; Wagner, E.; Kreitmeir, E.; Röesch, S.; von Krempelhuber, A.; Young, P.; Nichols, R.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN®) in 56-80-Year-Old Subjects. PLoS ONE 2016, 11, e0157335. [Google Scholar] [CrossRef]
- FDA Approves First Live, Non-Replicating Vaccine to Prevent Smallpox and Monkeypox. 2019. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-live-non-replicating-vaccine-prevent-smallpox-and-monkeypox (accessed on 30 March 2024).
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Götz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef]
- Hendrickson, R.C.; Wang, C.; Hatcher, E.L.; Lefkowitz, E.J. Orthopoxvirus genome evolution: The role of gene loss. Viruses 2010, 2, 1933–1967. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F.; Wittek, R.; Dumbell, K.R. CHPATER 4—The Pathogenesis, Pathology, and Immunology of Orthopoxvirus Infections. In The Orthopoxviruses; Fenner, F., Wittek, R., Dumbell, K.R., Eds.; Academic Press: Cambridge, MA, USA, 1989; pp. 85–141. [Google Scholar]
- Franceschi, V.; Parker, S.; Jacca, S.; Crump, R.W.; Doronin, K.; Hembrador, E.; Pompilio, D.; Tebaldi, G.; Estep, R.D.; Wong, S.W.; et al. BoHV-4-Based Vector Single Heterologous Antigen Delivery Protects STAT1(−/−) Mice from Monkeypoxvirus Lethal Challenge. PLoS Neglected Trop. Dis. 2015, 9, e0003850. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.S.; Hsiao, J.C.; Chang, Y.S.; Chang, W. A27L Protein Mediates Vaccinia Virus Interaction with Cell Surface Heparan Sulfate. J. Virol. 1998, 72, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.H.; Lou, H.; Whitbeck, J.C.; Ponce-de-León, M.; Atanasiu, D.; Eisenberg, R.J.; Cohen, G.H. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology 2009, 385, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Vanderplasschen, A.; Law, M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002, 83, 2915–2931. [Google Scholar] [CrossRef] [PubMed]
- Zajonc, D.M. Antibody Recognition of Immunodominant Vaccinia Virus Envelope Proteins. In Macromolecular Protein Complexes: Structure and Function; Harris, J.R., Marles-Wright, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 103–126. [Google Scholar]
- Paran, N.; Lustig, S. Complement-bound human antibodies to vaccinia virus B5 antigen protect mice from virus challenge. Expert Rev. Vaccines 2010, 9, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Smith, G.L. Antibody Neutralization of the Extracellular Enveloped Form of Vaccinia Virus. Virology 2001, 280, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kaever, T.; Meng, X.; Matho Michael, H.; Schlossman, A.; Li, S.; Sela-Culang, I.; Ofran, Y.; Buller, M.; Crump Ryan, W.; Parker, S.; et al. Potent Neutralization of Vaccinia Virus by Divergent Murine Antibodies Targeting a Common Site of Vulnerability in L1 Protein. J. Virol. 2014, 88, 11339–11355. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694. [Google Scholar] [CrossRef]
- Lustig, S.; Fogg, C.; Whitbeck, J.C.; Eisenberg Roselyn, J.; Cohen Gary, H.; Moss, B. Combinations of Polyclonal or Monoclonal Antibodies to Proteins of the Outer Membranes of the Two Infectious Forms of Vaccinia Virus Protect Mice against a Lethal Respiratory Challenge. J. Virol. 2005, 79, 13454–13462. [Google Scholar] [CrossRef]
- Hooper, J.W.; Custer, D.M.; Schmaljohn, C.S.; Schmaljohn, A.L. DNA Vaccination with Vaccinia Virus L1R and A33R Genes Protects Mice against a Lethal Poxvirus Challenge. Virology 2000, 266, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Custer, D.M.; Thompson, E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 2003, 306, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Reeman, S.; Gates, A.J.; Pulford, D.J.; Krieg, A.; Ulaeto, D.O. Protection of Mice from Lethal Vaccinia Virus Infection by Vaccinia Virus Protein Subunits with a CpG Adjuvant. Viruses 2017, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Thompson, E.; Wilhelmsen, C.; Zimmerman, M.; Ichou, M.A.; Steffen, S.E.; Schmaljohn, C.S.; Schmaljohn, A.L.; Jahrling, P.B. Smallpox DNA Vaccine Protects Nonhuman Primates against Lethal Monkeypox. J. Virol. 2004, 78, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Buchman, G.W.; Cohen, M.E.; Xiao, Y.; Richardson-Harman, N.; Silvera, P.; DeTolla, L.J.; Davis, H.L.; Eisenberg, R.J.; Cohen, G.H.; Isaacs, S.N. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine 2010, 28, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Hubert, M.; Guivel-Benhassine, F.; Bruel, T.; Porrot, F.; Planas, D.; Vanhomwegen, J.; Wiedemann, A.; Burrel, S.; Marot, S.; Palich, R.; et al. Complement-dependent mpox-virus-neutralizing antibodies in infected and vaccinated individuals. Cell Host Microbe 2023, 31, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Heraud, J.-M.; Edghill-Smith, Y.; Ayala, V.; Kalisz, I.; Parrino, J.; Kalyanaraman, V.S.; Manischewitz, J.; King, L.R.; Hryniewicz, A.; Trindade, C.J.; et al. Subunit Recombinant Vaccine Protects against Monkeypox1. J. Immunol. 2006, 177, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Laferrière, C.; Ardakani, A. Target Product Profile Analysis of COVID-19 Vaccines in Phase III Clinical Trials and Beyond: An Early 2021 Perspective. Viruses 2021, 13, 418. [Google Scholar] [CrossRef]
- Hou, F.; Zhang, Y.; Liu, X.; Murad, Y.; Xu, J.; Yu, Z.; Hua, X.; Song, Y.; Ding, J.; Huang, H.; et al. Novel mRNA vaccines encoding Monkeypox virus M1R and A35R protect mice from a lethal virus challenge. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sang, Y.; Zhang, Z.; Liu, F.; Lu, H.; Yu, C.; Sun, H.; Long, J.; Cao, Y.; Mai, J.; Wang, X.; et al. Monkeypox virus quadrivalent mRNA vaccine induces antibody responses and cellular immunity and protects mice against Vaccinia virus. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, R.R.; Wang, Z.J.; Zhu, Y.L.; Tang, W.; Zhou, C.; Zhao, S.Q.; Wu, M.; Ming, T.; Deng, Y.Q.; Chen, Q.; et al. Rational development of multicomponent mRNA vaccine candidates against mpox. Emerg. Microbes Infect. 2023, 12, 2192815. [Google Scholar] [CrossRef]
- Alec, W.F.; Caroline, A.; Patricia, L.E.; Jeffrey, L.A.; Gwo-Yu, C.; Harini, N.; Tiffany, F.; Jason, G.; Juan, I.M.; Ruth, H.; et al. A monkeypox mRNA-lipid nanoparticle vaccine targeting virus binding, entry, and transmission drives protection against lethal orthopoxviral challenge. bioRxiv 2022. [Google Scholar] [CrossRef]
- Fang, Z.; Monteiro, V.S.; Renauer, P.A.; Shang, X.; Suzuki, K.; Ling, X.; Bai, M.; Xiang, Y.; Levchenko, A.; Booth, C.J.; et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023, 33, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Zuiani, A.; Dulberger, C.L.; De Silva, N.S.; Marquette, M.; Lu, Y.-J.; Palowitch, G.M.; Dokic, A.; Sanchez-Velazquez, R.; Schlatterer, K.; Sarkar, S.; et al. A multivalent mRNA monkeypox virus vaccine (BNT166) protects mice and macaques from orthopoxvirus disease. Cell 2024, 187, 1363–1373. [Google Scholar] [CrossRef]
- Paran, N.; Lustig, S.; Zvi, A.; Erez, N.; Israely, T.; Melamed, S.; Politi, B.; Ben-Nathan, D.; Schneider, P.; Lachmi, B.; et al. Active vaccination with vaccinia virus A33 protects mice against lethal vaccinia and ectromelia viruses but not against cowpoxvirus; elucidation of the specific adaptive immune response. Virol. J. 2013, 10, 229. [Google Scholar] [CrossRef]
- Fogg, C.; Lustig, S.; Whitbeck, J.C.; Eisenberg Roselyn, J.; Cohen Gary, H.; Moss, B. Protective Immunity to Vaccinia Virus Induced by Vaccination with Multiple Recombinant Outer Membrane Proteins of Intracellular and Extracellular Virions. J. Virol. 2004, 78, 10230–10237. [Google Scholar] [CrossRef]
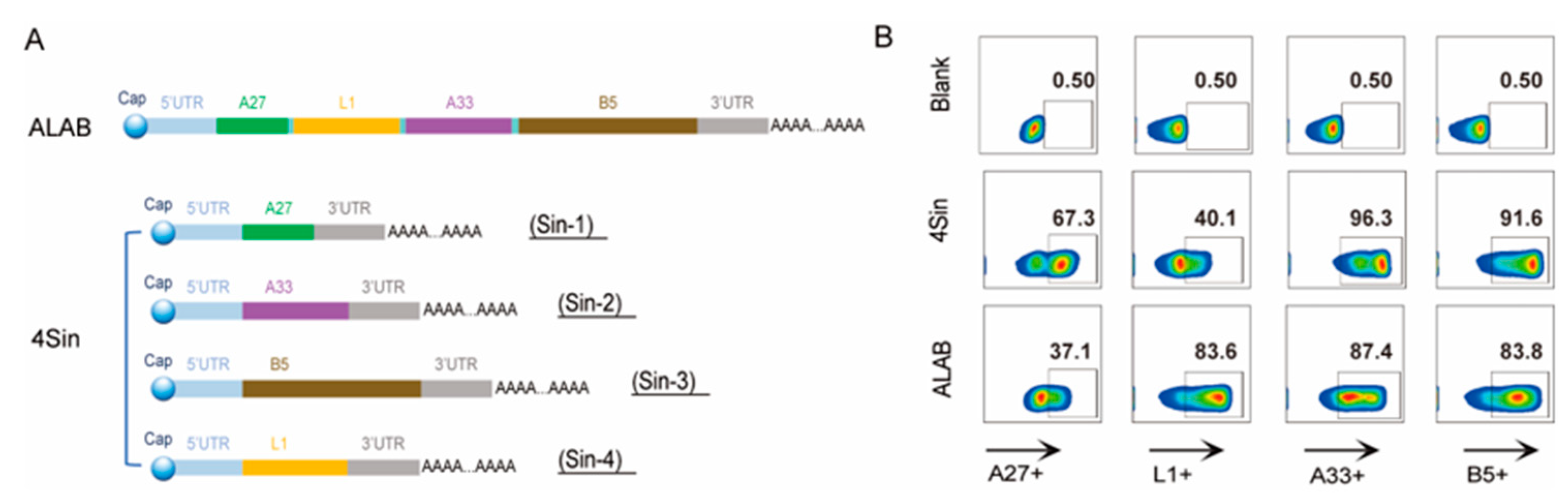




| Sample ID | EE (%) | Size (nm) | PDI |
|---|---|---|---|
| Empty-LNP | - | 76 | 0.07 |
| ALAB-LNP | 90 | 80 | 0.05 |
| 4Sin-LNP | 89 | 83 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Li, S.; Wen, Y.; Geng, X.; Yin, Q.; Wang, Y.; Xiong, Y.; Liu, Z. A Quadrivalent mRNA Immunization Elicits Potent Immune Responses against Multiple Orthopoxviral Antigens and Neutralization of Monkeypox Virus in Rodent Models. Vaccines 2024, 12, 385. https://doi.org/10.3390/vaccines12040385
Su C, Li S, Wen Y, Geng X, Yin Q, Wang Y, Xiong Y, Liu Z. A Quadrivalent mRNA Immunization Elicits Potent Immune Responses against Multiple Orthopoxviral Antigens and Neutralization of Monkeypox Virus in Rodent Models. Vaccines. 2024; 12(4):385. https://doi.org/10.3390/vaccines12040385
Chicago/Turabian StyleSu, Caixia, Sha Li, Yang Wen, Xiya Geng, Quanyi Yin, Yun Wang, Yelin Xiong, and Zhihua Liu. 2024. "A Quadrivalent mRNA Immunization Elicits Potent Immune Responses against Multiple Orthopoxviral Antigens and Neutralization of Monkeypox Virus in Rodent Models" Vaccines 12, no. 4: 385. https://doi.org/10.3390/vaccines12040385






