mRNA Vaccine for Alzheimer’s Disease: Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaccine Preparation
2.3. Immunization of Mice
2.4. Vaccine Administration to Non-Human Primates
2.5. Detection of Aβ-Specific Antibodies
2.6. Epitope Mapping of Aβ-Specific Antibodies
2.7. Detection of Aβ Plaques in Human Brain Tissues through Immunofluorescence and Confocal Microscopy
2.8. Statistical Analysis
3. Results
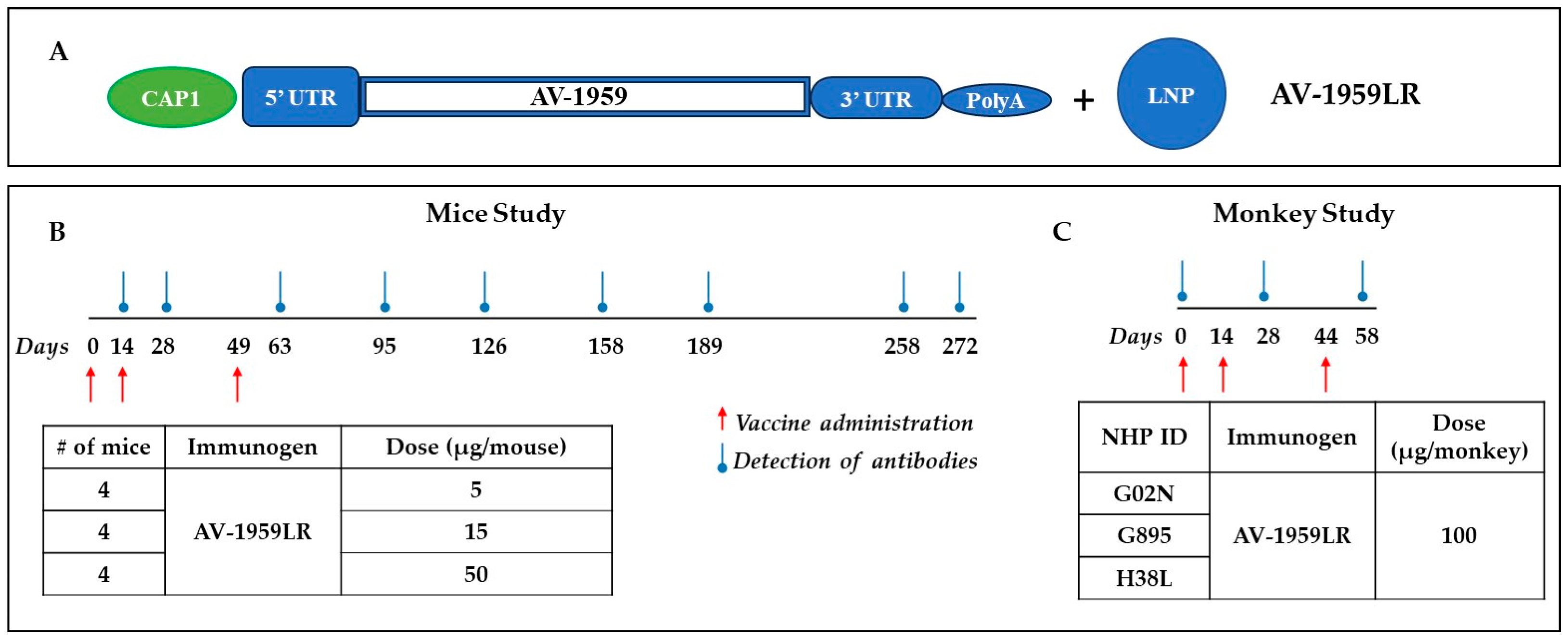
3.1. AV-1959LR, an mRNA-Based Vaccine: The Counterpart of the AV-1959D DNA Vaccine
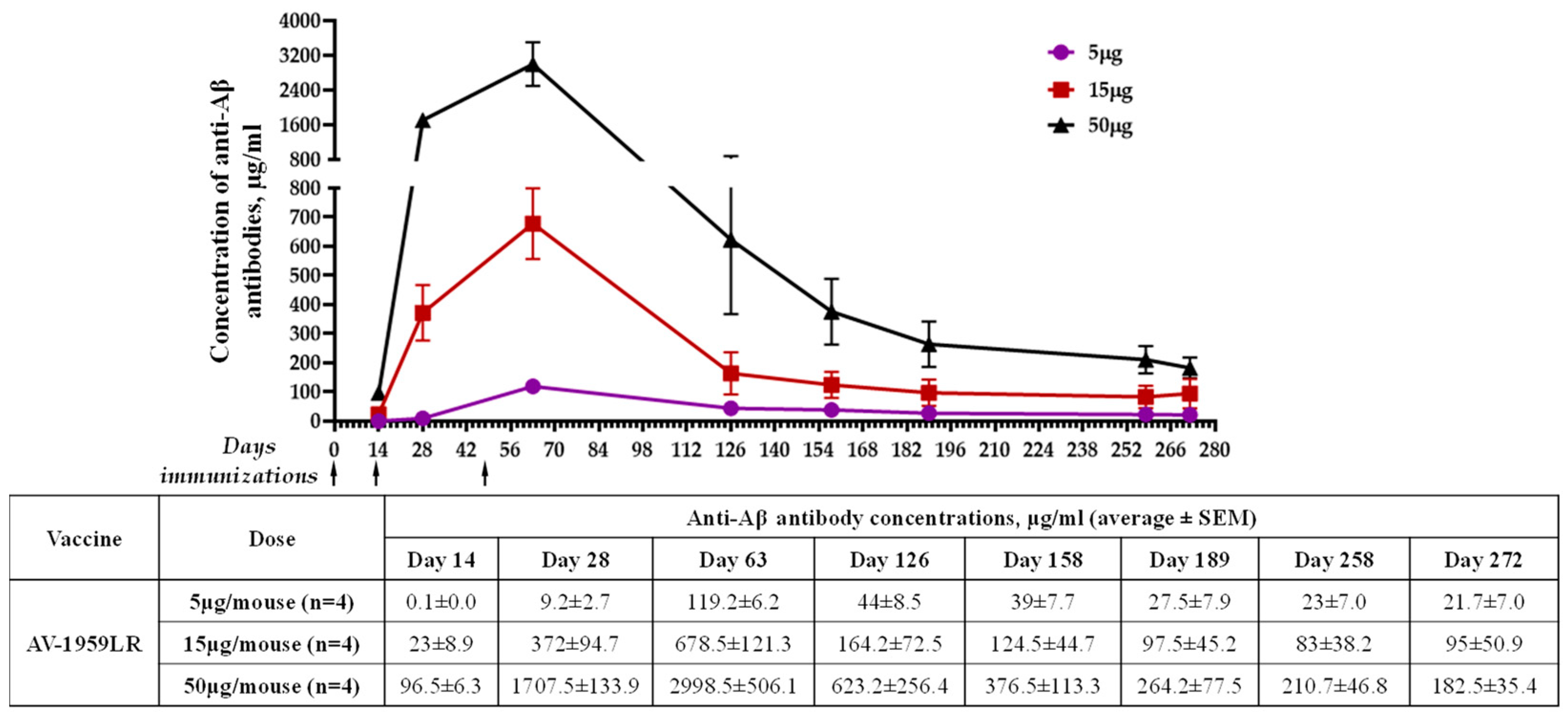
3.2. Immunogenicity and Dose–Response to AV-1959LR Vaccine in Mice
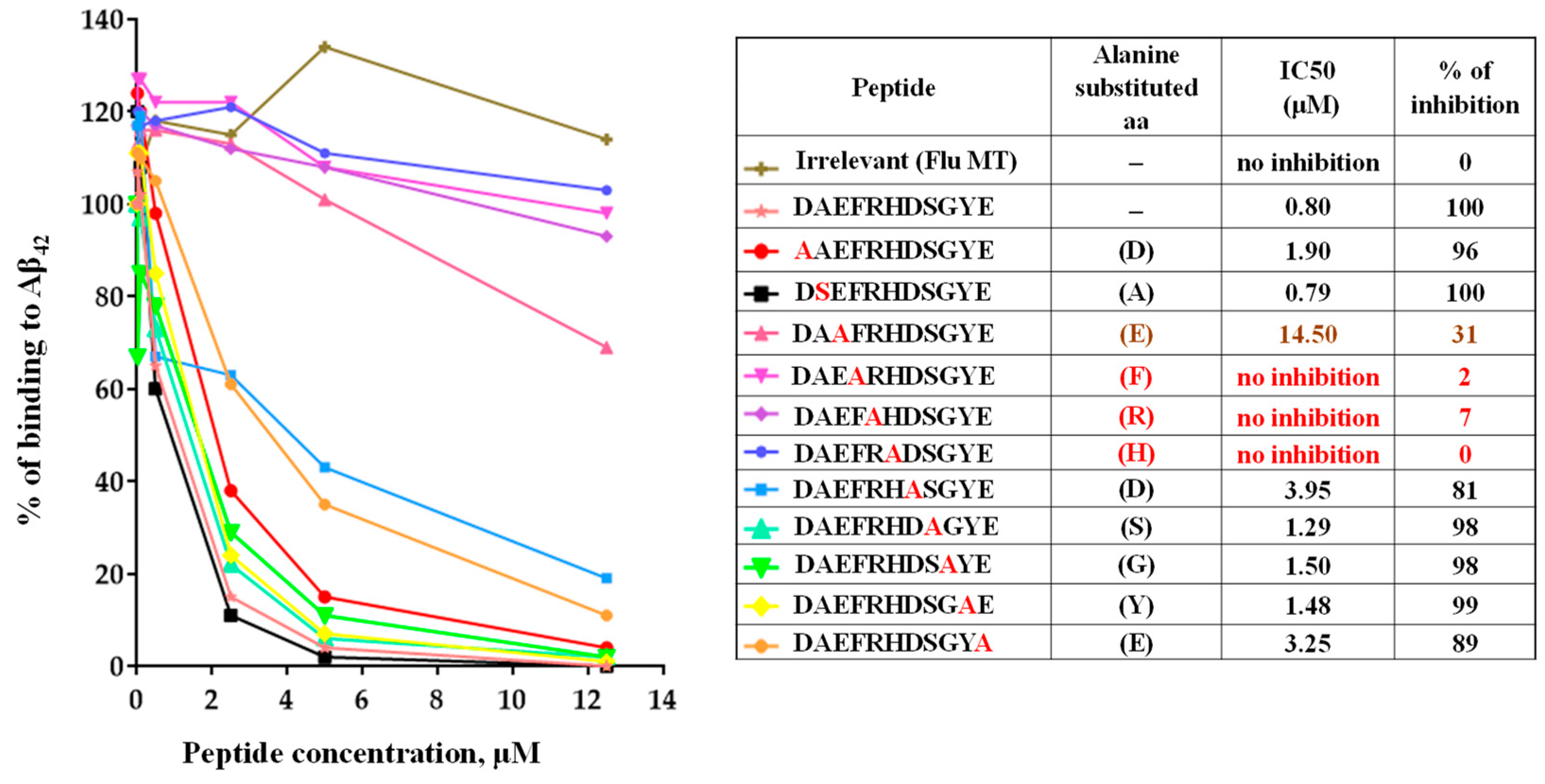
3.3. Epitope Mapping of Anti-Aβ Antibodies Generated by AV-1959LR in Mice
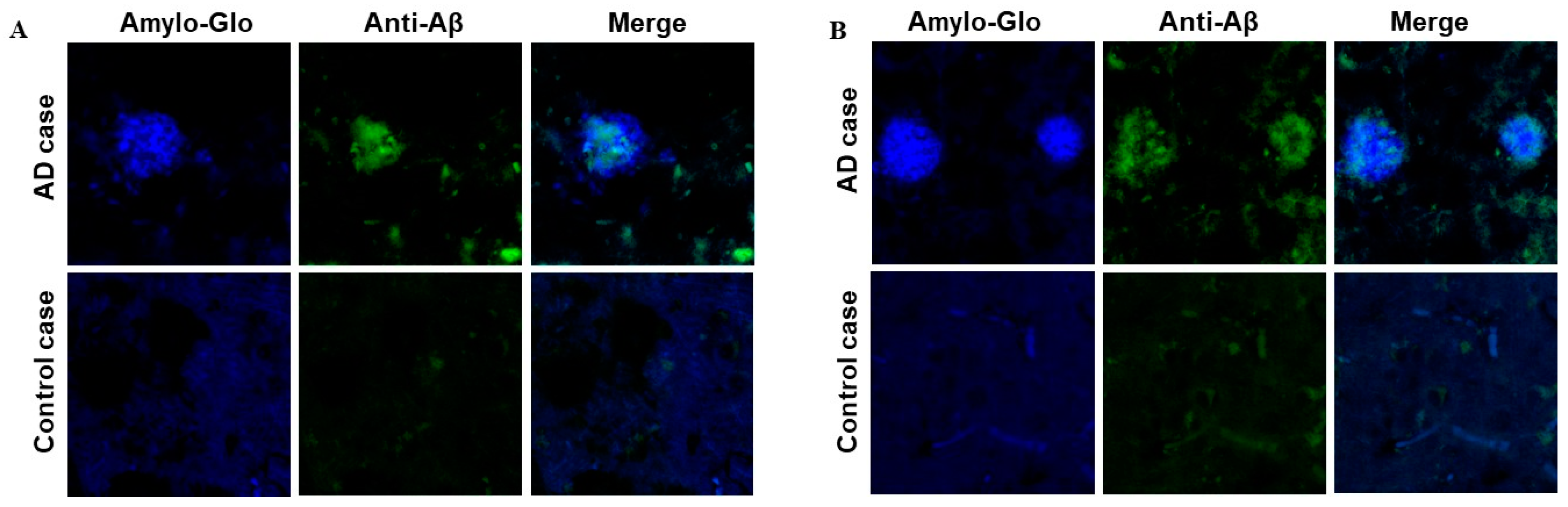
3.4. Binding of Anti-Aβ Antibodies to Amyloid Plaques
3.5. Immunogenicity of AV-1959LR mRNA Vaccine in NHPs
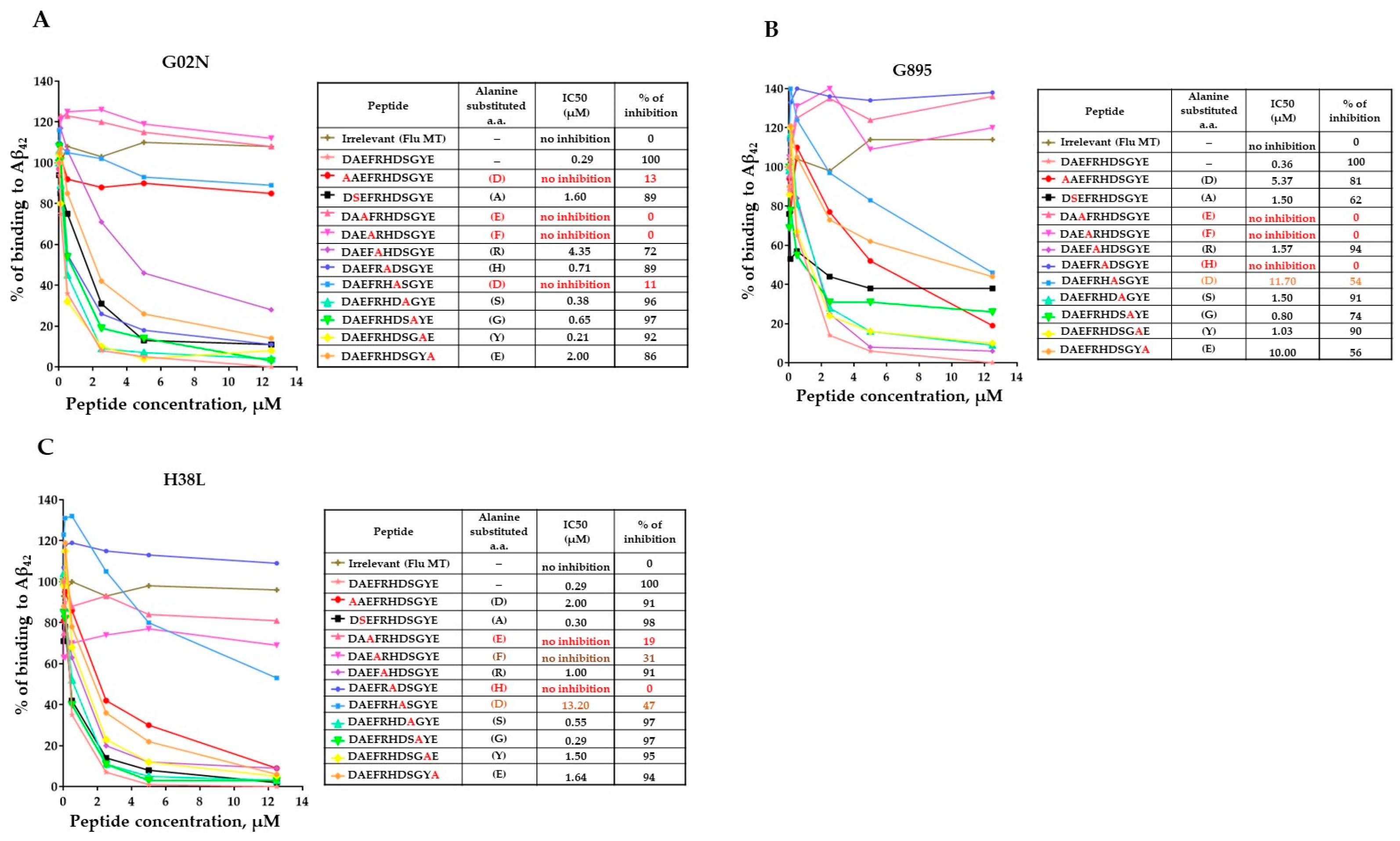
3.6. Epitope Mapping of Anti-Aβ Antibodies Generated by AV-1959LR in Non-Human Primates
3.7. Binding of Anti-Aβ Antibodies to Amyloid Plaques
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.; Cummings, J.L.; Dhadda, S.; Swanson, C.J.; Reyderman, L.; Kanekiyo, M.; Koyama, A.; Irizarry, M.; Kramer, L.D.; Bateman, R.J. Lecanemab in patients with early Alzheimer’s disease: Detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimer’s Res. Ther. 2022, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Ghochikyan, A.; Davtyan, H.; Petrushina, I.; Hovakimyan, A.; Movsesyan, N.; Davtyan, A.; Kiyatkin, A.; Cribbs, D.H.; Agadjanyan, M.G. Refinement of a DNA based Alzheimer’s disease epitope vaccine in rabbits. Hum. Vaccin. Immunother. 2013, 9, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.F.; Davtyan, H.; Petrushina, I.; Hovakimyan, A.; Davtyan, A.; Hannaman, D.; Cribbs, D.H.; Agadjanyan, M.G.; Ghochikyan, A. Epitope-based DNA vaccine for Alzheimer’s disease: Translational study in macaques. Alzheimer’s Dement. 2014, 10, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Davtyan, H.; Ghochikyan, A.; Petrushina, I.; Hovakimyan, A.; Davtyan, A.; Cribbs, D.H.; Agadjanyan, M.G. The MultiTEP platform-based Alzheimer’s disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimer’s Dement. 2014, 10, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Davtyan, H.; Zagorski, K.; Rajapaksha, H.; Hovakimyan, A.; Davtyan, A.; Petrushina, I.; Kazarian, K.; Cribbs, D.H.; Petrovsky, N.; Agadjanyan, M.G.; et al. Alzheimer’s disease Advax(CpG)- adjuvanted MultiTEP-based dual and single vaccines induce high-titer antibodies against various forms of tau and Abeta pathological molecules. Sci. Rep. 2016, 6, 28912. [Google Scholar] [CrossRef]
- Davtyan, H.; Hovakimyan, A.; Kiani Shabestari, S.; Antonyan, T.; Coburn, M.A.; Zagorski, K.; Chailyan, G.; Petrushina, I.; Svystun, O.; Danhash, E.; et al. Testing a MultiTEP-based combination vaccine to reduce Abeta and tau pathology in Tau22/5xFAD bigenic mice. Alzheimer’s Res. Ther. 2019, 11, 107. [Google Scholar] [CrossRef]
- Hovakimyan, A.; Zagorski, K.; Chailyan, G.; Antonyan, T.; Melikyan, L.; Petrushina, I.; Batt, D.G.; King, O.; Ghazaryan, M.; Donthi, A.; et al. Immunogenicity of MultiTEP platform technology-based Tau vaccine in non-human primates. NPJ Vaccines 2022, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Sidney, J.; Southwood, S.; Ruppert, J.; Oseroff, C.; Maewal, A.; Snoke, K.; Serra, H.M.; Kubo, R.T.; Sette, A.; et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1994, 1, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; del Guercio, M.F.; Maewal, A.; Qiao, L.; Fikes, J.; Chesnut, R.W.; Paulson, J.; Bundle, D.R.; DeFrees, S.; Sette, A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000, 164, 1625–1633. [Google Scholar] [CrossRef]
- James, E.A.; Bui, J.; Berger, D.; Huston, L.; Roti, M.; Kwok, W.W. Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int. Immunol. 2007, 19, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Panina-Bordignon, P.; Tan, A.; Termijtelen, A.; Demotz, S.; Corradin, G.; Lanzavecchia, A. Universally immunogenic T cell epitopes: Promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 1989, 19, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Faner, R.; James, E.; Huston, L.; Pujol-Borrel, R.; Kwok, W.W.; Juan, M. Reassessing the role of HLA-DRB3 T-cell responses: Evidence for significant expression and complementary antigen presentation. Eur. J. Immunol. 2010, 40, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Pei, W.; Ge, H.; Liang, Q.; Luo, Y.; Sharp, F.R.; Lu, A.; Ran, R.; Graham, S.H.; Chen, J. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J. Neurosci. 2002, 22, 5423–5431. [Google Scholar] [CrossRef] [PubMed]
- Derhovanessian, E.; Solana, R.; Larbi, A.; Pawelec, G. Immunity, ageing and cancer. Immun. Ageing 2008, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Petrushina, I.; Hovakimyan, A.; Harahap-Carrillo, I.S.; Davtyan, H.; Antonyan, T.; Chailyan, G.; Kazarian, K.; Antonenko, M.; Jullienne, A.; Hamer, M.M.; et al. Characterization and preclinical evaluation of the cGMP grade DNA based vaccine, AV-1959D to enter the first-in-human clinical trials. Neurobiol. Dis. 2020, 139, 104823. [Google Scholar] [CrossRef] [PubMed]
- Querol-Vilaseca, M.; Colom-Cadena, M.; Pegueroles, J.; Nunez-Llaves, R.; Luque-Cabecerans, J.; Munoz-Llahuna, L.; Andilla, J.; Belbin, O.; Spires-Jones, T.L.; Gelpi, E.; et al. Nanoscale structure of amyloid-beta plaques in Alzheimer’s disease. Sci. Rep. 2019, 9, 5181. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, C.M.C.; de Groot, N.G.; Bontrop, R.E. Comparative genetics of the major histocompatibility complex in humans and nonhuman primates. Int. J. Immunogenet. 2020, 47, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.C.; DeVit, M.; Johnston, S.A. Genetic immunization is a simple method for eliciting an immune response. Nature 1992, 356, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ugen, K.E.; Srikantan, V.; Agadjanyan, M.G.; Dang, K.; Refaeli, Y.; Sato, A.I.; Boyer, J.; Williams, W.V.; Weiner, D.B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1993, 90, 4156–4160. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.B.; Donnelly, J.J.; Parker, S.E.; Rhodes, G.H.; Felgner, P.L.; Dwarki, V.J.; Gromkowski, S.H.; Deck, R.R.; DeWitt, C.M.; Friedman, A.; et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993, 259, 1745–1749. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magne, R.; Gomard, E.; Guillet, J.G.; Levy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; Pavithra, K.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-generation DNA vaccine platforms and delivery systems: Advances, challenges and prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef] [PubMed]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Green, T.D.; Montefiori, D.C.; Ross, T.M. Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d. J. Virol. 2003, 77, 2046–2055. [Google Scholar] [CrossRef][Green Version]
- Jazayeri, S.D.; Poh, C.L. Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Vet. Res. 2019, 50, 78. [Google Scholar] [CrossRef] [PubMed]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Hogan, M.J.; Pardi, N. mRNA Vaccines in the COVID-19 Pandemic and Beyond. Annu. Rev. Med. 2022, 73, 17–39. [Google Scholar] [CrossRef]
- Sheridan, C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021, 39, 1479–1482. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Ghochikyan, A.; Vasilevko, V.; Petrushina, I.; Tran, M.; Sadzikava, N.; Babikyan, D.; Movsesyan, N.; Tian, W.; Ross, T.M.; Cribbs, D.H.; et al. Generation and chracterization of the humoral immune response to DNA immunization with a chimeric b-amyloid-interleukin-4 minigene. Eur. J. Immunol. 2003, 33, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.K.; Moreno, S.; Leder, C.; Pavlenko, M.; King, A.; Pisa, P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol. Ther. 2006, 13, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.K.; Eriksson, F.; Timmons, J.A.; Gerhardt, J.; Nyman, U.; Gudmundsdotter, L.; Brave, A.; Wahren, B.; Pisa, P. Skin electroporation: Effects on transgene expression, DNA persistence and local tissue environment. PLoS ONE 2009, 4, e7226. [Google Scholar] [CrossRef] [PubMed]
- Olkhanud, P.B.; Mughal, M.; Ayukawa, K.; Malchinkhuu, E.; Bodogai, M.; Feldman, N.; Rothman, S.; Lee, J.H.; Chigurupati, S.; Okun, E.; et al. DNA immunization with HBsAg-based particles expressing a B cell epitope of amyloid beta-peptide attenuates disease progression and prolongs survival in a mouse model of Alzheimer’s disease. Vaccine 2012, 30, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Davtyan, H.; Hovakimyan, A.; Zagorski, K.; Davtyan, A.; Petrushina, I.; Agdashian, D.; Murthy, V.; Cribbs, D.H.; Agadjanyan, M.G.; Ghochikyan, A. BTX AgilePulse(TM) system is an effective electroporation device for intramuscular and intradermal delivery of DNA vaccine. Curr. Gene Ther. 2014, 14, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P.; et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Leppek, K.; Byeon, G.W.; Kladwang, W.; Wayment-Steele, H.K.; Kerr, C.H.; Xu, A.F.; Kim, D.S.; Topkar, V.V.; Choe, C.; Rothschild, D.; et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 2022, 13, 1536. [Google Scholar] [CrossRef] [PubMed]


| Nucleic Acid Based Vaccine | Mice (Immune Haplotype) | Administration | Humoral Immune Response, Binding to Aβ42 Peptide after 3rd Immunization | Reference | |||
|---|---|---|---|---|---|---|---|
| Dose (µg/mouse) | Route | Device | |||||
| Concentration of Anti-Aβ Antibodies, µg/mL (Average ± SEM) | Isotype (IgG1/IgG2ab Ratio) | ||||||
| DNA | C57BL/6 (H-2b haplotype) | 20 | ID | _ | 15.3 ± 12.2 | 6.3 | [46] |
| DNA | C57BL/6 (H-2b haplotype) | 20 | ID | BTX | 645.0 ± 574.1 | 8.2 | [46] |
| DNA | C57BL/6 (H-2b haplotype) | 20 | IM | _ | 18.8 ± 9.3 | 1.2 | [46] |
| DNA | C57BL/6 (H-2b haplotype) | 20 | IM | BTX | 472.3 ± 229.6 | 1.1 | [46] |
| DNA | C57BL/6 (H-2b haplotype) | 10 | IM | TDS-IM EP | 155.1 ± 22.0 | 0.6 | [8] |
| RNA | C57BL/6 (H-2b haplotype) | 5 15 50 | IM | LNP (ALC-0315 ) | 119.3 ± 6.2 678.5 ± 121.3 2998.5 ± 506.7 | 1.5 | - |
| Nucleic Acid Based Vaccine | NHP | Administration | Endpoint Titers of Anti-Aβ Antibodies after 3rd Immunization Average ± SEM | Reference | ||
|---|---|---|---|---|---|---|
| Dose (µg/monkey) | Route | Device | ||||
| DNA | Macaca fascicularis | 2000 | ID | Pharmajet | 4950.2 ± 4264.1 | - |
| DNA | Rhesus macaque | 400 | IM | TDS-IM EP | 2420.7 ± 1929.4 | [7] |
| DNA | Rhesus macaque | 4000 | IM | TDS-IM EP | 7040.0 ± 2410.2 | [7] |
| RNA | Macaca fascicularis | 100 | IM | LNP (ALC-0315) | 8567.2 ± 5040.9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hovakimyan, A.; Chilingaryan, G.; King, O.; Capocchi, J.K.; Chadarevian, J.P.; Davtyan, H.; Kniazev, R.; Agadjanyan, M.G.; Ghochikyan, A. mRNA Vaccine for Alzheimer’s Disease: Pilot Study. Vaccines 2024, 12, 659. https://doi.org/10.3390/vaccines12060659
Hovakimyan A, Chilingaryan G, King O, Capocchi JK, Chadarevian JP, Davtyan H, Kniazev R, Agadjanyan MG, Ghochikyan A. mRNA Vaccine for Alzheimer’s Disease: Pilot Study. Vaccines. 2024; 12(6):659. https://doi.org/10.3390/vaccines12060659
Chicago/Turabian StyleHovakimyan, Armine, Garri Chilingaryan, Olga King, Joia Kai Capocchi, Jean Paul Chadarevian, Hayk Davtyan, Roman Kniazev, Michael G. Agadjanyan, and Anahit Ghochikyan. 2024. "mRNA Vaccine for Alzheimer’s Disease: Pilot Study" Vaccines 12, no. 6: 659. https://doi.org/10.3390/vaccines12060659
APA StyleHovakimyan, A., Chilingaryan, G., King, O., Capocchi, J. K., Chadarevian, J. P., Davtyan, H., Kniazev, R., Agadjanyan, M. G., & Ghochikyan, A. (2024). mRNA Vaccine for Alzheimer’s Disease: Pilot Study. Vaccines, 12(6), 659. https://doi.org/10.3390/vaccines12060659






