COVID-19 Vaccination in Pregnancy: Pilot Study of Plasma MicroRNAs Associated with Inflammatory Cytokines after COVID-19 mRNA Vaccination
Abstract
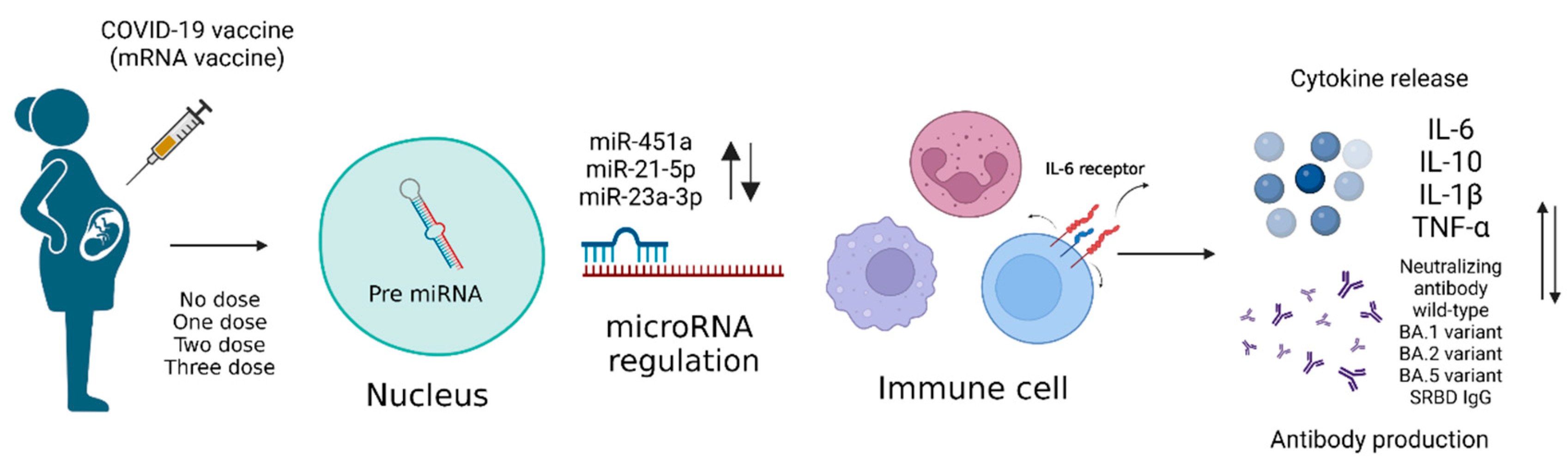
:1. Introduction
2. Materials and Methods
2.1. Participants and Sample Collection
2.2. microRNA qPCR Analysis
2.3. ELISA
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
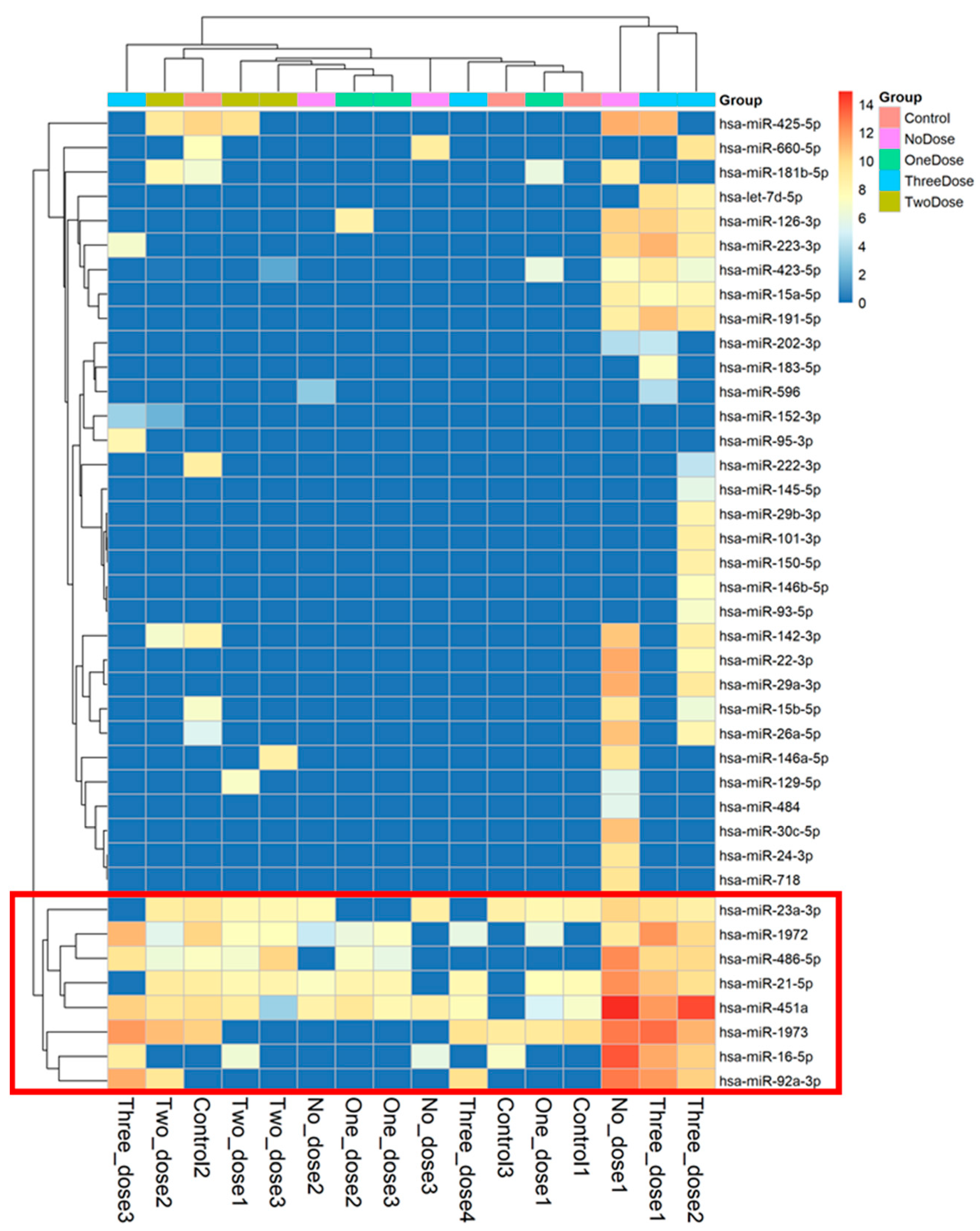
3.2. The Different Expression Levels of microRNA
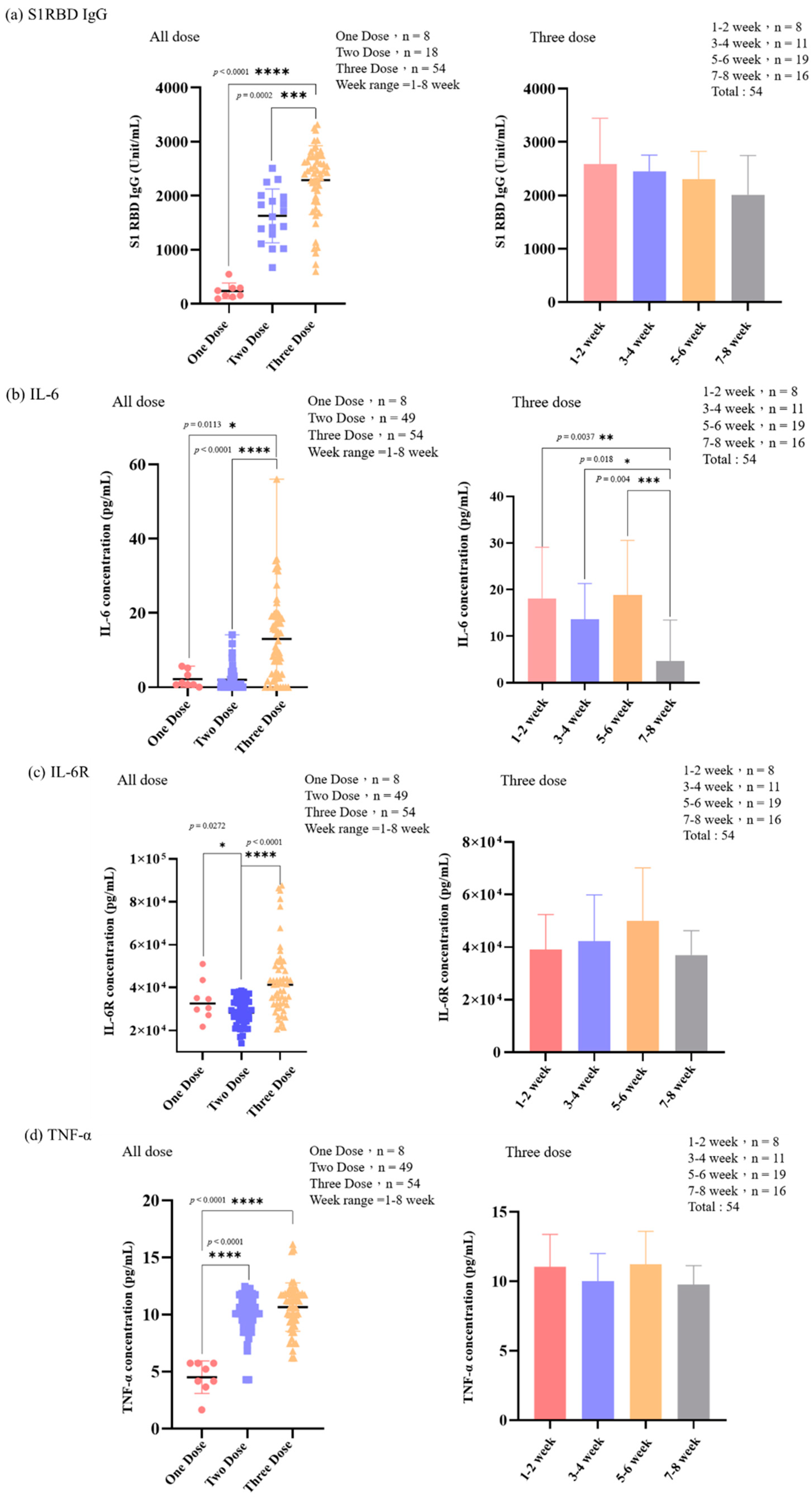
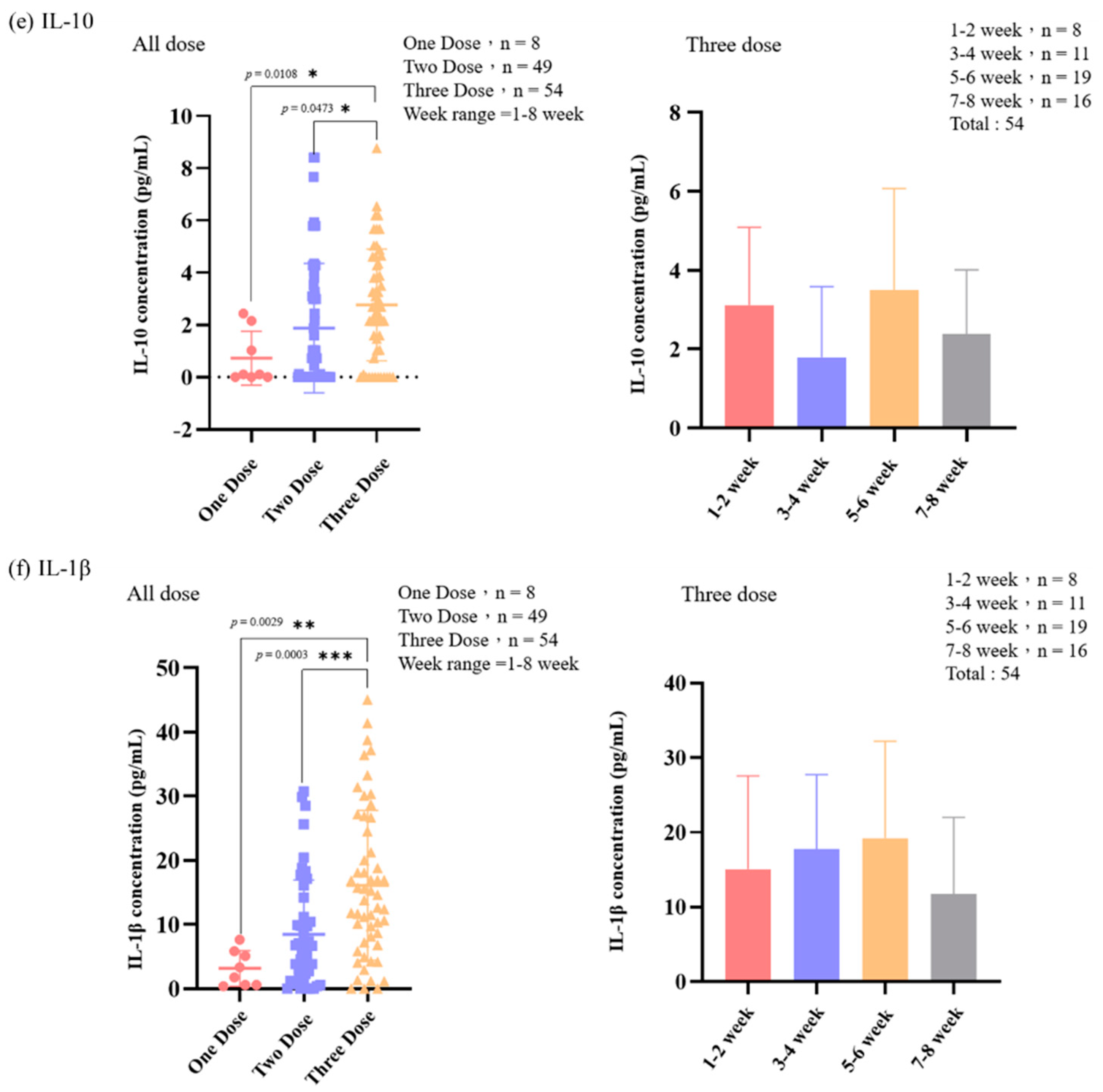
3.3. The Level of IL-6, IL-6R, TNF-α, IL-10, IL-1β, and S1RBD IgG in Samples from Patients Receiving Different Doses of COVID-19 Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Omer, S.B.; Malani, P.N. Winter of Omicron—The evolving COVID-19 pandemic. JAMA 2022, 327, 319–320. [Google Scholar] [CrossRef]
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Corey, L.; Fernandes, P.; Gilbert, P.B.; Hotez, P.J.; Rao, S.; Santos, M.R.; Schuitemaker, H.; Watson, M.; Arvin, A. Prospects for a safe COVID-19 vaccine. Sci. Transl. Med. 2020, 12, eabe0948. [Google Scholar] [CrossRef] [PubMed]
- Frenck Jr, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; McPhee, R.; Huang, W.; Bennett, H.; Pajon, R.; Nestorova, B.; Leav, B.; mRNA-1273 Study Group. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021, 39, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; Biswell, R. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021, 384, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Male, V. Are COVID-19 vaccines safe in pregnancy? Nat. Rev. Immunol. 2021, 21, 200–201. [Google Scholar] [CrossRef]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’Brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2022, 226, 236.e1–236.e14. [Google Scholar] [CrossRef] [PubMed]
- Cosma, S.; Carosso, A.R.; Cusato, J.; Borella, F.; Carosso, M.; Bovetti, M.; Filippini, C.; D’Avolio, A.; Ghisetti, V.; Di Perri, G. Coronavirus disease 2019 and first-trimester spontaneous abortion: A case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2021, 224, 391.e1–391.e7. [Google Scholar] [CrossRef] [PubMed]
- Phoswa, W.N.; Khaliq, O.P. Is pregnancy a risk factor of COVID-19? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, M.; Zhu, Z.; Liu, Y. Coronavirus disease 2019 (COVID-19) and pregnancy: A systematic review. J. Matern.-Fetal Neonatal Med. 2022, 35, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Creisher, P.S.; Burd, I. COVID-19 vaccine testing in pregnant females is necessary. J. Clin. Investig. 2021, 131, e147553. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Örtqvist, A.K.; Dahlqwist, E.; Ljung, R.; Skår, F.; Oakley, L.; Macsali, F.; Pasternak, B.; Gjessing, H.K.; Håberg, S.E. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA 2022, 327, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.B.; Dhinsa, T.; Alton, G.D.; Török, E.; Dimanlig-Cruz, S.; Regan, A.K.; Sprague, A.E.; Buchan, S.A.; Kwong, J.C.; Wilson, S.E. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA 2022, 327, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Erson-Bensan, A.E. Introduction to microRNAs in biological systems. In miRNomics: MicroRNA Biology and Computational Analysis; Humana Press: Totowa, NJ, USA, 2014; pp. 1–14. [Google Scholar]
- Lai, E.C. Micro RNAs are complementary to 3’ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002, 30, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gao, Y.; Li, Z.; Miao, Y.; Huang, Z.; Liu, X.; Xie, L.; Li, H.; Wen, W.; Zheng, Y.; et al. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020, 10, e200. [Google Scholar] [CrossRef]
- Farr, R.J.; Rootes, C.L.; Rowntree, L.C.; Nguyen, T.H.; Hensen, L.; Kedzierski, L.; Cheng, A.C.; Kedzierska, K.; Au, G.G.; Marsh, G.A. Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLoS Pathog. 2021, 17, e1009759. [Google Scholar] [CrossRef]
- Miyashita, Y.; Yoshida, T.; Takagi, Y.; Tsukamoto, H.; Takashima, K.; Kouwaki, T.; Makino, K.; Fukushima, S.; Nakamura, K.; Oshiumi, H. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. NPJ Vaccines 2022, 7, 16. [Google Scholar] [CrossRef]
- Liu, D.; Liu, C.; Wang, X.; Ingvarsson, S.; Chen, H. MicroRNA-451 suppresses tumor cell growth by down-regulating IL6R gene expression. Cancer Epidemiol. 2014, 38, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, Y.; Li, J.; Liu, C.; Zhu, L.; Zhang, J.; Yu, Y.; Wang, W.-J.; Lei, G.; Yan, J. Downregulated miR-451a as a feature of the plasma cfRNA landscape reveals regulatory networks of IL-6/IL-6R-associated cytokine storms in COVID-19 patients. Cell. Mol. Immunol. 2021, 18, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pham, A.; Kang, L.; Walker, S.A.; Davidovich, I.; Iannotta, D.; TerKonda, S.P.; Shapiro, S.; Talmon, Y.; Pham, S.; et al. Effects of Adipose-Derived Biogenic Nanoparticle-Associated microRNA-451a on Toll-like Receptor 4-Induced Cytokines. Pharmaceutics 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Terao, M.; Fratelli, M.; Kurosaki, M.; Zanetti, A.; Guarnaccia, V.; Paroni, G.; Tsykin, A.; Lupi, M.; Gianni, M.; Goodall, G.J. Induction of miR-21 by retinoic acid in estrogen receptor-positive breast carcinoma cells: Biological correlates and molecular targets. J. Biol. Chem. 2011, 286, 4027–4042. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-L.; Chang, A.-C.; Huang, C.-C.; Tsai, C.-H.; Lin, C.-C.; Tang, C.-H. Myostatin promotes interleukin-1β expression in rheumatoid arthritis synovial fibroblasts through inhibition of miR-21-5p. Front. Immunol. 2017, 8, 1747. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-D.; Lin, F.-M.; Wu, W.-Y.; Liang, C.; Huang, W.-C.; Chan, W.-L.; Tsai, W.-T.; Chen, G.-Z.; Lee, C.-J.; Chiu, C.-M. miRTarBase: A database curates experimentally validated microRNA–target interactions. Nucleic Acids Res. 2011, 39, D163–D169. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Consortium, G.O. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, A.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database 2015, 2015, bav028. [Google Scholar] [CrossRef] [PubMed]
- Schriml, L.M.; Arze, C.; Nadendla, S.; Chang, Y.-W.W.; Mazaitis, M.; Felix, V.; Feng, G.; Kibbe, W.A. Disease Ontology: A backbone for disease semantic integration. Nucleic Acids Res. 2012, 40, D940–D946. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; He, Q.-Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. BioSystems 2016, 12, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [PubMed]
- Ljungman, P. Vaccination of immunocompromised patients. Clin. Microbiol. Infect. 2012, 18, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.A.; Engel, S.M.; Sperling, R.S.; Kellerman, L.; Lo, Y.; Wallenstein, S.; Escribese, M.M.; Garrido, J.L.; Singh, T.; Loubeau, M.; et al. Characterizing the Pregnancy Immune Phenotype: Results of the Viral Immunity and Pregnancy (VIP) Study. J. Clin. Immunol. 2012, 32, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Sperling, R.S.; Moran, T.M.; Kraus, T.A. The influence of pregnancy on systemic immunity. Immunol. Res. 2012, 54, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the Complexity of the Immune System during Pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Christian, L.M.; Porter, K.; Karlsson, E.; Schultz-Cherry, S.; Iams, J.D. Serum Proinflammatory Cytokine Responses to Influenza Virus Vaccine among Women during Pregnancy versus Non-Pregnancy. Am. J. Reprod. Immunol. 2013, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, V.; Demos, R.; Snyder-Cappione, J.; Parker, S.E.; Shaik-Dasthagirisaheb, Y.; Hunnewell, J.; Boateng, J.; Clarke, K.; Yuen, R.; Barnett, E.; et al. Cytokine levels in maternal and infant blood after COVID-19 vaccination during pregnancy in comparison with unvaccinated controls. J. Reprod. Immunol. 2023, 156, 103821. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.; Wang, F.; Wang, J.; Liu, Q.; Saaoud, F.; Wang, Y.; Singh, U.P.; Chen, H.; Luo, M.; Ai, W.; et al. Overexpression of microRNA-155 enhances the efficacy of dendritic cell vaccine against breast cancer. Oncoimmunology 2020, 9, 1724761. [Google Scholar] [CrossRef] [PubMed]
- de Candia, P.; Torri, A.; Pagani, M.; Abrignani, S. Serum microRNAs as Biomarkers of Human Lymphocyte Activation in Health and Disease. Front. Immunol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, D.M.; Cancro, M.P.; Scherle, P.A.; Nordan, R.P.; Van Snick, J.; Gerhard, W.; Rudikoff, S. T cell derived IL-6 is differentially required for antigen-specific antibody secretion by primary and secondary B cells. J. Immunol. 1989, 143, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Lue, C.; Kiyono, H.; McGhee, J.R.; Fujihashi, K.; Kishimoto, T.; Hirano, T.; Mestecky, J. Recombinant human interleukin 6 (rhIL-6) promotes the terminal differentiation of in vivo-activated human B cells into antibody-secreting cells. Cell Immunol. 1991, 132, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Mara, A.B.; Gavitt, T.D.; Tulman, E.R.; Miller, J.M.; He, W.; Reinhardt, E.M.; Ozyck, R.G.; Goodridge, M.L.; Silbart, L.K.; Szczepanek, S.M.; et al. Vaccination with Mycoplasma pneumoniae membrane lipoproteins induces IL-17A driven neutrophilia that mediates Vaccine-Enhanced Disease. NPJ Vaccines. 2022, 7, 86. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Idiz, U.O.; Yurttas, T.T.; Degirmencioglu, S.; Orhan, B.; Erdogan, E.; Sevik, H.; Sevinc, M.M. Immunophenotyping of lymphocytes and monocytes and the status of cytokines in the clinical course of COVID-19 patients. J. Med. Virol. 2022, 94, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]


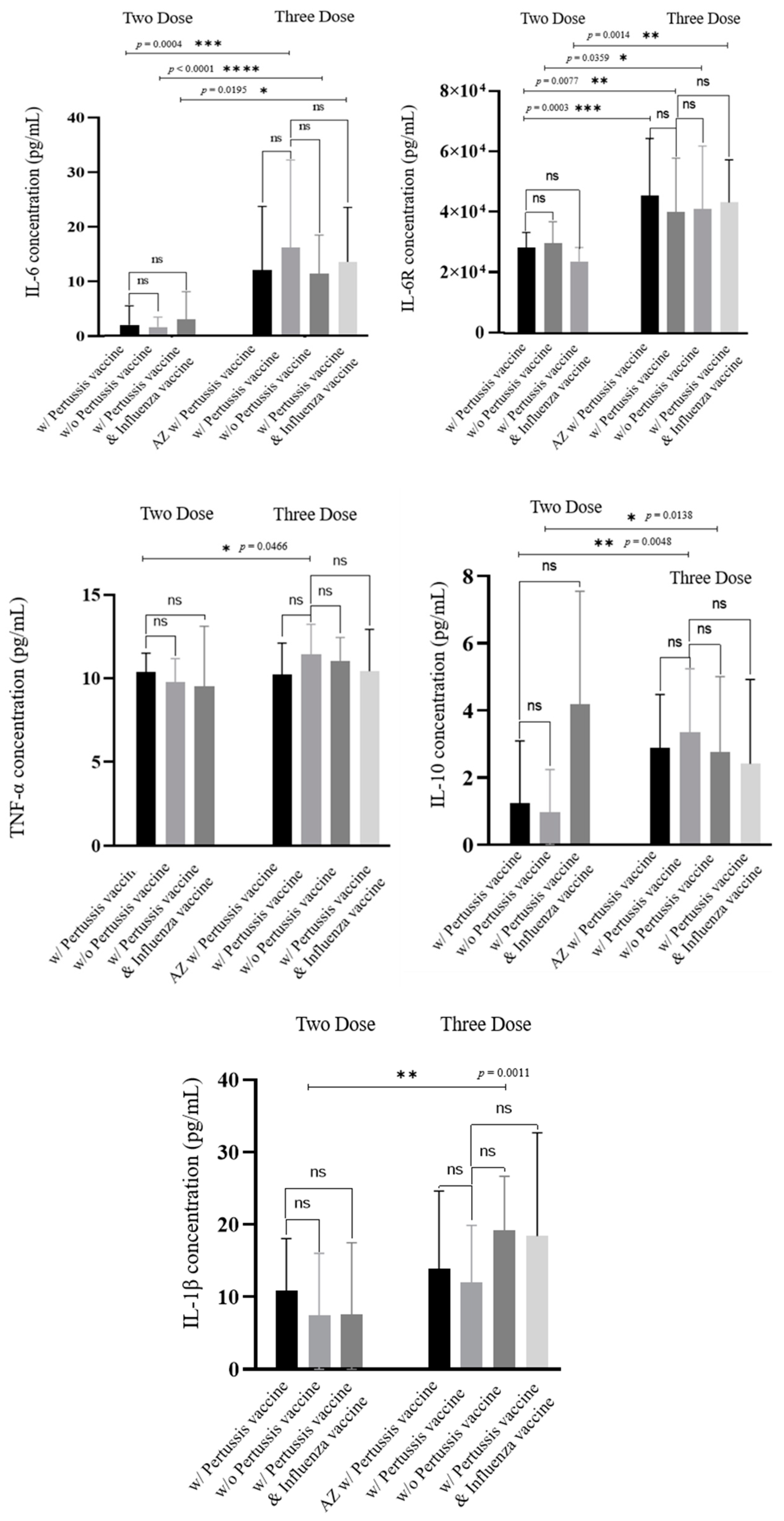
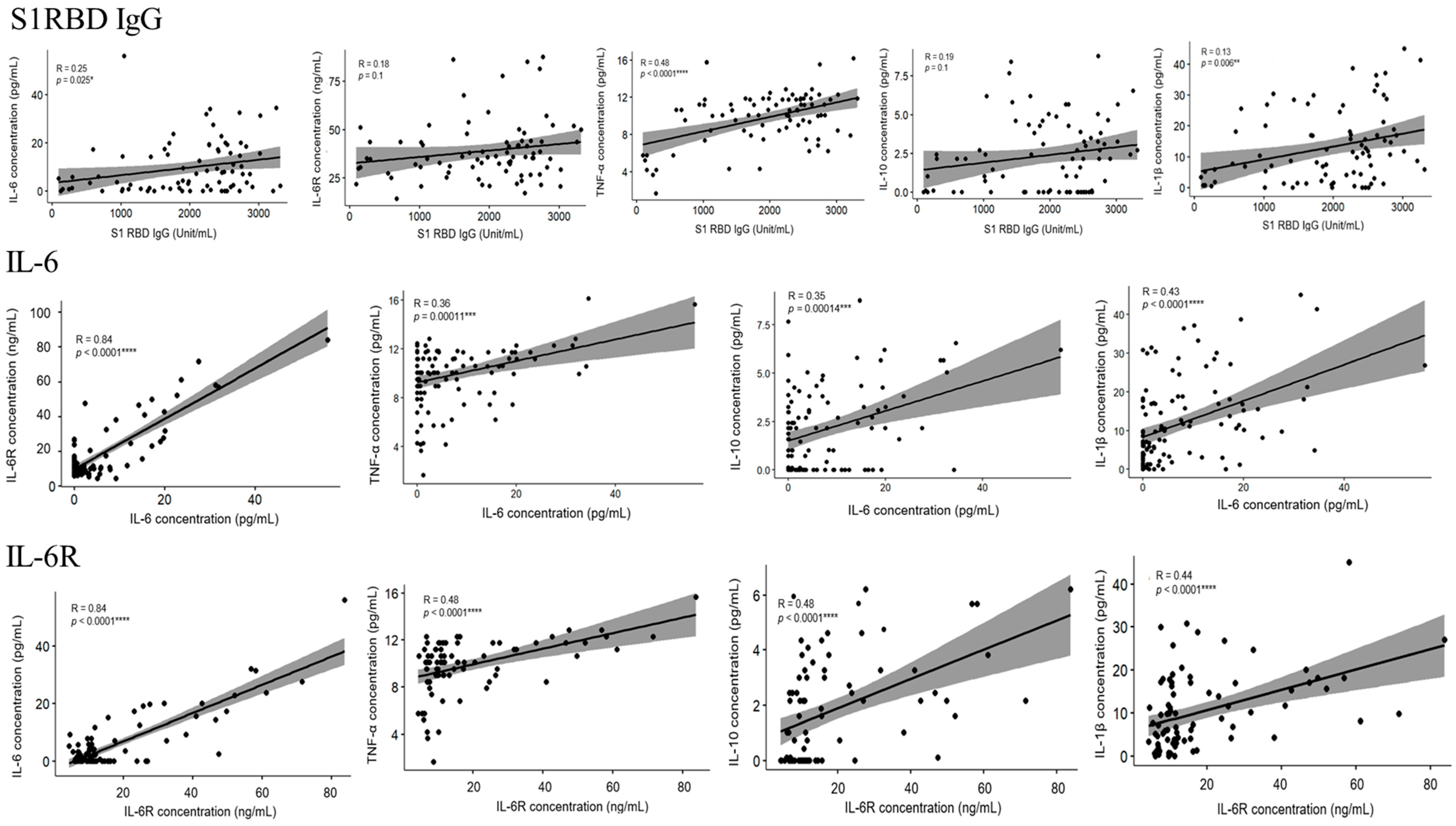
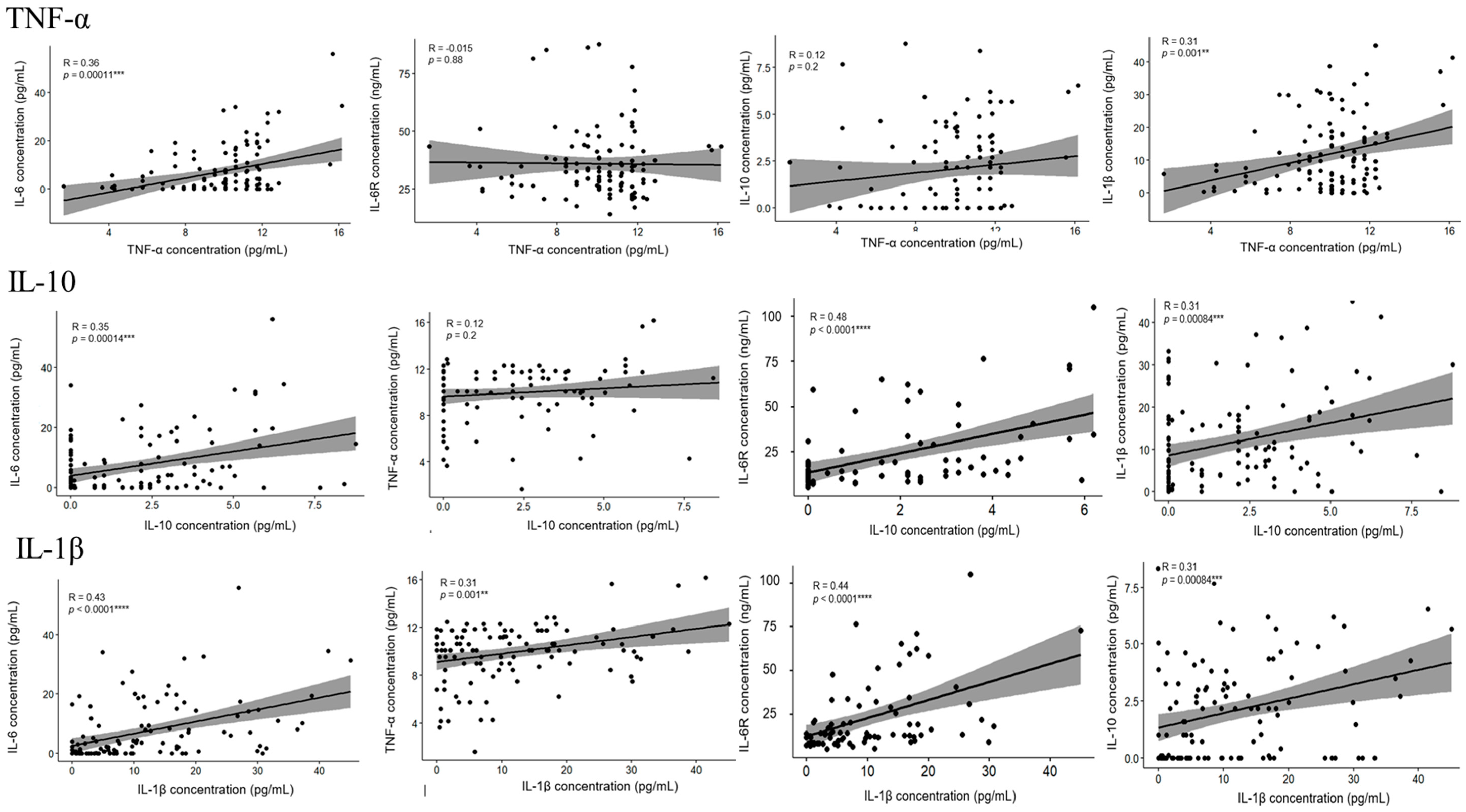
| Clinical Data | ||||||
|---|---|---|---|---|---|---|
| Sample ID | Parity | Age (Year) | BMI | Weeks of Gestation at Delivery (Weeks) | The Interval between the Last Dose of the COVID-19 Vaccine and the Day of Delivery (Weeks) | Kind of COVID-19 Vaccine for the First/Second/Third Dose |
| Control 1 | - | 23 | 19.8 | - | - | BNT/BNT/Moderna |
| Control 2 | - | 23 | 18.62 | - | - | BNT/BNT/Moderna |
| Control 3 | - | 23 | 21 | - | - | BNT/BNT/Moderna |
| No dose 1 | 3 | 40 | 28.38 | 39 | - | - |
| No dose 2 | 1 | 40 | 26.95 | 37 | - | - |
| No dose 3 | 4 | 38 | 30.12 | 36 | - | - |
| One dose 1 | 1 | 36 | 25 | 36 | 15 | Moderna/-/- |
| One dose 2 | 2 | 25 | 26.8 | 37 | 6 | Moderna/-/- |
| One dose 3 | 0 | 37 | 25 | 39 | 6 | Moderna/-/- |
| Two dose 1 | 3 | 40 | 25.7 | 39 | 8 | Moderna/Moderna/- |
| Two dose 2 | 0 | 31 | 25.71 | 38 | 5 | Moderna/Moderna/- |
| Two dose 3 | 1 | 33 | 28 | 40 | 4 | Moderna/Moderna/- |
| Three dose 1 | 2 | 35 | 21.8 | 40 | 6 | Moderna/Moderna/Moderna |
| Three dose 2 | 1 | 24 | 27.6 | 38 | 5 | Moderna/Moderna/Moderna |
| Three dose 3 | 1 | 32 | 23.82 | 40 | 5 | Moderna/Moderna/Moderna |
| Three dose 4 | 2 | 35 | 23.52 | 39 | 6 | Moderna/Moderna/Moderna |
| Variable | Included in the Analysis | ||
|---|---|---|---|
| One Dose a | Two Dose b | Three Dose c | |
| Age of mothers (years) | 34.25 * ± 6.58 ** IQR 38–28.5 | 32.49 ± 4.66 IQR 35–28 | 33.57 ± 5.36 IQR 37.75–30 |
| Parity | 1.375 * ± 0.74 ** IQR 1.25–0 | 1.31 ± 0.55 IQR 2–1 | 1.57 ± 0.63 IQR 2–1 |
| BMI (body mass index) | 26.93 * ± 4.13 ** IQR 28.19–23.68 | 27 ± 4.41 IQR 30.3–23.82 | 27.29 ± 3.99 IQR 29.61–24.3 |
| Weeks of gestation at the first dose of COVID-19 vaccination (weeks) | 33.5 ± 2.27 IQR 35–32.5 | 23.06 ± 10.26 IQR 28–24 | 0.74 ± 8.93 IQR 4.75–(−5.57) |
| Weeks of gestation at the second dose of COVID-19 vaccination (weeks) | - | 32.82 ± 1.91 IQR 34–31 | 14.48 ± 7.29 IQR 20–9.5 |
| Weeks of gestation at the third dose of COVID-19 vaccination (weeks) | - | - | 33.33 ± 2.49 IQR 35–31 |
| The interval between the first dose of COVID-19 vaccination and the collection of blood samples (the day of delivery) (weeks) | 4.75 ± 1.91 IQR 6–3.75 | 15.27 ± 10.08 IQR 15–10 | 37.43 ± 9.05 IQR 42–34 |
| The interval between the second dose of COVID-19 vaccination and the collection of blood samples (the day of delivery) (weeks) | - | 5.67 ± 1.76 IQR 7–5 | 23.72 ± 7.39 IQR 29–19 |
| The interval between the third dose of COVID-19 vaccination and the collection of blood samples (day of delivery) (weeks) | - | - | 5.11 ± 2.03 IQR 7–4 |
| Weeks of gestation at delivery (weeks) | 38.25 ± 0.89 IQR 39–37.75 | 38.49 ± 1.17 IQR 39–38 | 38.44 ± 1.11 IQR 39–38 |
| Weight of newborn (g) | 3123.13 * ± 363.52 ** IQR 3205–2992.5 | 3089.9 ± 360.78 IQR 3235–2900 | 3103.89 ± 311.99 IQR 3293.75–2883.8 |
| Sex of newborn | |||
| Male | 4 (50% ***) | 22 (44.9% ***) | 28 (51.9% ***) |
| Female | 4 (50% ***) | 27 (55.1% ***) | 26 (48.1% ***) |
| miR-451a | ||
|---|---|---|
| Pathway Description | Gene Name | p-Value |
| Interleukin-4 and Interleuki-13 Signaling | AKT1/MMP2/MMP9/BCL2/ MYC/IL6R | 2.60 × 10−7 |
| Vascular Inflammations | AKT1/MMP2/MMP9/ADAM10/CRP/ ETS1/CAV1 | 4.09 × 10−7 |
| Immune Thrombocytopenic Purpura | MIF/ABCB1/MMP9/BCL2/IKBKB/ ADAM10/CRP | 9.75 × 10−7 |
| Acquired Immunodeficiency Syndrome | ABCB1/MMP2/MMP9/BCL2/ MYC/CRP | 2.31 × 10−6 |
| Pelvic Inflammatory Disease | MMP2/MMP9/CRP | 1.32 × 10−5 |
| Target Cytokine related to COVID-19 [23,24,25] | IL-6R/IL-6/TNF-α [23,24,25] | |
| miR-21-5p | ||
|---|---|---|
| Pathway Description | Gene Name | p-Value |
| Inflammation | PTEN/FAS/EGFR/IL1B/ICAM1/PLAT/PTX3/TNFAIP3/CCL20/DUSP10/PPARA/NTF3/FASLG/SMAD7/MMP2/VEGFA/MMP9/STAT3/MYD88/IL12A/CXCL10/CLU/CCR7/TLR2 | 2.79 × 10−11 |
| Interleukin-4 and Interleukin-13 Signaling | BCL2/SOCS5/MYC/IL1B/ICAM1/FASLG/SOX2/MMP2/VEGFA/MMP9/STAT3/BCL6/IL12A/FOXO3 | 6.62 × 10−10 |
| Acquired Immunodeficiency Syndrome | BCL2/PTEN/FAS/MYC/IL1B/ICAM1/SP1/FASLG/MMP2/VEGFA/MMP9/SLK/STAT3/BCL6/MYD88/CXCL10 | 1.96 × 10−9 |
| Immune Thrombocytopenic Purpura | RASGRP1/BCL2/JAG1/TIMP3/PTEN/FAS/IL1B/TNFAIP3/FASLG/SMAD7/VEGFA/MMP9/STAT3/BCL6/IL12A/CXCL10/GP5 | 3.41 × 10−8 |
| Vascular Inflammations | TIMP3/PTEN/PPIF/IL1B/ICAM1/PTX3/SMARCA4/SMAD7/MMP2/VEGFA/MMP9/AKT2/STAT3/FOXO3/CXCL10/TLR2 | 4.90 × 10−8 |
| Target Cytokine related to COVID-19 [26,27] | IL-1β/IL-12 [26,27] | |
| miR-23a-3p | ||
|---|---|---|
| Pathway Description | Gene Name | p-Value |
| Inflammation | CXCL12/PTEN/CDH1/IRF1/FAS/MT2A/STS/VCAM1/TNFAIP3/TLR6/FGF2/FOXP3/SIRT1 | 2.30 × 10−5 |
| Human Immunodeficiency Virus Infectious Disease | CXCL12/CDH1/IRF1/FAS/VCAM1/SOD2/ MEF2C/FOXP3/BCL2/SIRT1 | 2.96 × 10−5 |
| Experimental Autoimmune Encephalomyelitis | FOXO3/VCAM1/ADAM17/FGF2/ TGFB2/SIRT1 | 5.85 × 10−5 |
| Autoimmune Lymphoproliferative Syndrome | FAS/TNFAIP3/XIAP/FOXP3/BCL2 | 8.79 × 10−5 |
| Immune Thrombocytopenic Purpura | CXCL12/HES1/PTEN/FAS/HOXB4/STS/ TNFAIP3/FGF2/FOXP3/BCL2 | 0.000127 |
| Target Cytokine or Protein related to COVID-19 [24] | IL-6/BCL2 [24] | |
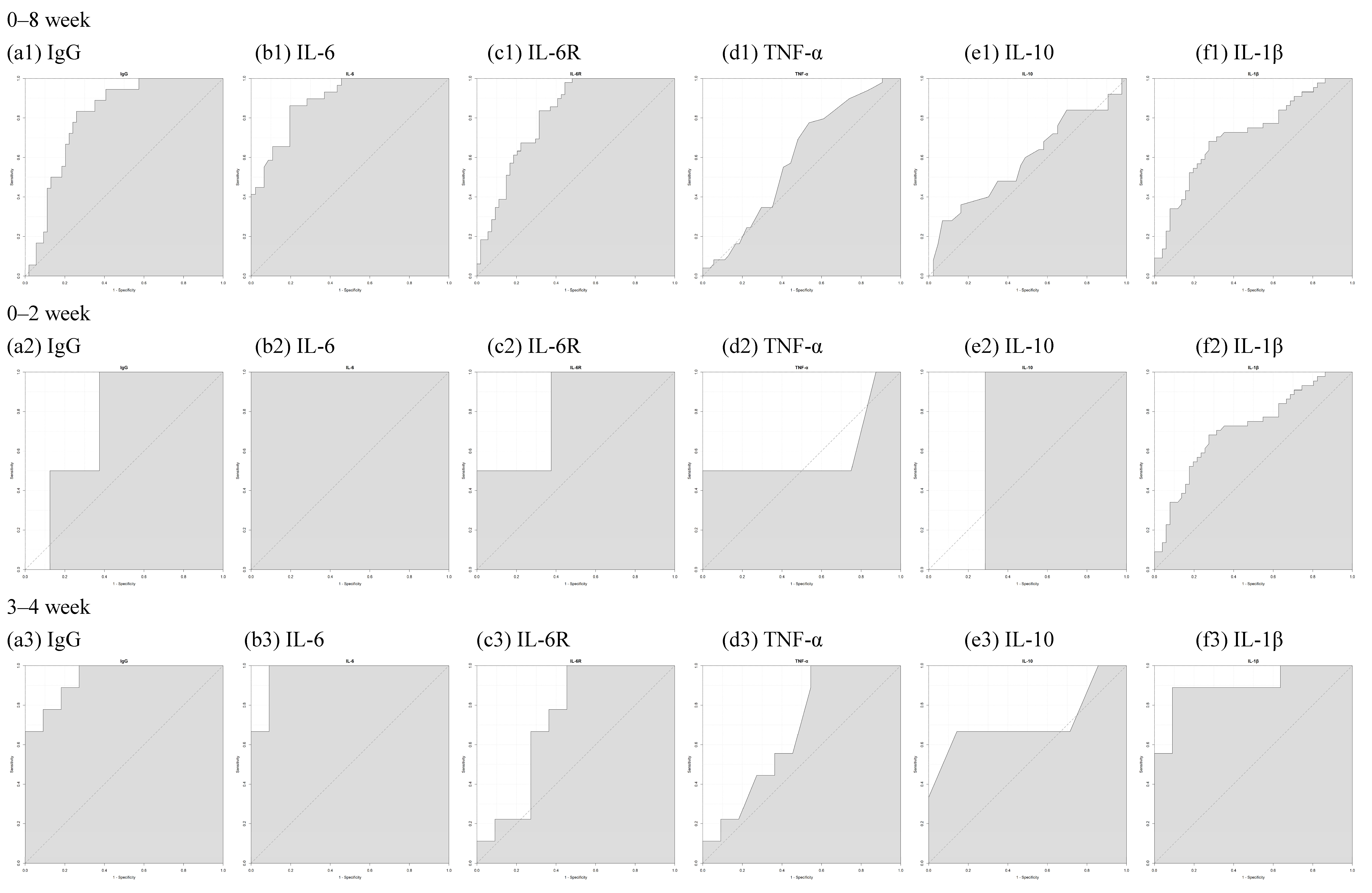
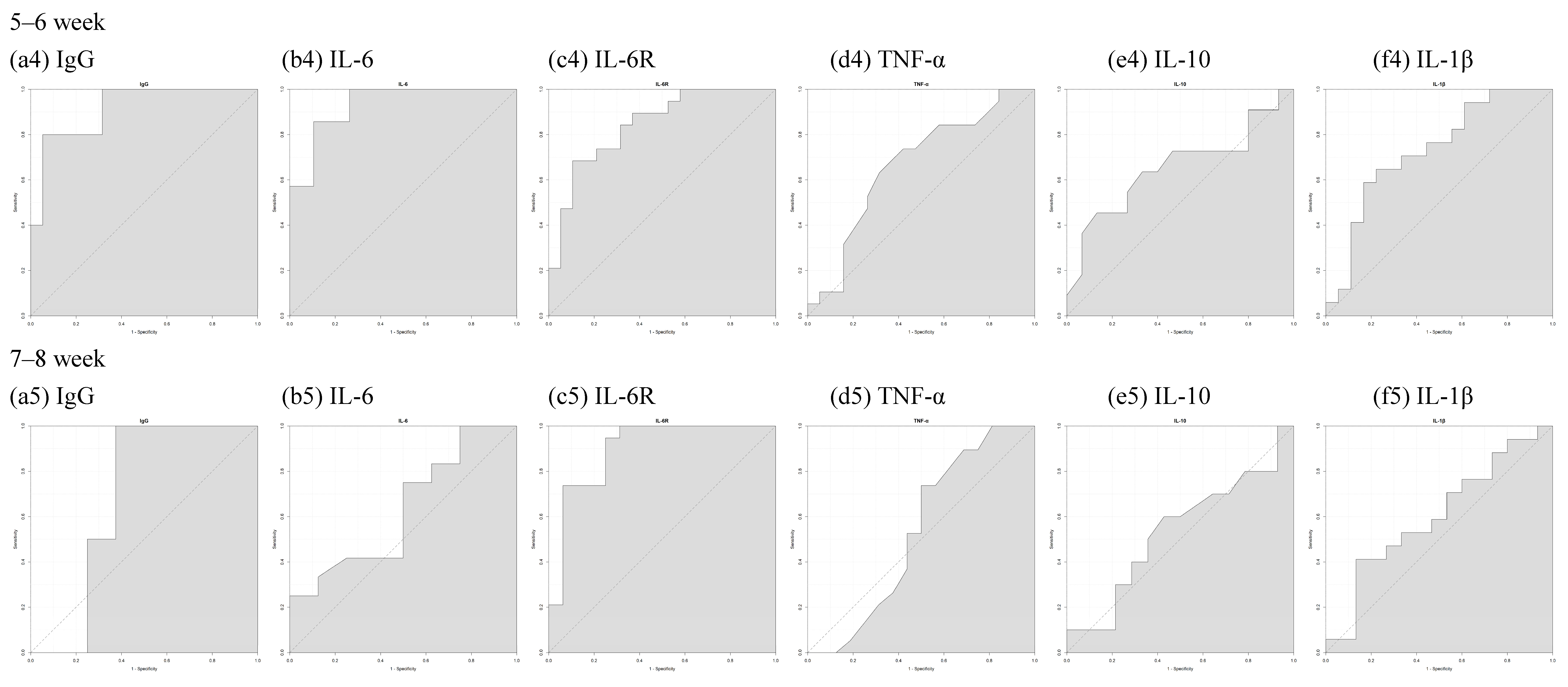
| 0–8 Week | 0–2 Week | 3–4 Week | 5–6 Week | 7–8 Week | |
|---|---|---|---|---|---|
| S1RBD IgG | 0.8086 | 0.75 | 0.9394 | 0.9158 | 0.6875 |
| IL-6 | 0.8887 | 1 | 0.9697 | 0.9323 | 0.6302 |
| IL-6R | 0.8107 | 0.8125 | 0.7273 | 0.8421 | 0.898 |
| TNF-α | 0.5911 | 0.5938 | 0.6717 | 0.6648 | 0.5362 |
| IL-10 | 0.5865 | 0.7143 | 0.7143 | 0.6545 | 0.5357 |
| IL-1β | 0.7146 | 0.8571 | 0.899 | 0.7288 | 0.6039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-J.; Lin, Y.-P.; Chen, W.-C.; Cheng, M.-H.; Hong, J.-J.; Hu, S.-Y.; Shen, C.-F.; Cheng, C.-M. COVID-19 Vaccination in Pregnancy: Pilot Study of Plasma MicroRNAs Associated with Inflammatory Cytokines after COVID-19 mRNA Vaccination. Vaccines 2024, 12, 658. https://doi.org/10.3390/vaccines12060658
Shen C-J, Lin Y-P, Chen W-C, Cheng M-H, Hong J-J, Hu S-Y, Shen C-F, Cheng C-M. COVID-19 Vaccination in Pregnancy: Pilot Study of Plasma MicroRNAs Associated with Inflammatory Cytokines after COVID-19 mRNA Vaccination. Vaccines. 2024; 12(6):658. https://doi.org/10.3390/vaccines12060658
Chicago/Turabian StyleShen, Ching-Ju, Yen-Pin Lin, Wei-Chun Chen, Mei-Hsiu Cheng, Jun-Jie Hong, Shu-Yu Hu, Ching-Fen Shen, and Chao-Min Cheng. 2024. "COVID-19 Vaccination in Pregnancy: Pilot Study of Plasma MicroRNAs Associated with Inflammatory Cytokines after COVID-19 mRNA Vaccination" Vaccines 12, no. 6: 658. https://doi.org/10.3390/vaccines12060658
APA StyleShen, C.-J., Lin, Y.-P., Chen, W.-C., Cheng, M.-H., Hong, J.-J., Hu, S.-Y., Shen, C.-F., & Cheng, C.-M. (2024). COVID-19 Vaccination in Pregnancy: Pilot Study of Plasma MicroRNAs Associated with Inflammatory Cytokines after COVID-19 mRNA Vaccination. Vaccines, 12(6), 658. https://doi.org/10.3390/vaccines12060658








