Analysis of Protection and Immune Response against Teladorsagia circumcincta in Goats Immunised with Thiol-Binding Proteins from Adult Worms
Abstract
1. Introduction
2. Materials and Methods
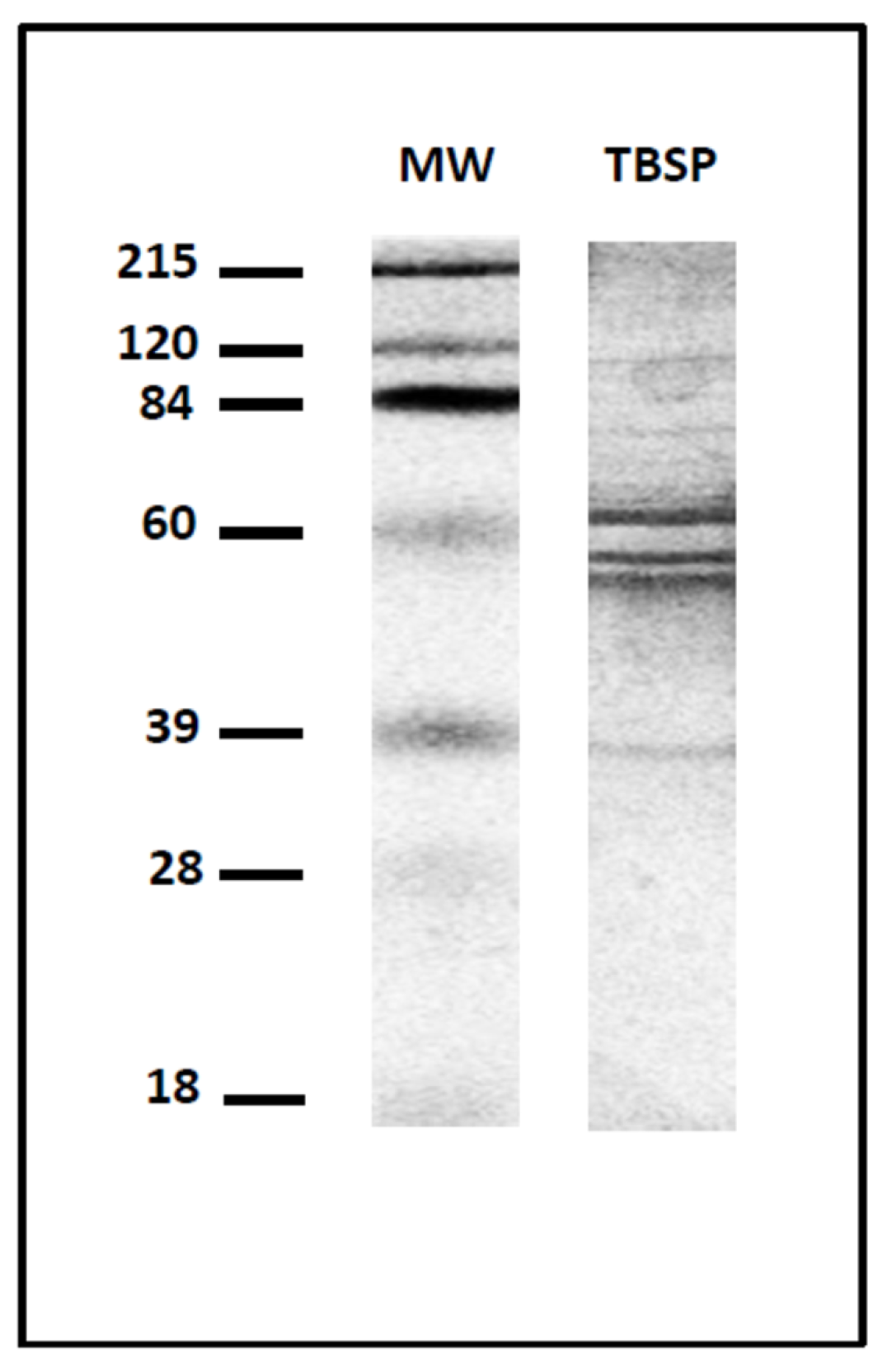
2.1. Preparation of Homogenates of Adult Worms of T. circumcincta and Enriched Protein Fractions by Thiol-Sepharose Chromatography
2.2. Experimental Design
2.3. Parasitological and Biopathological Analysis
2.4. Determination of Systemic and Local Humoral (IgG and IgA Isotypes) Response
2.5. Histology and Immunohistochemistry of Abomasal Mucosa
2.6. Determination of Relative Cytokine Gene Expression by Real-Time PCR (RT-PCR)
| Cytokine | Primers 5′ to 3′ | Size (bp) | MgCl2 (mM) | Tm (°C) | Accession Number/Ref. |
|---|---|---|---|---|---|
| B-act | CCAACCGTGAGAAGATGACCC CCCAGAGTCCATGACAATGCC | 122 | 5 | 85.0 | AF481159 |
| IL-2 | GTGAAGTCATTGCTGCTGGA TGTTCAGGTTTTTGCTTGGA | 202 | 3 | 81.0 | [28] |
| IL-4 | GCTGGTCTGCTTACTGGTATG CGATGTGAGGATGTTCAGC | 100 | 5 | 80.0 | FJ936316 |
| IL-10 | GTGGAGCAGGTGAAGAGAGTC TGGGTCGGATTTCAGAGG | 198 | 3 | 82.0 | AF458378 |
| INFγ | AGATAACCAGGTCATTCAAAGGAG GGCGACAGGTCATTCATCAC | 180 | 3 | 82.5 | U34232 |
| IL-17 | TGCTACTGCTTCTGAGTCTGGTGGC TGACCCTCACATGCTGTGGGAAGTT | 111 | 0 | 83.5 | [29] |
2.7. Statistical Analysis
3. Results
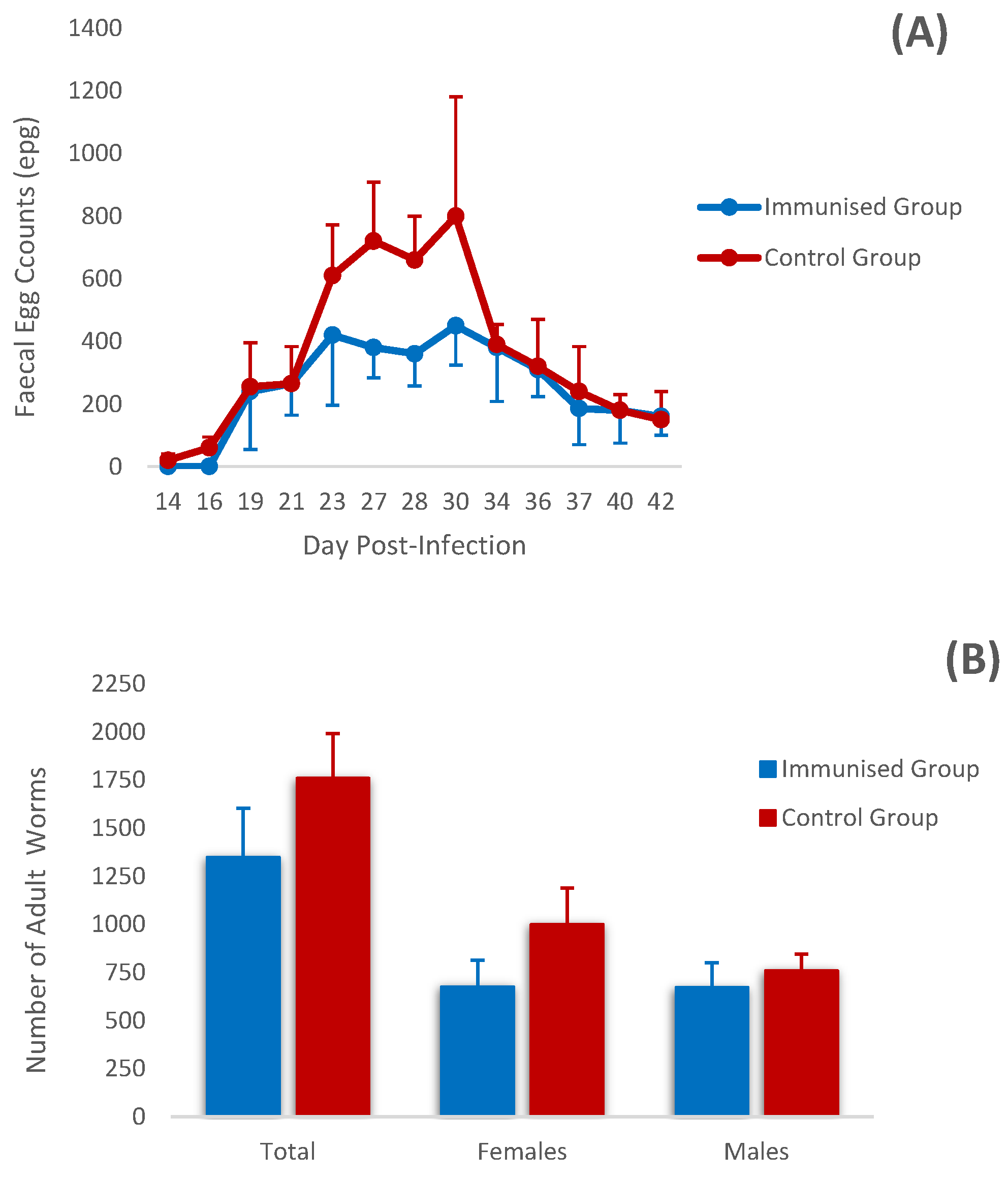
3.1. Parasitological Analysis
3.2. Biopathological Analysis
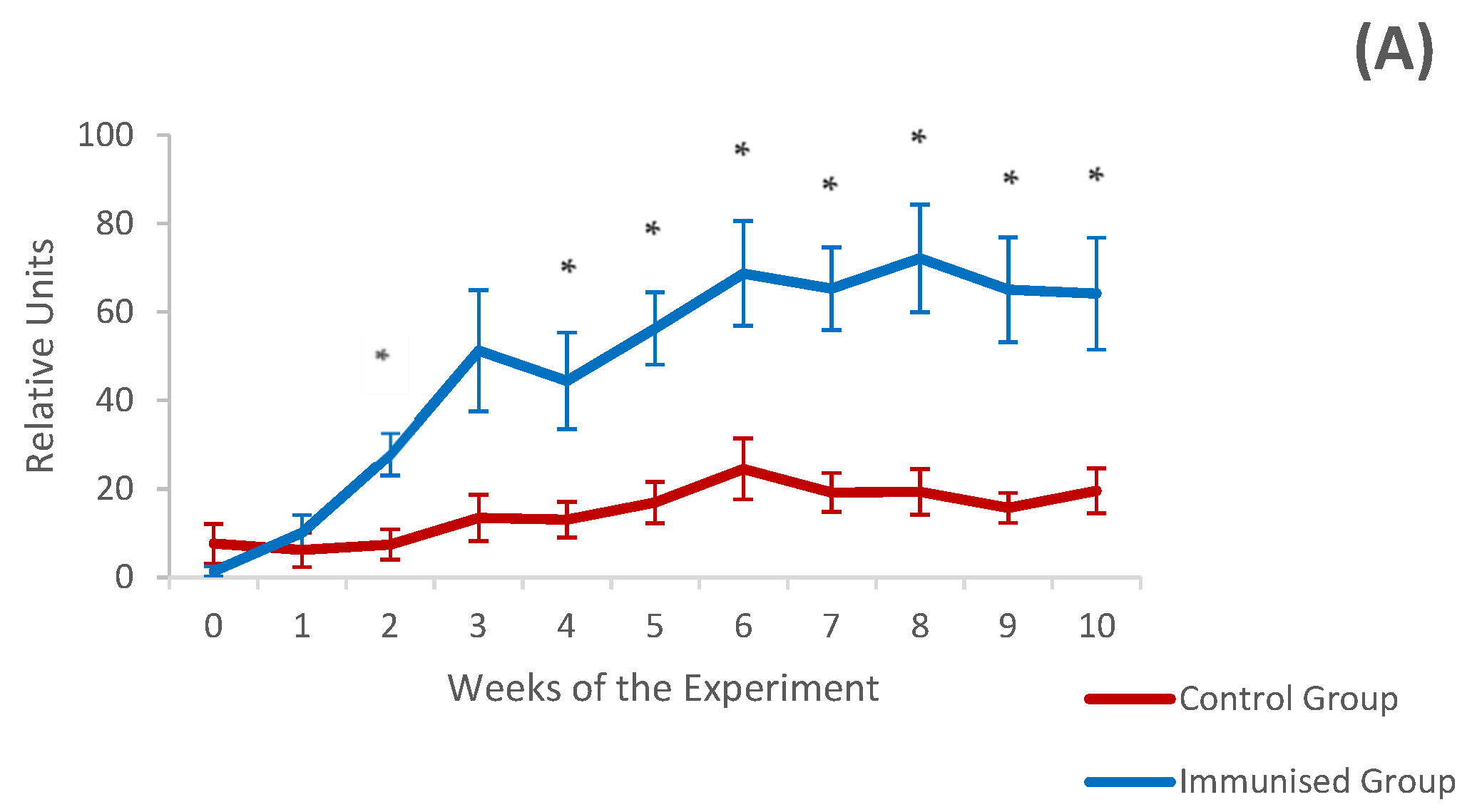
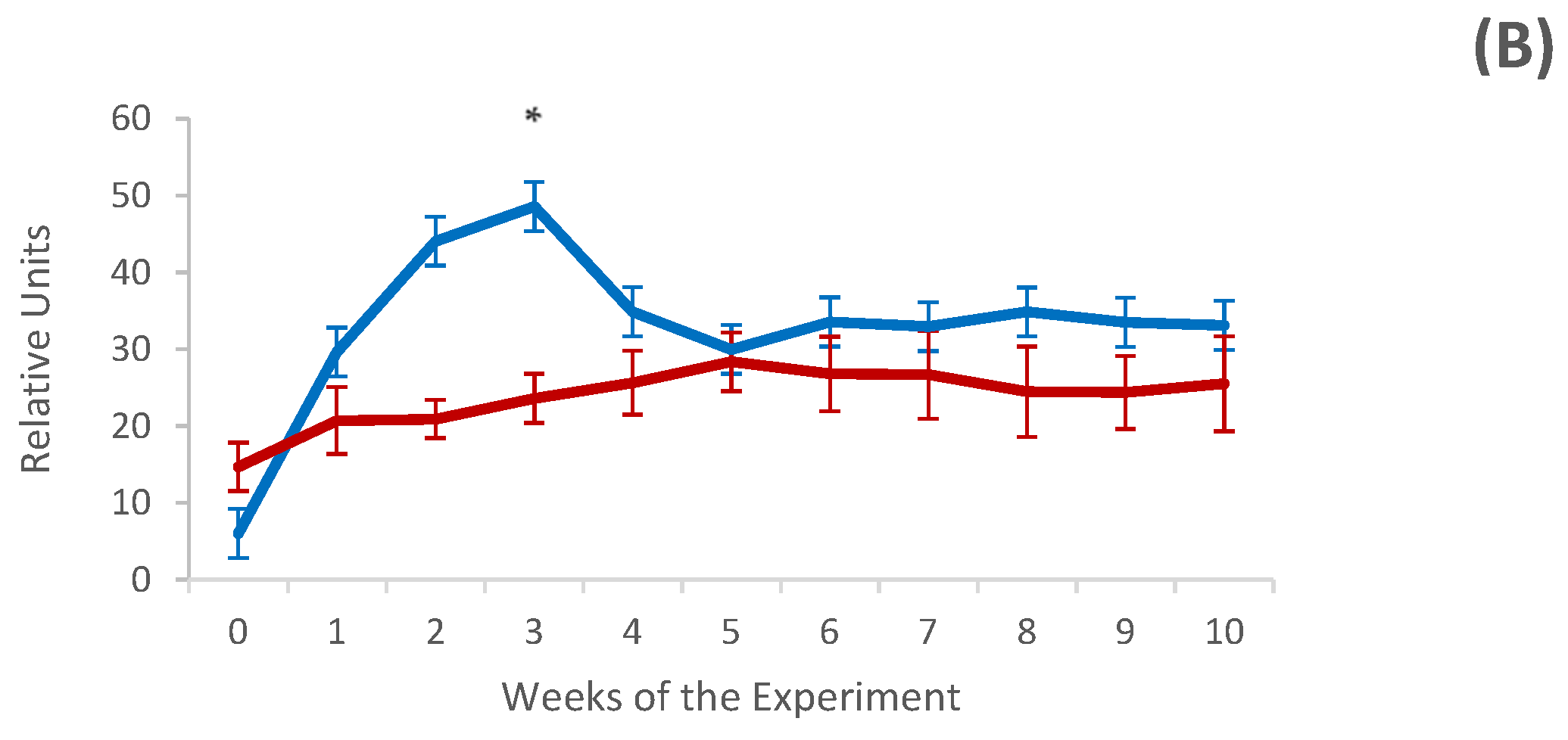
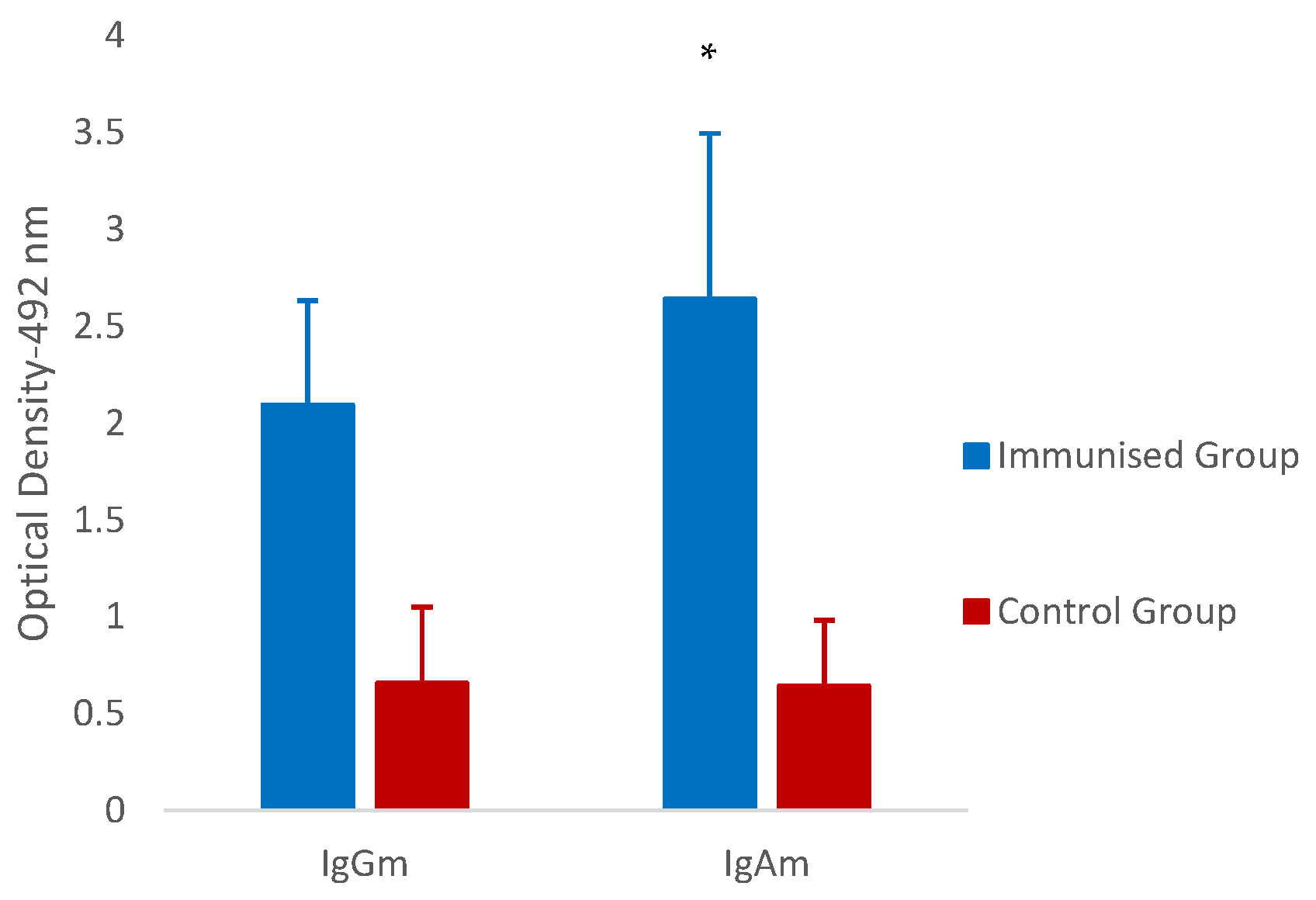
3.3. Systemic and Local Humoral Response
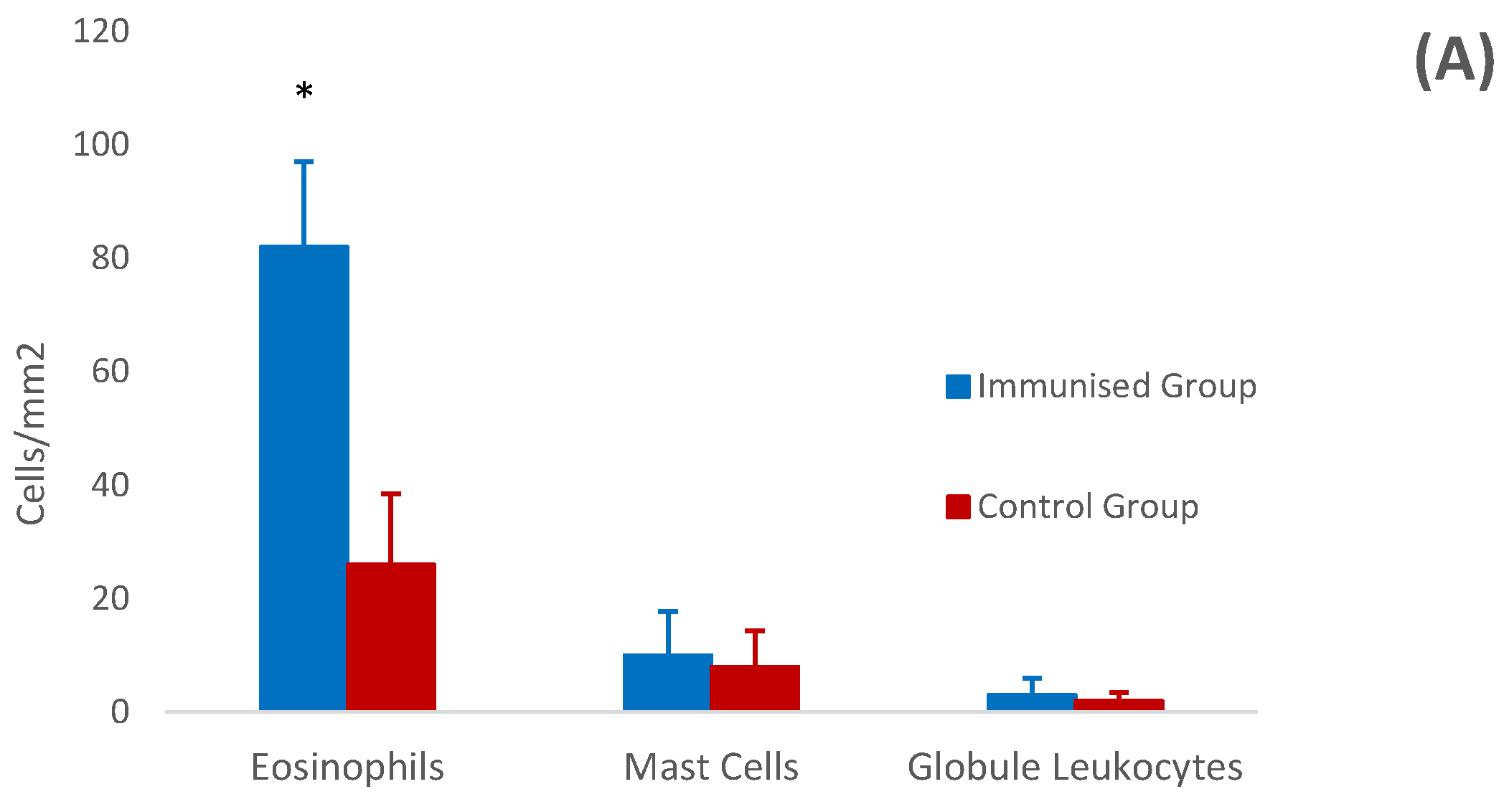
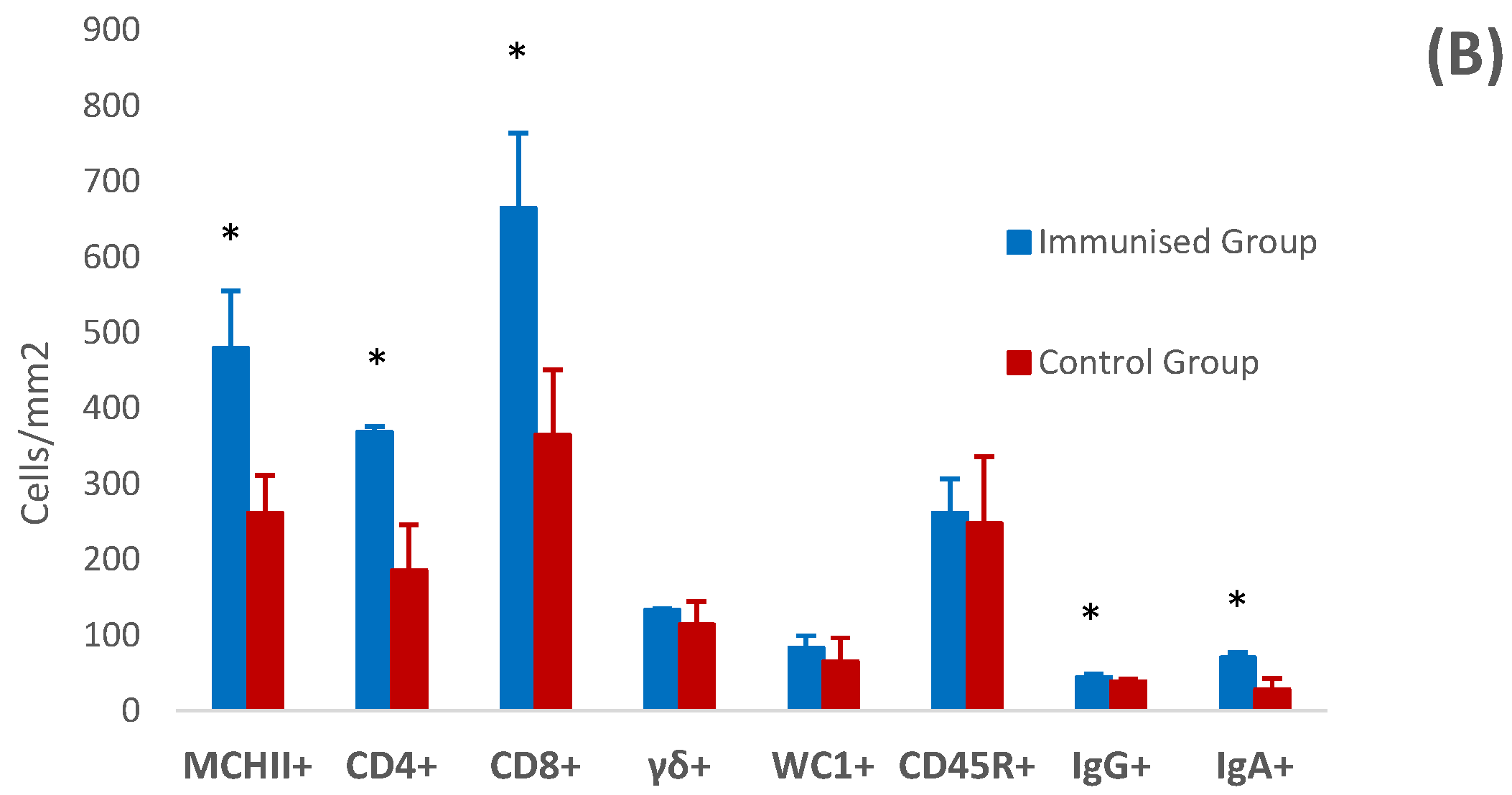
3.4. Histology and Immunohistochemistry of Abomasal Mucosa
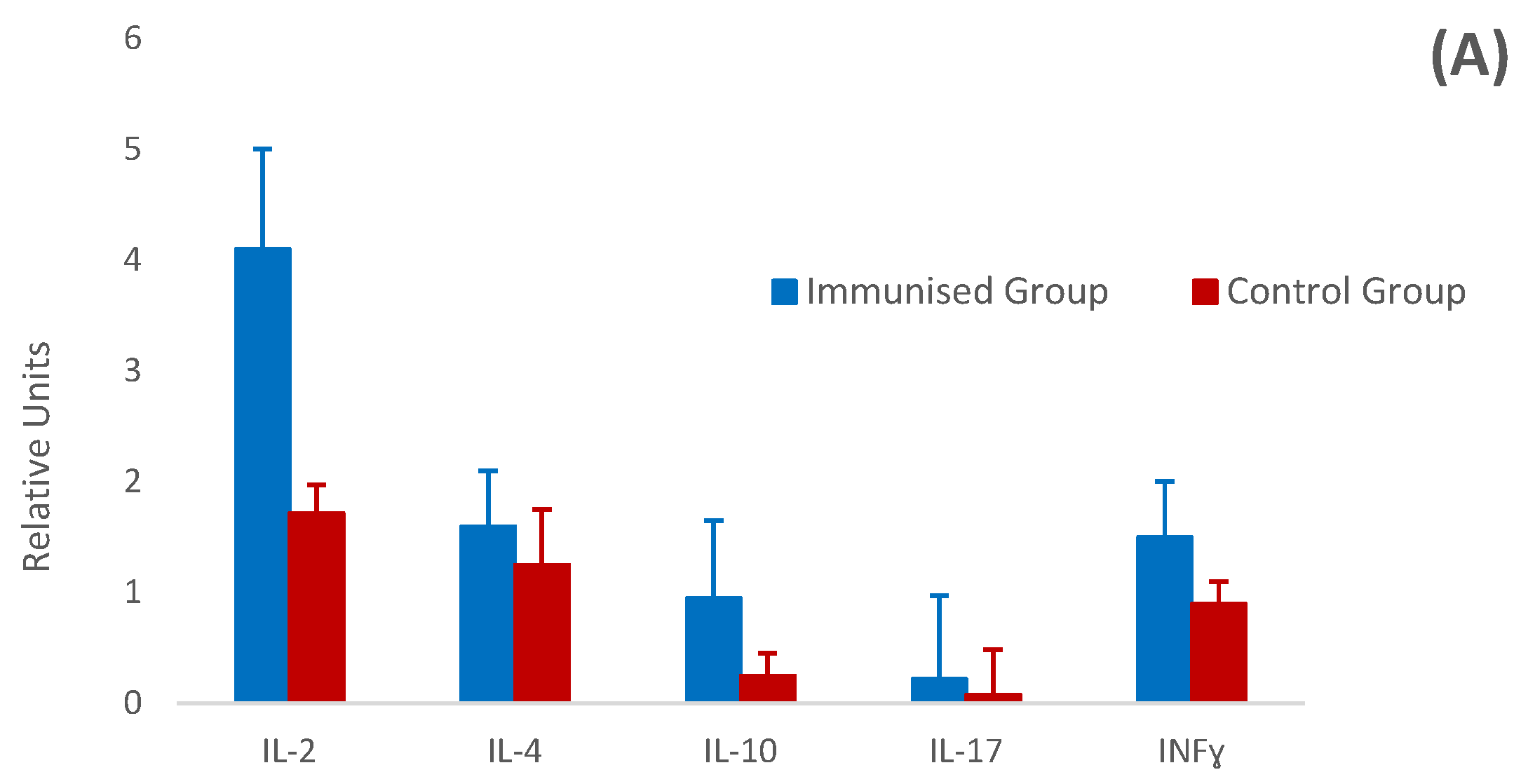
3.5. Relative Cytokine Gene Expression by Real-Time PCR (RT-PCR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chartier, C.; Reche, B. Gastrointestinal helminths and lungworms of French dairy goats: Prevalence and geographical distribution in Poitou-Charentes. Vet. Res. Commun. 1992, 16, 327–355. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Gutiérrez, A.C.; Rodríguez-Ponce, E.; Viera, J.A.; Hernández, S. Abomasal nematodes in goats from the subtropical island of Grand Canary (Spain). Vet. Res. 1997, 28, 259–270. [Google Scholar] [PubMed]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. (Eds.) Parasites of sheep and goats (Parasites of the digestive system). In Veterinary Parasitology, 4th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016; pp. 436–474. [Google Scholar]
- Papadopoulus, E.; Gallidis, E.; Ptochos, S. Anthelmintic resistance in sheep in Europe: A selected review. Vet. Parasitol. 2012, 189, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Paraud, C.; Chartier, C. Facing anthelmintic resistance in goats. In Sustainable Goat Production in Adverse Environments; Simões, J., Gutiérrez, C., Eds.; Springer International Publishing: New York, NY, USA, 2017; Volume I, pp. 267–292. [Google Scholar]
- European Food Safety Authority. Report for 2010 on the Results from the Monitoring of Veterinary Medicinal Product Residues and Other Substances in Live Animals and Animal Products. 2012. Available online: http://www.efsa.europa.eu/fr/supporting/pub/212e (accessed on 12 April 2024).
- Stears, M.J.; Doligalska, M.; Donskow-Schemelter, K. Alternatives to anthelmintics for the control of nematodes in livestock. Parasitology 2007, 134, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Le Jambre, L.F.; Windon, R.G.; Smith, W.D. Vaccination against Haemonchus contortus: Performance of native parasite gut membrane glycoproteins in Merino lambs grazing contaminated pasture. Vet. Parasitol. 2008, 153, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Britton, C.; Emery, D.L.; McNeilly, T.M.; Nisbet, A.J.; Stear, M.J. The potential for vaccines against scour worms of small ruminants. Int. J. Parasitol. 2020, 50, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, A.J.; McNeilly, T.N.; Wildblood, L.A.; Morrison, A.A.; Barteley, D.J.; Bartley, I.; Longhi, C.; McKendrick, I.J.; Palarea-Almendralejo, J.; Matthews, J.B. Successful immunisation against a parasitic nematode by vaccination with recombinant proteins. Vaccine 2013, 31, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Meyvis, Y.; Geldholf, P.; Gevaert, K.; Timmerman, E.; Vercruysse, J.; Claerebout, E. Vaccination against Ostertagia ostertagi with subfractions of the protective ES-thiol fraction. Vet. Parasitol. 2007, 149, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.M.; Smith, W.D. Protective immunization of calves against Ostertagia ostertagi using fourth stage larval extracts. Parasite Immunol. 2010, 32, 656–663. [Google Scholar] [CrossRef]
- Hoste, H.; Sotiraki, S.; Landau, S.Y.; Jackson, F.; Beveridge, I. Goat-nematode interactions: Think differently. Trends Parasitol. 2010, 26, 376–381. [Google Scholar] [CrossRef]
- Onzima, R.B.; Mukiibi, R.; Ampaire, A.; Benda, K.K.; Kanis, E. Between-breed variations in resistance/resilience to gastrointestinal nematodes among indigenous goat breeds in Uganda. Trop. Anim. Health. Prod. 2017, 49, 1763–1769. [Google Scholar] [CrossRef]
- Ruiz, A.; Molina, J.M.; González, J.F.; Conde, M.M.; Martín, S.; Hernández, Y.I. Immunoprotection in goats against Haemonchus contortus after immunization with cysteine protease enriched protein fractions. Vet. Res. 2004, 35, 565–572. [Google Scholar] [CrossRef]
- Molina, J.M.; Martín, S.; Hernández, Y.I.; González, J.F.; Ferrer, O.; Ruiz, A. Immunoprotective effect of cysteine proteinase fractions from two Haemonchus contortus strains adapted to sheep and goats. Vet. Parasitol. 2012, 188, 53–59. [Google Scholar] [CrossRef]
- Martín, S.; Molina, J.M.; Hernández, Y.I.; Ferrer, O.; Muñoz, M.C.; López, A.; Ortega, L.; Ruiz, A. Influence of immunoprotection on genetic variability of cysteine proteinases from Haemonchus contortus adult worms. Int. J. Parasitol. 2015, 45, 831–840. [Google Scholar] [CrossRef]
- Molina, J.M.; Hernández, Y.I.; Martín, S.; Ferrer, O.; Rodríguez, F.; Ruiz, A. Immune response in goats vaccinated with thiol-binding proteins from Haemonchus contortus. Parasite Immunol. 2018, 40, e12569. [Google Scholar] [CrossRef]
- Molina, J.M.; Hernández, Y.I.; Ferrer, O.; Conde, M.M.; Rodríguez, F.; Ruiz, A. Immunization with thiol-binding proteins from Haemonchus contortus adult worms partially protects goats against infection during prepatency. Exp. Parasitol. 2023, 248, 108512. [Google Scholar] [CrossRef]
- Smith, W.D.; Smith, S.K.; Murray, J.M. Protection studies with integral membrane protein fractions of Haemonchus contortus. Parasite Immunol. 1994, 16, 231–241. [Google Scholar] [CrossRef]
- Geldhof, P.; Claerebout, E.; Knox, D.; Vercauteren, I.; Looszova, A.; Vercruysse, J. Vaccination of calves against Ostertagia ostertagi with cysteine proteinase enriched protein fractions. Parasite Immunol. 2002, 24, 263–270. [Google Scholar] [CrossRef]
- Thienpont, D.; Rochette, F.; Van Parijs, O.F.J. Diagnosing helmintiasis through coproscopical examination. Janssen Res. Found. 1979; 187. [Google Scholar]
- MAFF. Manual of Veterinary Parasitological Laboratory Diagnostic Techniques, 3rd ed.; Ministry of Agriculture, Fisheries and Food: London, UK, 1989.
- Dorny, P.; Vercruysse, J. Evaluation of a micromethod for the routine determination of serum pepsinogen in cattle. Res. Vet. Sci. 1998, 65, 259–262. [Google Scholar] [CrossRef]
- Ortega, L.; Quesada, J.; Ruiz, A.; Conde, M.M.; Ferrer, O.; Rodríguez, F.; Molina, J.M. Local immune response of Canarian Majorera goats infected with Teladorsagia circumcincta. Parasites Vectors 2022, 15, 25. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, J.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbour Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Craig, N.M.; Miller, H.R.P.; Smith, W.D.; Knight, P.A. Cytokine expression in naïve and previously infected lambs after challenge with Teladorsagia circumcincta. Vet. Inmunol. Immunopathol. 2007, 120, 47–54. [Google Scholar] [CrossRef]
- Yan, X.; Huang, Y.; Wang, H.; Du, M.; Hess, B.W.; Ford, S.P.; Nathanielsz, P.W.; Zhu, M.J. Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm. Bowel Dis. 2011, 17, 1513–1522. [Google Scholar] [CrossRef]
- Knox, D.P.; Smith, S.K.; Smith, W.D. Immunization with an affinity purified protein extract from the adult parasite protects lambs against infection with Haemonchus contortus. Parasitol. Immunol. 1999, 21, 201–210. [Google Scholar] [CrossRef]
- Martín, S.; Molina, J.M.; Hernández, Y.; Pérez, D.; Matos, L.; López, A.M.; Ortega, L.; Quesada, J.; Ruiz, A. Inmunoprotección y variabilidad genética de proteinasas tipo cisteína de Haemonchus contortus en ovinos inmunizados a diferentes edades. Parasitol. latinoam. 2011, 70, 178–188. [Google Scholar]
- García Casas, C.; Moreno, A.; Caporte, J.; de la Haba, M.R. Characterization of the Canary racial goat groups with eritrocyte genetic markers. Small Rum. Res. 1992, 7, 361–368. [Google Scholar]
- Knox, D.P.; Smith, W.D. Vaccination against gastrointestinal nematode parasites of ruminants using gut-expressed antigens. Vet. Parasitol. 2001, 100, 21–32. [Google Scholar] [CrossRef]
- Beraldi, D.; Craig, B.H.; Bishop, S.C.; Hopkins, J.; Pemberton, J.M. Phenotypic analysis of host parasite interactions in lambs infected with Teladorsagia circumcincta. Int. J. Parasitol. 2008, 38, 1567–1577. [Google Scholar] [CrossRef]
- Bakker, N.; Vervelde, L.; Kanobana, K.; Knox, D.P.; Cornelissen, A.W.C.A.; de Vries, E.; Yatsuda, A.P. Vaccination against the nematode Haemonchus contortus with a thiol-binding fraction from the excretory/secretory products (ES). Vaccine 2004, 22, 618–628. [Google Scholar] [CrossRef]
- Geldhof, P.; Vercauteren, I.; Vercruysse, J.; Knox, D.P.; Van Den Broeck, W.; Claerebout, E. Validation of the protective Ostertagia ostertagi ES-thiol antigens with different adjuvantia. Parasite Immunol. 2004, 26, 37–43. [Google Scholar] [CrossRef]
- Halliday, A.M.; Smith, W.D. Attempts to immunize sheep against Teladorsagia circumcincta using fourth-stage larval extracts. Parasite Immunol. 2011, 33, 554–560. [Google Scholar] [CrossRef]
- González, J.F.; Hernández, J.N.; Machín, C.; Pérez-Hernández, T.; Wright, H.W.; Corripio-Miyar, Y.; Price, D.R.G.; Matthews, J.B.; McNeilly, T.N.; Nisbet, A.J. Impacts of breed type and vaccination on Teladorsagia circumcincta infection in native sheep in Gran Canaria. Vet. Res. 2019, 50, 29. [Google Scholar] [CrossRef]
- Stear, M.J.; Bishop, S.C.; Duncan, J.L.; McKellar, Q.A.; Murray, M. The repeatability of faecal egg counts, peripheral eosinophil counts, and plasma pepsinogen concentrations during deliberate infections with Ostertagia circumcincta. Int. J. Parasitol. 1995, 25, 375–380. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Brown, A.E.; Green, R.S.; Miller, C.M.; Leathwick, D.M. The immune response of sheep to larval challenge with Ostertagia circumcincta and O. ostertagi. Vet. Parasitol. 1999, 84, 125–135. [Google Scholar] [CrossRef]
- Pérez-Hernández, T.; Corripio-Miyar, Y.; Hernández, J.N.; Machín, C.; Paz-Sánchez, Y.; Hayward, A.D.; Wright, H.W.; Price, D.R.G.; Matthews, J.B.; McNeilly, T.N.; et al. Differences in the protection elicited by a recombinant Teladorsagia circumcincta vaccine in weaned lambs of two Canarian sheep breeds. Vet. Parasitol. 2022, 306, 109722. [Google Scholar]
- Castilla Gómez de Agüero, V.; Valderas-García, E.; González Del Palacio, L.; Giráldez, F.J.; Balaña-Fouce, R.; Martínez-Valladares, M. Secretory IgA as biomarker for gastrointestinal nematodes natural infection in different breed sheep. Animals 2023, 13, 2189. [Google Scholar] [CrossRef]
- Balic, A.; Bowles, V.M.; Meeusen, E.N.T. The immunobiology of gastrointestinal nematode infections in rumiants. Adv. Parasitol. 2000, 45, 181–241. [Google Scholar]
- Shakya, K.P.; Miller, J.E.; Horohov, D.W. A Th2 type of immune response is associated with increased resistance to Haemonchus contortus in naturally infected Gulf Coast Native lambs. Vet. Parasitol. 2009, 163, 57–66. [Google Scholar] [CrossRef]
- Hendawy, S.H.M. Immunity to gastrointestinal nematodes in ruminants: Effector cell mechanisms and cytokines. J. Parasit. Dis. 2018, 42, 471–482. [Google Scholar] [CrossRef]
- McRae, K.M.; Stear, M.J.; Good, B.; Keane, O.M. The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 2015, 37, 605–613. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. Th2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Gill, H.S.; Altmann, K.; Cross, M.L.; Husband, A.J. Induction of T helper 1- and T helper 2-type immune responses during Haemonchus contortus infection in sheep. Immunology 2000, 99, 458–463. [Google Scholar] [CrossRef]
- Claerebout, E.; Vercauteren, I.; Geldhof, P.; Zarlenga, D.S.; Goddeeris, B.M.; Vercruysse, J. Cytokine responses in immunised and non-immunised calves after Ostertagia ostertagi infection. Parasite Immunol. 2005, 27, 325–331. [Google Scholar] [CrossRef]
- Liu, W.; McNeilly, T.N.; Mitchell, M.; Burgess, S.T.G.; Nisbet, A.J.; Matthews, J.B.; Babayan, S.A. Vaccine-induced time- and age-dependent mucosal immunity to gastrointestinal parasite infection. NPJ Vaccines 2022, 7, 78. [Google Scholar] [CrossRef]
- Venturina, V.M.; Gossner, A.G.; Hopkins, J. The immunology and genetics of resistance of sheep to Teladorsagia circumcincta. Vet. Res. Commun. 2013, 37, 171–181. [Google Scholar] [CrossRef]
- Andronicos, N.; Hunt, P.; Windon, R. Expression of genes in gastrointestinal and lymphatic tissues during parasite infection in sheep genetically resistant or susceptible to Trichostrongylus colubriformis and Haemonchus contortus. Int. J. Parasitol. 2010, 40, 417–429. [Google Scholar] [CrossRef]
- González-Hernández, A.; Van Coppernolle, S.; Borloo, J.; Van Meulder, F.; Paerewijck, O.; Peelaers, I.; Leclercq, G.; Claerebout, E.; Geldhof, P. Host protective ASP-based vaccine against the parasitic nematode Ostertagia ostertagi triggers NK cell activation and mixed IgG1-IgG2 response. Sci. Rep. 2016, 6, 29496. [Google Scholar] [CrossRef]
- Balic, A.; Bowles, V.M.; Meeusen, E.N. Mechanisms of immunity to Haemonchus contortus infection in sheep. Parasite. Immunol. 2002, 24, 39–46. [Google Scholar] [CrossRef]
- Halliday, A.M.; Morrison, W.I.; Smith, W.D. Kinetics of the local cellular response in the gastric lymph of immune and susceptible sheep to infection with Teladorsagia circumcincta. Parasite Immunol. 2009, 31, 402–411. [Google Scholar] [CrossRef]
- Halliday, A.M.; McAllister, H.C.; Smith, W.D. Kinetics of the local immune response in the gastric lymph of lambs after primary and challenge infection with Teladorsagia circumcincta. Parasite Immunol. 2010, 32, 81–90. [Google Scholar] [CrossRef]
- Aboshady, H.M.; Stear, M.J.; Johansson, A.; Jonas, E.; Bambou, J.C. Immunoglobulins as biomarkers for gastrointestinal nematodes resistance in small ruminants: A systematic review. Sci. Rep. 2020, 10, 7765. [Google Scholar] [CrossRef]
- Stears, M.; Preston, S.; Piedrafita, D.; Donskow-Łysoniewska, K. The immune response to nematode infection. Int. J. Mol. Sci. 2023, 24, 2283. [Google Scholar] [CrossRef]
- González, J.F.; Hernández, A.; Meeusen, E.N.T.; Rodríguez, F.; Molina, J.M.; Jaber, R.; Raadsma, H.W.; Piedrafita, D. Fecundity in adult Haemonchus contortus parasites is correlated with abomasal tissue eosinophils and γδ-T cells in resistant Canarian Hair Breed sheep. Vet. Parasitol. 2011, 78, 286–292. [Google Scholar] [CrossRef]
- Meeusen, E.N.T.; Balic, A.; Bowles, V. Cells, cytokines and other molecules associated with rejection of gastrointestinal nematode parasites. Vet. Immunol Immunopathol. 2005, 108, 121–125. [Google Scholar] [CrossRef]
- Pearson, M.; Ranit, N.; Loukas, A. Blunting the knife: Development of vaccines targeting digestive proteases of blood-feeding helminth parasites. Biol. Chem. 2010, 8, 901–911. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, L.; Quesada, J.; Ruiz, A.; Conde-Felipe, M.M.; Ferrer, O.; Muñoz, M.d.C.; Molina, J.A.; Rodríguez, F.; Molina, J.M. Analysis of Protection and Immune Response against Teladorsagia circumcincta in Goats Immunised with Thiol-Binding Proteins from Adult Worms. Vaccines 2024, 12, 437. https://doi.org/10.3390/vaccines12040437
Ortega L, Quesada J, Ruiz A, Conde-Felipe MM, Ferrer O, Muñoz MdC, Molina JA, Rodríguez F, Molina JM. Analysis of Protection and Immune Response against Teladorsagia circumcincta in Goats Immunised with Thiol-Binding Proteins from Adult Worms. Vaccines. 2024; 12(4):437. https://doi.org/10.3390/vaccines12040437
Chicago/Turabian StyleOrtega, Leire, Jessica Quesada, Antonio Ruiz, Magnolia María Conde-Felipe, Otilia Ferrer, María del Carmen Muñoz, José Adrián Molina, Francisco Rodríguez, and José Manuel Molina. 2024. "Analysis of Protection and Immune Response against Teladorsagia circumcincta in Goats Immunised with Thiol-Binding Proteins from Adult Worms" Vaccines 12, no. 4: 437. https://doi.org/10.3390/vaccines12040437
APA StyleOrtega, L., Quesada, J., Ruiz, A., Conde-Felipe, M. M., Ferrer, O., Muñoz, M. d. C., Molina, J. A., Rodríguez, F., & Molina, J. M. (2024). Analysis of Protection and Immune Response against Teladorsagia circumcincta in Goats Immunised with Thiol-Binding Proteins from Adult Worms. Vaccines, 12(4), 437. https://doi.org/10.3390/vaccines12040437