Nasopharyngeal Carriage of Streptococcus pneumoniae in Tunisian Healthy under-Five Children during a Three-Year Survey Period (2020 to 2022)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Laboratory Methods
2.3. Data Analysis
3. Results
3.1. Prevalence of Pneumococcal Nasopharyngeal Carriage
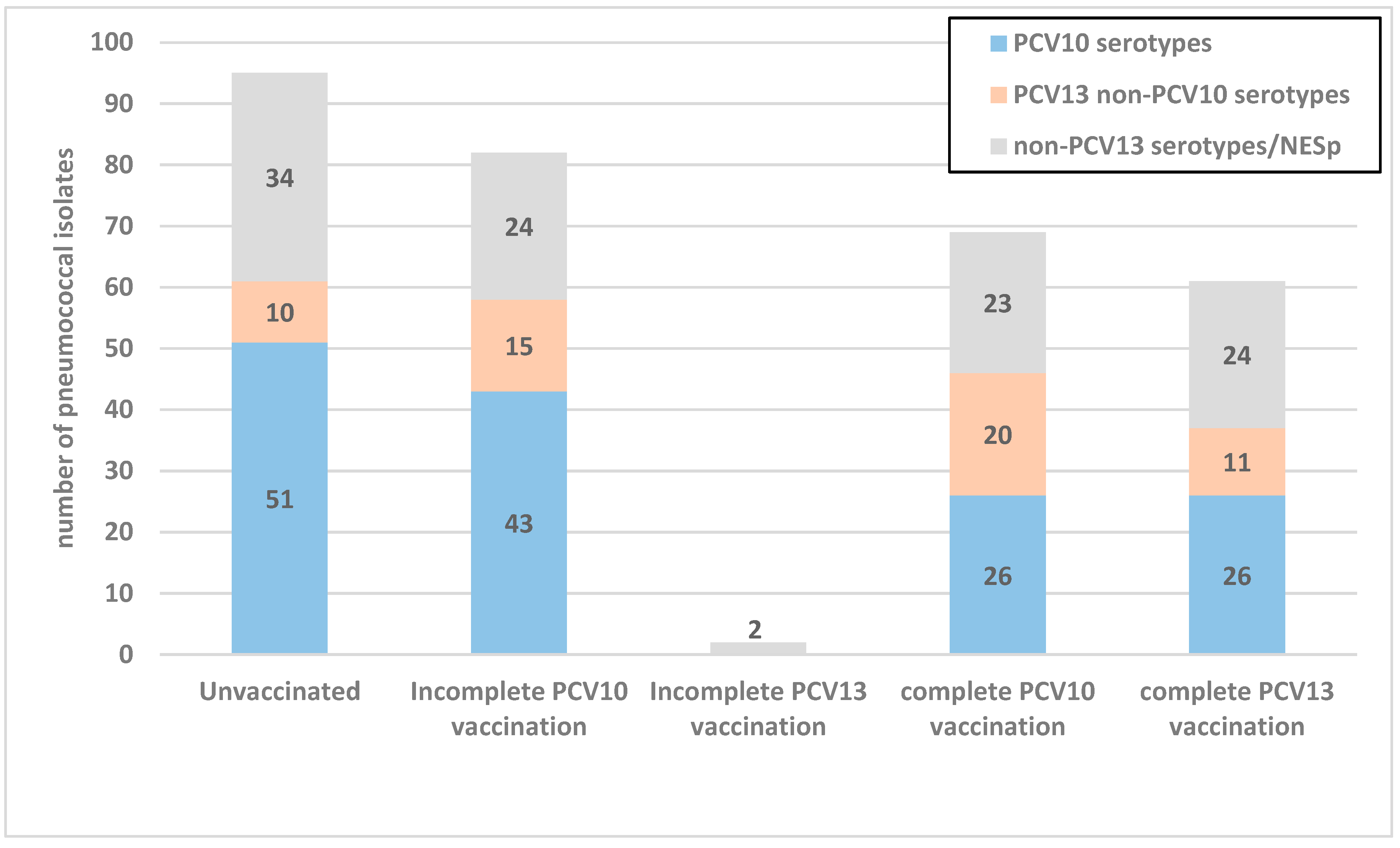
3.2. Serotype Distribution of S. pneumoniae Carriage Isolates
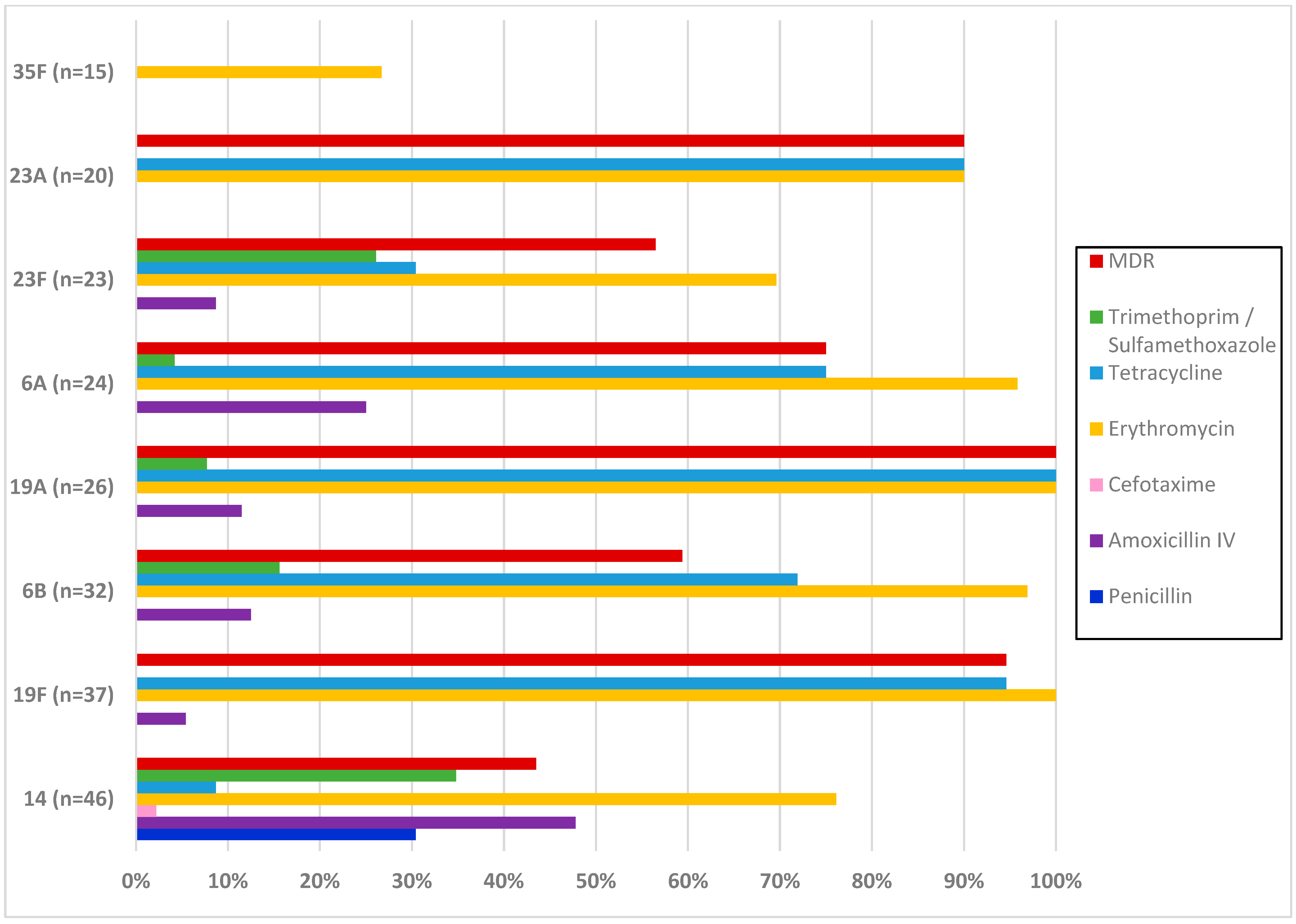
3.3. Antimicrobial Resistance of S. pneumoniae Carriage Isolates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–2015. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; De Groot, R.; Hermans, P. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’brien, K.L. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef]
- Manna, S.; Werren, J.P.; Ortika, B.D.; Bellich, B.; Pell, C.L.; Nikolaou, E.; Gjuroski, I.; Lo, S.; Hinds, J.; Tundev, O.; et al. Streptococcus pneumoniae serotype 33G: Genetic, serological, and structural analysis of a new capsule type. Microbiol. Spectr. 2024, 12, e03579-23. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Ito, M.; Takahashi, A.; Habadera, S.; Kobayashi, N. High prevalence of antimicrobial resistance in non-vaccine serotypes of non-invasive/colonization isolates of Streptococcus pneumoniae: A cross-sectional study eight years after the licensure of conjugate vaccine in Japan. J. Infect. Public Health 2020, 13, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, F.; Saad, J.S.; McGee, L.; van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A New Pneumococcal Capsule Type, 10D, is the 100th Serotype and Has a Large cps Fragment from an Oral Streptococcus. mBio 2020, 11, e00937-20. [Google Scholar] [CrossRef]
- Watkins, E.R.; Kalizang’Oma, A.; Gori, A.; Gupta, S.; Heyderman, R.S. Factors affecting antimicrobial resistance in Streptococcus pneumoniae following vaccination introduction. Trends Microbiol. 2022, 30, 1135–1145. [Google Scholar] [CrossRef]
- Satzke, C.; Turner, P.; Virolainen-Julkunen, A.; Adrian, P.V.; Antonio, M.; Hare, K.M.; Henao-Restrepo, A.M.; Leach, A.J.; Klugman, K.P.; Porter, B.D.; et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013, 32, 165–179. [Google Scholar] [CrossRef]
- Pai, R.; Gertz, R.E.; Beall, B. Sequential Multiplex PCR Approach for Determining Capsular Serotypes of Streptococcus pneumoniae Isolates. J. Clin. Microbiol. 2006, 44, 124–131. [Google Scholar] [CrossRef]
- Ziane, H.; Manageiro, V.; Ferreira, E.; Bektache, S.; Tazir, M.; Caniça, M. Capsular typing of Streptococcus pneumoniae isolated in an Algerian hospital using a new multiplex PCR-based scheme. J. Microbiol. Methods 2015, 119, 243–246. [Google Scholar] [CrossRef]
- Kawaguchiya, M.; Urushibara, N.; Aung, M.S.; Kudo, K.; Ito, M.; Sumi, A.; Kobayashi, N. Clonal lineages and antimicrobial resistance of nonencapsulated Streptococcus pneumoniae in the post-pneumococcal conjugate vaccine era in Japan. Int. J. Infect. Dis. 2021, 105, 695–701. [Google Scholar] [CrossRef]
- Golden, A.R.; Rosenthal, M.; Fultz, B.; Nichol, K.A.; Adam, H.J.; Gilmour, M.W.; Baxter, M.R.; Hoban, D.J.; Karlowsky, J.A.; Zhanel, G.G. Characterization of MDR and XDR Streptococcus pneumoniae in Canada, 2007–2013. J. Antimicrob. Chemother. 2015, 70, 2199–2202. [Google Scholar] [CrossRef]
- Tvedskov, E.S.F.; Hovmand, N.; Benfield, T.; Tinggaard, M. Pneumococcal carriage among children in low and lower-middle-income countries: A systematic review. Int. J. Infect. Dis. 2022, 115, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Mameli, C.; Daprai, L.; Garlaschi, M.L.; Dilillo, D.; Bedogni, G.; Faccini, M.; Gramegna, M.; Torresani, E.; Emanuela, B.; et al. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine 2014, 32, 527–534. [Google Scholar] [CrossRef]
- Koliou, M.G.; Andreou, K.; Lamnisos, D.; Lavranos, G.; Iakovides, P.; Economou, C.; Soteriades, E.S. Risk factors for carriage of Streptococcus pneumoniae in children. BMC Pediatr. 2018, 18, 144. [Google Scholar] [CrossRef]
- Løvlie, A.; Vestrheim, D.F.; Aaberge, I.S.; Steens, A. Changes in pneumococcal carriage prevalence and factors associated with carriage in Norwegian children, four years after introduction of PCV13. BMC Infect. Dis. 2020, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Nguyen, C.D.; Dunne, E.M.; Mulholland, E.K.; Mungun, T.; Pomat, W.S.; Rafai, E.; Satzke, C.; Weinberger, D.M.; Russell, F.M. Using pneumococcal carriage studies to monitor vaccine impact in low- and middle-income countries. Vaccine 2019, 37, 6299–6309. [Google Scholar] [CrossRef] [PubMed]
- Jochems, S.P.; Weiser, J.N.; Malley, R.; Ferreira, D.M. The immunological mechanisms that control pneumococcal carriage. PLoS Pathog. 2017, 13, e1006665. [Google Scholar] [CrossRef]
- Hausdorff, W.P.; Feikin, D.R.; Klugman, K.P. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 2005, 5, 83–93. [Google Scholar] [CrossRef]
- Tam, P.-Y.I.; Thielen, B.K.; Obaro, S.K.; Brearley, A.M.; Kaizer, A.M.; Chu, H.; Janoff, E.N. Childhood pneumococcal disease in Africa—A systematic review and meta-analysis of incidence, serotype distribution, and antimicrobial susceptibility. Vaccine 2017, 35, 1817–1827. [Google Scholar] [CrossRef]
- Johnson, H.L.; Deloria-Knoll, M.; Levine, O.S.; Stoszek, S.K.; Freimanis Hance, L.; Reithinger, R.; Muenz, L.R.; O’Brien, K.L. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children under Five: The Pneumococcal Global Serotype Project. PLoS Med. 2010, 7, e1000348. [Google Scholar] [CrossRef]
- Adetifa, I.M.O.; Adamu, A.L.; Karani, A.; Waithaka, M.; Odeyemi, K.A.; Okoromah, C.A.N.; Bello, M.M.; Abubakar, I.S.; Inem, V.; Scott, J.A.G. Nasopharyngeal Pneumococcal Carriage in Nigeria: A two-site, population-based survey. Sci. Rep. 2018, 8, 3509. [Google Scholar] [CrossRef]
- Lindstrand, A.; Galanis, I.; Darenberg, J.; Morfeldt, E.; Naucler, P.; Blennow, M.; Alfven, T.; Henriques-Normark, B.; Örtqvist, Å. Unaltered pneumococcal carriage prevalence due to expansion of non-vaccine types of low invasive potential 8 years after vaccine introduction in Stockholm, Sweden. Vaccine 2016, 34, 4565–4571. [Google Scholar] [CrossRef]
- Kandasamy, R.; Voysey, M.; Collins, S.; Berbers, G.; Robinson, H.; Noel, I.; Hughes, H.; Ndimah, S.; Gould, K.; Fry, N.; et al. Persistent Circulation of Vaccine Serotypes and Serotype Replacement After 5 Years of Infant Immunization with 13-Valent Pneumococcal Conjugate Vaccine in the United Kingdom. J. Infect. Dis. 2020, 221, 1361–1370. [Google Scholar] [CrossRef]
- Swarthout, T.D.; Fronterre, C.; Lourenço, J.; Obolski, U.; Gori, A.; Bar-Zeev, N.; Everett, D.; Kamng’ona, A.W.; Mwalukomo, T.S.; Mataya, A.A.; et al. High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat. Commun. 2020, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Murad, C.; Dunne, E.M.; Sudigdoadi, S.; Fadlyana, E.; Tarigan, R.; Pell, C.L.; Watts, E.; Nguyen, C.D.; Satzke, C.; Hinds, J.; et al. Pneumococcal carriage, density, and co-colonization dynamics: A longitudinal study in Indonesian infants. Int. J. Infect. Dis. 2019, 86, 73–81. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Trzciński, K.; Lu, Y.-J.; Bogaert, D.; Brandes, A.; Galagan, J.; Anderson, P.W.; Malley, R.; Lipsitch, M. Pneumococcal Capsular Polysaccharide Structure Predicts Serotype Prevalence. PLoS Pathog. 2009, 5, e1000476. [Google Scholar] [CrossRef]
- Song, J.H.; Dagan, R.; Klugman, K.P.; Fritzell, B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine 2012, 30, 2728–2737. [Google Scholar] [CrossRef]
- Varghese, R.; Veeraraghavan, B. Decoding the Penicillin Resistance of Streptococcus pneumoniae for Invasive and Noninvasive Infections. Microb. Drug Resist. 2021, 27, 942–950. [Google Scholar] [CrossRef]
- Högberg, L.; Geli, P.; Ringberg, H.; Melander, E.; Lipsitch, M.; Ekdahl, K. Age- and Serogroup-Related Differences in Observed Durations of Nasopharyngeal Carriage of Penicillin-Resistant Pneumococci. J. Clin. Microbiol. 2007, 45, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayed, N.; Ktari, S.; Mezghani, S.; Mnif, B.; Mahjoubi, F.; Hammami, A. Relationship Between Serotypes and Antimicrobial Nonsusceptibility of Streptococcus pneumoniae Clinical Isolates in Tunisia. Microb. Drug Resist. 2022, 28, 370–377. [Google Scholar] [CrossRef] [PubMed]
| Factors | Carriage Prevalence (%) | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|---|
| OR | CI95% | p | OR | CI95% | p | ||
| Age, months | |||||||
| 2–24 (n = 779) | 20.3 | 1.768 | 1.362–2.295 | <0.001 | 1.570 | 1.052–2.345 | 0.027 |
| 25–59 (n = 477) | 31 | ||||||
| Vaccination status | |||||||
| Unvaccinated (n = 375) | 25.3 | 1.00 | 1.00 | ||||
| Incomplete PCV vaccination (n = 431) | 19.3 | 0.703 | 0.503–0.982 | 0.039 | 0.821 | 0.524–1.287 | 0.390 |
| Complete PCV vaccination (n = 450) | 28.4 | 1.172 | 0.859–1.597 | 0.317 | 1.105 | 0.782–1.560 | 0.572 |
| Presence of siblings | |||||||
| Yes (n = 814) | 27.4 | 1.632 | 1.228–2.168 | 0.001 | 1.485 | 1.099–2.006 | 0.010 |
| No (n = 442) | 18.8 | ||||||
| Childcare attendance | |||||||
| Yes (n = 795) | 28.2 | 1.813 | 1.364–2.410 | <0.001 | 1.131 | 0.789–1.623 | 0.503 |
| No (n = 461) | 17.8 | ||||||
| History of respiratory tract infections | |||||||
| Yes (n = 426) | 32.4 | 1.888 | 1.450–2.459 | <0.001 | 1.942 | 1.412–2.670 | <0.001 |
| No (n = 830) | 20.2 | ||||||
| Antibiotic treatment within the last 3 months | |||||||
| Yes (n = 409) | 30.1 | 1.560 | 1.194–2.039 | 0.001 | 1.190 | 0.863–1.642 | 0.289 |
| No (n = 847) | 21.6 | ||||||
| History of hospitalization | |||||||
| Yes (n = 102) | 34.3 | 1.702 | 1.106–2.619 | 0.015 | 1.727 | 1.083–2.754 | 0.022 |
| No (n = 1154) | 23.5 | ||||||
| Serotypes | Total n (%) | First Survey Period n (%) | Second Survey Period n (%) | Third Survey Period n (%) | p |
|---|---|---|---|---|---|
| PCV10 serotypes | |||||
| 14 | 46 (14.9) | 12 (11.4) | 20 (19.2) | 14 (14) | 0.591 |
| 19F | 37 (12) | 22 (21) | 9 (8.7) | 6 (6) | 0.001 |
| 6B | 32 (10.4) | 11 (10.5) | 8 (7.7) | 13 (13) | 0.564 |
| 23F | 23 (7.4) | 5 (4.8) | 8 (7.7) | 10 (10) | 0.153 |
| 18C | 5 (1.6) | 2 (1.9) | 3 (2.9) | 0 (0) | N/A |
| 7F | 2 (0.6) | 0 (0) | 0 (0) | 2 (2) | N/A |
| 9V | 1 (0.3) | 0 (0) | 1 (1) | 0 (0) | N/A |
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N/A |
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N/A |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N/A |
| PCV13 non-PCV10 serotypes | |||||
| 19A | 26 (8.4) | 5 (4.8) | 8 (7.7) | 13 (13) | 0.034 |
| 6A | 24 (7.8) | 3 (2.9) | 10 (9.6) | 11 (11) | 0.029 |
| 3 | 6 (1.9) | 1 (1) | 1 (1) | 4 (4) | N/A |
| Non-PCV13 serotypes/NESp | |||||
| 23A | 20 (6.5) | 5 (4.8) | 14 (13.5) | 1 (1) | 0.297 |
| 35F | 15 (4.9) | 8 (7.6) | 5 (4.8) | 2 (2) | N/A |
| 11A/D | 13 (4.2) | 3 (2.9) | 3 (2.9) | 7 (7) | N/A |
| 24F | 10 (3.2) | 2 (1.9) | 3 (2.9) | 5 (5) | N/A |
| 35B | 9 (2.9) | 5 (4.8) | 1 (1) | 3 (3) | N/A |
| 16F | 7 (2.3) | 3 (2.9) | 1 (1) | 3 (3) | N/A |
| 34 | 5 (1.6) | 1 (1) | 3 (2.9) | 1 (1) | N/A |
| 6C | 5 (1.6) | 2 (1.9) | 2 (1.9) | 1 (1) | N/A |
| 10A | 3 (1) | 3 (2.9) | 0 (0) | 0 (0) | N/A |
| 9N | 3 (1) | 0 (0) | 2 (1.9) | 1 (1) | N/A |
| 13 | 2 (0.6) | 1 (1) | 0 (0) | 1 (1) | N/A |
| 15A | 2 (0.6) | 1 (1) | 0 (0) | 1 (1) | N/A |
| 22F | 2 (0.6) | 1 (1) | 1 (1) | 0 (0) | N/A |
| 15B/C | 1 (0.3) | 0 (0) | 1 (1) | 0 (0) | N/A |
| 38 | 1 (0.3) | 1 (1) | 0 (0) | 0 (0) | N/A |
| NESp | 9 (2.9) | 8 (7.6) | 0 (0) | 1 (1) | N/A |
| Total | 309 | 105 | 104 | 100 | - |
| Antibiotic | Total | First Survey Period | Second Survey Period | Third Survey Period | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | ||
| Penicillin MIC: 0.06–2 | 15.9 | 79.3 | 4.9 | 16.2 | 81.9 | 1.9 | 12.5 | 79.8 | 7.7 | 19 | 76 | 5 | N/A |
| Penicillin (meningitis) MIC: 0.06–0.06 | 15.9 | - | 84.1 | 16.2 | - | 83.8 | 12.5 | - | 87.5 | 19 | - | 81 | |
| Amoxicillin IV MIC: 1–2 | 67.3 | 17.8 | 14.9 | 71.4 | 19 | 9.5 | 71.2 | 10.6 | 18.3 | 59 | 24 | 17 | 0.129 |
| Amoxicillin IV (meningitis) MIC: 0.5–0.5 | 45.3 | - | 54.7 | 45.7 | - | 54.3 | 43.3 | - | 56.7 | 47 | - | 53 | |
| Amoxicillin per os MIC: 0.5–1 | 45.3 | 22 | 32.7 | 45.7 | 25.7 | 28.6 | 43.3 | 27.9 | 28.8 | 47 | 12 | 41 | |
| Cefotaxime MIC: 0.5–2 | 90.9 | 8.7 | 0.3 | 94.3 | 4.8 | 1 | 87.5 | 12.5 | 0 | 91 | 9 | 0 | N/A |
| Cefotaxime (meningitis) MIC: 0.5–0.5 | 90.9 | - | 9.1 | 94.3 | - | 5.7 | 87.5 | - | 12.5 | 91 | - | 9 | |
| Erythromycin | 21.7 | 0 | 78.3 | 22.9 | 0 | 77.1 | 23.1 | 0 | 76.9 | 19 | 0 | 81 | 0.507 |
| Clindamycin | 31.1 | - | 68.9 | 36.2 | - | 63.8 | 30.8 | - | 69.2 | 26 | - | 74 | 0.115 |
| Tetracycline | 46.6 | - | 53.4 | 42.9 | - | 57.1 | 51 | - | 49 | 46 | - | 54 | 0.642 |
| Trimethoprim/ Sulfamethoxazole | 80.9 | 3.2 | 15.9 | 91.4 | 1 | 7.6 | 79.8 | 1 | 19.2 | 71 | 8 | 21 | 0.008 |
| MDR | 56.6 | 51.4 | 58.7 | 60 | 0.214 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ayed, N.; Ktari, S.; Jdidi, J.; Gargouri, O.; Smaoui, F.; Hachicha, H.; Ksibi, B.; Mezghani, S.; Mnif, B.; Mahjoubi, F.; et al. Nasopharyngeal Carriage of Streptococcus pneumoniae in Tunisian Healthy under-Five Children during a Three-Year Survey Period (2020 to 2022). Vaccines 2024, 12, 393. https://doi.org/10.3390/vaccines12040393
Ben Ayed N, Ktari S, Jdidi J, Gargouri O, Smaoui F, Hachicha H, Ksibi B, Mezghani S, Mnif B, Mahjoubi F, et al. Nasopharyngeal Carriage of Streptococcus pneumoniae in Tunisian Healthy under-Five Children during a Three-Year Survey Period (2020 to 2022). Vaccines. 2024; 12(4):393. https://doi.org/10.3390/vaccines12040393
Chicago/Turabian StyleBen Ayed, Nourelhouda, Sonia Ktari, Jihen Jdidi, Omar Gargouri, Fahmi Smaoui, Haifa Hachicha, Boutheina Ksibi, Sonda Mezghani, Basma Mnif, Faouzia Mahjoubi, and et al. 2024. "Nasopharyngeal Carriage of Streptococcus pneumoniae in Tunisian Healthy under-Five Children during a Three-Year Survey Period (2020 to 2022)" Vaccines 12, no. 4: 393. https://doi.org/10.3390/vaccines12040393
APA StyleBen Ayed, N., Ktari, S., Jdidi, J., Gargouri, O., Smaoui, F., Hachicha, H., Ksibi, B., Mezghani, S., Mnif, B., Mahjoubi, F., & Hammami, A. (2024). Nasopharyngeal Carriage of Streptococcus pneumoniae in Tunisian Healthy under-Five Children during a Three-Year Survey Period (2020 to 2022). Vaccines, 12(4), 393. https://doi.org/10.3390/vaccines12040393






