A Narrative Review on the Promising Potential of Graphene in Vaccine Design: Evaluating the Benefits and Drawbacks of Carbon Nanoplates in Nanovaccine Production
Abstract
1. Introduction
- -
- Pathogen variability: Many pathogens, such as influenza virus and HIV, have the ability to rapidly mutate and change their antigenic properties. This variability can make it challenging to develop broadly effective vaccines that can provide protection against multiple strains or variants of the same pathogen. Vaccine developers must continuously monitor pathogen evolution and update vaccine compositions to maintain effectiveness.
- -
- Immune system complexity: The human immune system is highly complex, and individual responses to vaccines can vary significantly based on factors such as age, genetics, and underlying health conditions. Certain populations, like the elderly or immunocompromised individuals, may have a weaker or less robust immune response to vaccines. Designing vaccines that can elicit a consistent and protective immune response across diverse populations is an ongoing challenge.
- -
- Vaccine safety and adverse events: Ensuring the safety of vaccines is of paramount importance, and vaccine developers must thoroughly evaluate the potential for adverse events or side effects. Even rare adverse events can raise public concerns and affect vaccine acceptance, necessitating robust safety monitoring and communication strategies.
- -
- Vaccine stability and storage: Many vaccines require specific storage conditions, such as cold-chain maintenance, to preserve their potency and efficacy. Maintaining the cold chain can be particularly challenging in resource-limited settings or areas with limited infrastructure, which can impact vaccine availability and distribution. Developing more thermostable vaccine formulations or alternative delivery methods can help address these challenges.
- -
- Regulatory and ethical considerations: Vaccine development and approval processes are subject to strict regulatory requirements to ensure safety and efficacy. Navigating the regulatory landscape and addressing ethical concerns, such as equitable access and fair distribution, can add complexity to the vaccine development process.
- -
- Vaccine hesitancy and misinformation: Vaccine hesitancy, driven by factors such as misinformation, distrust, and personal beliefs, can hinder the uptake and effectiveness of vaccines. Addressing vaccine hesitancy requires a multifaceted approach, including effectively communicating, providing education, and addressing the underlying concerns of the public [2,3,4].
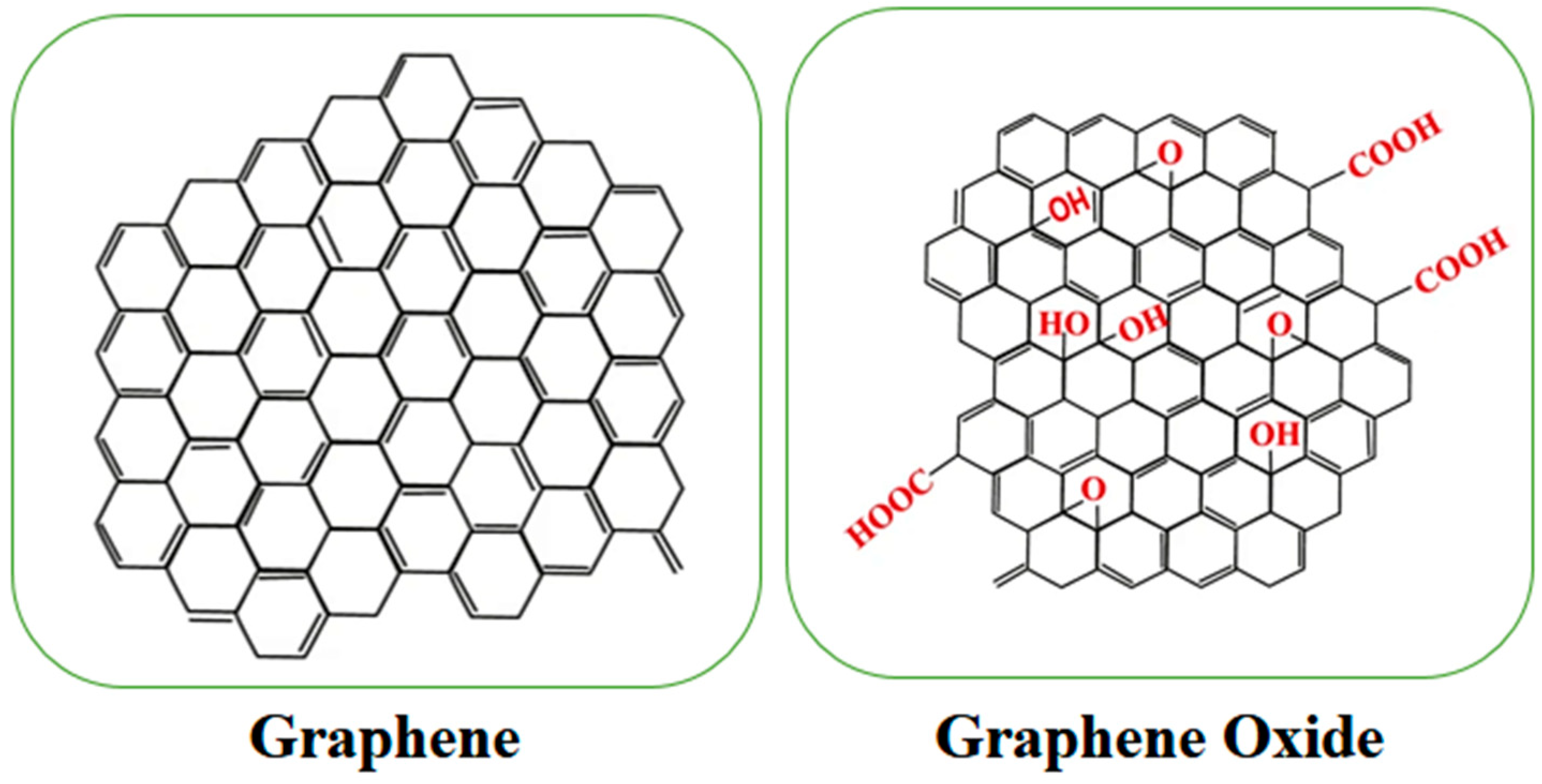
2. Graphene Material Structure
3. Graphene and Graphene Oxide Properties
3.1. Graphene
3.1.1. Mechanical Properties
3.1.2. Electrical Properties
3.1.3. Thermal Properties
3.1.4. Optical Properties
3.1.5. Surface Area and Adsorption
3.1.6. Graphene Applications
3.2. Graphene Oxide
3.2.1. High Surface Area
3.2.2. Hydrophilicity
3.2.3. Tunable Electronic Properties
3.2.4. Mechanical Strength
3.2.5. Applications of Graphene Materials in Vaccine Design
3.2.6. Antigen Delivery
3.2.7. Adjuvant Properties
- -
- Activating pattern recognition receptors (PRRs) on immune cells, triggering signaling cascades that promote immune responses.
- -
- Inducing inflammasome activation, leading to the release of pro-inflammatory cytokines.
- -
- Enhancing the recruitment and activation of immune cells at the site of vaccine administration.
- -
- Improving the uptake and processing of vaccine antigens by APCs.
4. Controlled Release
- -
- pH-responsive release: Graphene-based materials can be designed to release the cargo in response to changes in pH, which can occur in different physiological environments or upon internalization by cells [47].
- -
- Enzymatic or redox-triggered release: The release can be triggered by the presence of specific enzymes or changes in redox conditions, which can be exploited to target specific cellular or extracellular environments.
- -
- Stimuli-responsive release: External stimuli, such as temperature, light, or magnetic fields, can be used to control the release of vaccine components from the graphene-based delivery system [27].
5. Improved Vaccine Stability
6. Mucosal Vaccines
7. Needle-Free Vaccination
8. Disadvantages of Graphene in Vaccine Design
9. Future Trends in the Area of Graphene
- Personalized vaccines: Graphene-based materials can be tailored to deliver personalized vaccine formulations based on an individual’s genetic makeup, immune response, and disease susceptibility. This can lead to more effective and targeted vaccination strategies.
- Multivalent vaccines: Graphene’s ability to carry multiple antigens or adjuvants simultaneously can enable the development of multivalent vaccines that protect against multiple pathogens or strains, reducing the need for multiple vaccine doses.
- Nanoparticle-based vaccines: Graphene-based nanoparticles can be engineered to deliver vaccine components directly to immune cells, enhancing antigen uptake and presentation. This can lead to more potent and durable immune responses.
- Smart vaccine systems: Graphene-based materials can be integrated with sensors or other smart technologies to monitor vaccine efficacy, storage conditions, and immune responses in real time. This can help optimize vaccine formulations and administration strategies.
- Next-generation adjuvants: Graphene-based materials can be functionalized with novel immunomodulatory agents or combined with other adjuvants to enhance the immune response to vaccines. This can lead to the development of more potent and effective adjuvant systems.
- Needle-free vaccine delivery: Graphene-based materials can be used to develop innovative needle-free vaccination methods, such as aerosolized or oral vaccine formulations. This can improve vaccine acceptance and simplify vaccine administration, particularly in resource-limited settings.
- Rapid vaccine development: Graphene’s ability to rapidly adsorb and deliver vaccine components can accelerate vaccine development and production, enabling faster responses to emerging infectious diseases or pandemics.
- Vaccine thermostability: Graphene-based materials can improve the thermostability of vaccines, reducing the need for cold-chain storage and increasing vaccine accessibility in regions with limited infrastructure.
- Immunomodulatory therapies: Graphene-based materials can be used to develop novel immunomodulatory therapies that target specific immune pathways or cells, providing new treatment options for autoimmune diseases or cancer.
- Combination therapies: Graphene-based materials can be combined with other nanomaterials or biomolecules to create hybrid vaccine systems with enhanced properties, such as improved antigen delivery, adjuvant activity, or controlled release capabilities.
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current Challenges in Vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G. Vaccines and Antivirals: Grand Challenges and Great Opportunities. Front. Virol. 2021, 1, 666548. [Google Scholar] [CrossRef]
- Lozano, D.; Larraga, V. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef]
- Mao, L.; Chen, Z.; Wang, Y.; Chen, C. Design and application of nanoparticles as vaccine adjuvants against human corona virus infection. J. Inorg. Biochem. 2021, 219, 111454. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Khurana, N. Aspects of Nanotechnology for COVID-19 Vaccine Development and Its Delivery Applications. Pharmaceutics 2023, 15, 451. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Amiri, A.; Bordbar, A.-K. New generation of drug delivery systems based on ginsenoside Rh2-, Lysine-and Arginine-treated highly porous graphene for improving anticancer activity. Sci. Rep. 2018, 8, 586. [Google Scholar] [CrossRef]
- Foroutan, T.; Nazemi, N.; Tavana, M.; Kassaee, M.Z.; Motamedi, E.; Sonieshargh, S.; Zare-Zardini, H. Suspended graphene oxide nanoparticle for accelerated multilayer osteoblast attachment. J. Biomed. Mater. Res. Part A 2018, 106, 293–303. [Google Scholar] [CrossRef]
- Farhangfar, S.D.; Fesahat, F.; Zare-Zardini, H.; Dehghan-Manshadi, M.; Zare, F.; Miresmaeili, S.M.; Vajihinejad, M.; Soltaninejad, H. In vivo study of anticancer activity of ginsenoside Rh2-containing arginine-reduced graphene in a mouse model of breast cancer. Iran. J. Basic Med. Sci. 2022, 25, 1442–1451. [Google Scholar]
- Farhangfar, S.D.; Fesahat, F.; Miresmaeili, S.M.; Zare-Zardini, H. Evaluating the blood toxicity of functionalized graphene-arginine with anticancer drug ginsenoside Rh2 in balb/c mouse model with breast cancer. Iran. J. Pediatr. Hematol. Oncol. 2022, 12, 10–16. [Google Scholar] [CrossRef]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Ordooei, M.; Amiri, A.; Karimi-Zarchi, M. Evaluation of toxicity of functionalized graphene oxide with ginsenoside Rh2, lysine and arginine on blood cancer cells (K562), red blood cells, blood coagulation and cardiovascular tissue: In vitro and in vivo studies. J. Taiwan Inst. Chem. Eng. 2018, 93, 70–78. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Lalire, T.; Longuet, C.; Taguet, A. Electrical properties of graphene/multiphase polymer nanocomposites: A review. Carbon 2024, 225, 119055. [Google Scholar] [CrossRef]
- Renteria, J.D.; Nika, D.L.; Balandin, A.A. Graphene thermal properties: Applications in thermal management and energy storage. Appl. Sci. 2014, 4, 525–547. [Google Scholar] [CrossRef]
- Najim, A.; Bajjou, O.; Bakour, A.; Boulghallat, M.; Rahmani, K. A fundamental study on the electronic and optical properties of graphene oxide under an external electric field. Mod. Phys. Lett. B 2024, 38, 2450032. [Google Scholar] [CrossRef]
- Farhangfar, S.D.; Fesahat, F.; Zare-Zardini, H.; Dehghan-Manshadi, M.; Zare, F.; Miresmaeili, S.M.; Vajihinejad, M.; Soltaninejad, H.; Ghorani-Azam, A. Behavioral studies of mice with breast cancer after treatment with new anticancer agent, Rh2-containing arginine-graphene. Acad. J. 2023, 38, 63–65. [Google Scholar]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Avouris, P.; Xia, F. Graphene applications in electronics and photonics. Mrs Bull. 2012, 37, 1225–1234. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in drug delivery nanosystems using graphene-based materials and carbon nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Priya Swetha, P.D.; Manisha, H.; Sudhakaraprasad, K. Graphene and graphene-based materials in biomedical science. Part. Part. Syst. Charact. 2018, 35, 1800105. [Google Scholar] [CrossRef]
- Imani, R.; Mohabatpour, F.; Mostafavi, F. Graphene-based nano-carrier modifications for gene delivery applications. Carbon 2018, 140, 569–591. [Google Scholar] [CrossRef]
- Roy, H.; Bhanja, S.; Panigrahy, U.P.; Theendra, V.K. Graphene-Based Nanovehicles for Drug Delivery. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–111. [Google Scholar]
- Huang, S.; Li, Y.; Zhang, S.; Chen, Y.; Su, W.; Sanchez, D.J.; Mai, J.D.; Zhi, X.; Chen, H.; Ding, X. A self-assembled graphene oxide adjuvant induces both enhanced humoral and cellular immune responses in influenza vaccine. J. Control. Release 2024, 365, 716–728. [Google Scholar] [CrossRef]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C 2018, 90, 764–780. [Google Scholar] [CrossRef] [PubMed]
- Vakili, B.; Karami-Darehnaranji, M.; Mirzaei, E.; Hosseini, F.; Nezafat, N. Graphene oxide as novel vaccine adjuvant. Int. Immunopharmacol. 2023, 125 Pt A, 111062. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Bottini, M.; Fadeel, B. Graphene and the Immune System: A Romance of Many Dimensions. Front. Immunol. 2017, 8, 673. [Google Scholar] [CrossRef]
- Cao, W.; He, L.; Cao, W.; Huang, X.; Jia, K.; Dai, J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef]
- Yin, Y.; Nguyen, T.L.; Wang, B.; Duong, H.T.T.; Lee, D.S.; Kim, J.-H.; Kim, J.; Jeong, J.H. Simultaneous delivery of DNA vaccine and hydrophobic adjuvant using reducible polyethylenimine-functionalized graphene oxide for activation of dendritic cells. J. Ind. Eng. Chem. 2019, 80, 870–876. [Google Scholar] [CrossRef]
- Xu, C.; Hong, H.; Lee, Y.; Park, K.S.; Sun, M.; Wang, T.; Aikins, M.E.; Xu, Y.; Moon, J.J. Efficient lymph node-targeted delivery of personalized cancer vaccines with reactive oxygen species-inducing reduced graphene oxide nanosheets. ACS Nano 2020, 14, 13268–13278. [Google Scholar] [CrossRef]
- Tan, J.; Ding, B.; Teng, B.; Ma, P.; Lin, J. Understanding structure–function relationships of nanoadjuvants for enhanced cancer vaccine efficacy. Adv. Funct. Mater. 2022, 32, 2111670. [Google Scholar] [CrossRef]
- Alphandéry, E. Nano dimensions/adjuvants in COVID-19 vaccines. J. Mater. Chem. B 2022, 10, 1520–1552. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, H.; Huang, D.; Feng, S.; Fujita, M.; Gao, X.D. Chitosan-Functionalized Graphene Oxide as a Potential Immunoadjuvant. Nanomaterials 2017, 7, 59. [Google Scholar] [CrossRef]
- Wang, X.; Cao, F.; Yan, M.; Liu, Y.; Zhu, X.; Sun, H.; Ma, G. Alum-functionalized graphene oxide nanocomplexes for effective anticancer vaccination. Acta Biomater. 2019, 83, 390–399. [Google Scholar] [CrossRef]
- Gao, A.; Chen, Y.; Liang, H.; Cui, X.; Zhang, A.; Cui, D. Developing an efficient MGCR microneedle nanovaccine patch for eliciting Th 1 cellular response against the SARS-CoV-2 infection. Theranostics 2023, 13, 4821–4835. [Google Scholar] [CrossRef]
- Zhao, L.; Shu, M.; Shi, K.; Tang, S.; Li, Z. Novel use of graphene oxide quantum dots in a pickering emulsion as a Chlamydia trachomatis vaccine adjuvant. Int. Immunopharmacol. 2023, 118, 110035. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, J.; Liu, Y.; Xu, J.; Luo, Y.; Feng, L.; Liu, Z.; Peng, R. Functionalized graphene oxide serves as a novel vaccine nano-adjuvant for robust stimulation of cellular immunity. Nanoscale 2016, 8, 3785–3795. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Wang, Z.; Song, Z.; Zhang, T.; Yu, L.; Yu, R.; Gao, Q.; Peng, S.; Jin, H.; Wang, D.; et al. Lentinan-functionalized graphene oxide hydrogel as a sustained antigen delivery system for vaccines. Int. J. Biol. Macromol. 2023, 253 Pt 1, 126629. [Google Scholar] [CrossRef]
- Liu, Z.; He, J.; Zhu, T.; Hu, C.; Bo, R.; Wusiman, A.; Hu, Y.; Wang, D. Lentinan-Functionalized Graphene Oxide Is an Effective Antigen Delivery System That Modulates Innate Immunity and Improves Adaptive Immunity. ACS Appl. Mater. Interfaces 2020, 12, 39014–39023. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, X.; Li, B.; Chen, T.; Yu, B.; Wang, X.; Dou, X.; Peng, B.; Hu, Q. Poria cocos polysaccharide-functionalized graphene oxide nanosheet induces efficient cancer immunotherapy in mice. Front. Bioeng. Biotechnol. 2022, 10, 1050077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shu, M.; Chen, H.; Shi, K.; Li, Z. Preparation of graphene oxide-stabilized Pickering emulsion adjuvant for Pgp3 recombinant vaccine and enhanced immunoprotection against Chlamydia Trachomatis infection. Front. Immunol. 2023, 14, 1148253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Colombi Ciacchi, L.; Wei, G. Recent Advances in the Synthesis of Graphene-Based Nanomaterials for Controlled Drug Delivery. Appl. Sci. 2017, 7, 1175. [Google Scholar] [CrossRef]
- Ayub, M.; Othman, M.H.D.; Khan, I.U.; Yusop, M.Z.M.; Kurniawan, T.A. Graphene-based nanomaterials as antimicrobial surface coatings: A parallel approach to restrain the expansion of COVID-19. Surf. Interfaces 2021, 27, 101460. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Dong, C.; Ren, T.; Li, Y.; Shi, D. Surface-engineered graphene-based nanomaterials for drug delivery. J. Biomed. Nanotechnol. 2014, 10, 2086–2106. [Google Scholar] [CrossRef] [PubMed]
- Alhourani, A.; Førde, J.-L.; Eichacker, L.A.; Herfindal, L.; Hagland, H.R. Improved pH-Responsive Release of Phenformin from Low-Defect Graphene Compared to Graphene Oxide. ACS Omega 2021, 6, 24619–24629. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Asadi, M.; Akhavan, O. Graphene-based nanomaterials in fighting the most challenging viruses and immunogenic disorders. ACS Biomater. Sci. Eng. 2022, 8, 54–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gu, H.; Sun, S.; Zhang, Y.; Hou, Y.; Li, C.; Zhao, Y.; Ma, P.; Lv, L.; Aji, S.; et al. Large-sized graphene oxide nanosheets increase DC–T-cell synaptic contact and the efficacy of DC vaccines against SARS-CoV-2. Adv. Mater. 2021, 33, e2102528. [Google Scholar] [CrossRef]
- Meng, C.; Zhi, X.; Li, C.; Li, C.; Chen, Z.; Qiu, X.; Ding, C.; Ma, L.; Lu, H.; Chen, D.; et al. Graphene Oxides Decorated with Carnosine as an Adjuvant To Modulate Innate Immune and Improve Adaptive Immunity in Vivo. ACS Nano 2016, 10, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Wang, Y.; Liu, J.; Tan, G.; Huang, F.; He, N.; Lu, Z. A novel therapeutic vaccine based on graphene oxide nanocomposite for tumor immunotherapy. Chin. Chem. Lett. 2022, 33, 4089–4095. [Google Scholar] [CrossRef]
- Mohamed, R.; Ramli, M.M.; Reshak, A.H.; Osman, N.H.; Aziz, M.Y.; Mansor, M.S. Revolutionizing cancer treatment by boosting dendritic cell vaccine efficacy with graphene oxide. Trends Immunother. 2023, 7, 2039. [Google Scholar] [CrossRef]
- Song, Y.; Mehl, F.; Zeichner, S.L. Vaccine Strategies to Elicit Mucosal Immunity. Vaccines 2024, 12, 191. [Google Scholar] [CrossRef]
- de Sousa, I.P.; Buttenhauser, K.; Suchaoin, W.; Partenhauser, A.; Perrone, M.; Matuszczak, B.; Bernkop-Schnürch, A. Thiolated graphene oxide as promising mucoadhesive carrier for hydrophobic drugs. Int. J. Pharm. 2016, 509, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.R., Jr.; Farazuddin, M.; Wong, P.T.; O’Konek, J.J. The unfulfilled potential of mucosal immunization. J. Allergy Clin. Immunol. 2022, 150, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wang, J. Mucosal vaccine delivery: A focus on the breakthrough of specific barriers. Acta Pharm. Sin. B 2022, 12, 3456–3474. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Gonzalez, G.X.; Ma, Y.; Song, Y.; Wang, S. Intranasal vaccination with influenza HA/GO-PEI nanoparticles provides immune protection against homo- and heterologous strains. Proc. Natl. Acad. Sci. USA 2021, 118, e2024998118. [Google Scholar] [CrossRef]
- Kim, M.-G.; Park, J.Y.; Shon, Y.; Kim, G.; Shim, G.; Oh, Y.-K. Nanotechnology and vaccine development. Asian J. Pharm. Sci. 2014, 9, 227–235. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Ahsan, W.; Aggarwal, G.; Kohli, K. Nanocarriers-Assisted Needle-Free Vaccine Delivery Through Oral and Intranasal Transmucosal Routes: A Novel Therapeutic Conduit. Front. Pharmacol. 2021, 12, 757761. [Google Scholar] [CrossRef] [PubMed]
- Pingle, P.; Joshi, I.; Sodhi, R.K.; Madan, J.; Mehra, N.K.; Singh, P.K.; Srivastava, S.; Khatri, D.K.; Singh, S.B. Needle-free technology for biomedical applications. In Multifunctional Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 149–173. [Google Scholar]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Zare, Y.; Rhee, K.Y.; Park, S.-J. Progressing of a power model for electrical conductivity of graphene-based composites. Sci. Rep. 2023, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
- Mettelman, R.C.; Allen, E.K.; Thomas, P.G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 2022, 55, 749–780. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Qin, M.; Sun, X. Recent progress in application of nanovaccines for enhancing mucosal immune responses. Acta Pharm. Sin. B 2023, 13, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Ban, G.; Hou, Y.; Shen, Z.; Jia, J.; Chai, L.; Ma, C. Potential Biomedical Limitations of Graphene Nanomaterials. Int. J. Nanomed. 2023, 18, 1695–1708. [Google Scholar] [CrossRef]
- Tajnur, R.; Rezwan, R.; Aziz, A. An update on vaccine status and the role of nanomedicine against SARS-CoV-2: A narrative review. Health Sci. Rep. 2023, 6, e1377. [Google Scholar] [CrossRef]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.; Robinson, J.M.; Cunningham, G.; Iqbal, R.; Larsen, S. The complexity and cost of vaccine manufacturing—An overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- Seifi, T.; Reza Kamali, A. Antiviral performance of graphene-based materials with emphasis on COVID-19: A review. Med. Drug Discov. 2021, 11, 100099. [Google Scholar] [CrossRef]
- Achawi, S.; Feneon, B. Structure-Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity. Nanomaterials 2021, 11, 2963. [Google Scholar] [CrossRef]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. Acs Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef]

| Author | Title | Results | Ref. |
|---|---|---|---|
| Alphandéry | Nano dimensions/adjuvants in COVID-19 vaccines | The proposed COVID-19 vaccines contain various vaccine active principles and adjuvants such as graphene oxide that can potentially enhance their effectiveness and reduce the necessary dose, thereby facilitating mass vaccination and potentially bringing an end to the COVID-19 crisis. These adjuvants, inspired by cancer nano-vaccines, may improve the vaccine’s benefit-to-risk ratio and modulate the immune response by targeting specific regions and adjusting physical properties. | [34] |
| Huang et al. | A self-assembled graphene oxide adjuvant induces both enhanced humoral and cellular immune responses in influenza vaccine | Self-assembled nanoparticles based on graphene oxide quantum dots with the adjuvant ZnGC-R were designed for influenza vaccines, enhancing antigen utilization, DC recruitment, and antigen-presenting cell activation. This novel adjuvant induces robust CD4+ and CD8+ T-cell responses, promotes CD26+ B-cell proliferation, elicits higher levels of hemagglutination-inhibiting antibodies, and significantly boosts IgG responses, leading to 100% in vivo protection against H1N1 influenza. | [26] |
| Yan et al. | Chitosan-Functionalized Graphene Oxide as a Potential Immunoadjuvant | The study introduced CS-functionalized GO (GO-CS) as a safe and effective nanoadjuvant for vaccines, showing improved biocompatibility and immune response activation compared to non-functionalized GO. The GO-CS adjuvant also exhibited a smaller size, positive charge, and enhanced thermal stability, making it a promising candidate for vaccine development and immunotherapy. | [35] |
| Wang et al. | Alum-functionalized graphene oxide nanocomplexes for effective anticancer vaccination | The study describes the development of an aluminum-based adjuvant in the form of AlO(OH)-modified graphene oxide (GO) nanosheets (GO-AlO(OH)) as an effective vaccine adjuvant. GO-AlO(OH) not only maintains the induction of humoral immunity but also elicits a cellular immune response. Antigen-loaded GO-AlO(OH) nanocomplexes enhance the cellular uptake and cytosolic release of antigens, promote dendritic cell maturation, stimulate higher antigen-specific IgG titers and robust CD4+ and CD8+ T lymphocyte responses, and inhibit tumor growth in vivo. This novel formulation may serve as a facile and efficient approach for effective anticancer vaccination. | [36] |
| Gao et al. | Developing an efficient MGCR microneedle nanovaccine patch for eliciting Th 1 cellular response against the SARS-CoV-2 infection | The study aimed to enhance the immunogenicity of subunit vaccines for COVID-19 and other epidemics by creating a CpG 1018- and graphene oxide-based bi-adjuvant system. This system successfully delivered the Receptor-Binding Domain (RBD) of the SARS-CoV-2 spike protein, resulting in the graphene oxide-based complex adjuvant nanovaccine (GCR). The GCR nanovaccine induced robust antibody responses and Type 1 Cellular responses in CD8+ T cells. Furthermore, a microneedle patch vaccine (MGCR) was developed based on the GCR vaccine, which produced a similar antibody response, maintained high antibody levels over time, and increased the Tcm proportion in the mouse spleen. The MGCR vaccine also exhibited improved storage stability and could be administered without medical staff, enhancing vaccine distribution efficiency. This innovative vaccine system offers a promising approach to combating SARS-CoV-2 infection and future pandemics. | [37] |
| Zhao et al. | Novel use of graphene oxide quantum dots in a pickering emulsion as a Chlamydia trachomatis vaccine adjuvant | The study investigated the use of graphene oxide quantum dots (GOQDs) as a surfactant substitute in Pickering emulsions for the Chlamydia trachomatis Pgp3 recombinant vaccine. GOQD-stabilized Pickering emulsion (GQPE) enhances immune responses, prolonging the immune response and promoting dendritic cell recruitment. It is found to be a safe and effective adjuvant, stimulating various immune responses and improving immune response duration. | [38] |
| Xu et al. | Functionalized graphene oxide serves as a novel vaccine nano-adjuvant for robust stimulation of cellular immunity | The study explored graphene oxide (GO) as a vaccine adjuvant for immunotherapy using urease B (Ure B) as the model antigen. Dual-polymer-modified GO (GO-PEG-PEI) was developed as a positive modulator, promoting dendritic cell maturation and cytokine secretion. GO-PEG-PEI also effectively transports antigens into dendritic cells, making it a promising vaccine adjuvant. Compared to free Ure B and an aluminum-adjuvant-based vaccine (Alum-Ure B), GO-PEG-PEI-Ure B induces stronger cellular immunity, showcasing its potential in cancer immunotherapy. The study highlights the critical role of surface chemistry in the rational design of nanoadjuvants. | [39] |
| Jiao et al. | Lentinan-functionalized graphene oxide hydrogel as a sustained antigen delivery system for vaccines | The study introduced a lentinan-functionalized graphene oxide hydrogel (LNT-GO Gel) as a sustained antigen delivery system for vaccines. The LNT-GO Gel effectively encapsulates and releases antigens, enhances immune responses, and displays favorable safety and biodegradability, highlighting its potential as an adjuvant delivery platform for subunit vaccines. | [40] |
| Liu et al. | Lentinan-Functionalized Graphene Oxide Is an Effective Antigen Delivery System That Modulates Innate Immunity and Improves Adaptive Immunity | The study synthesized graphene oxide grafted with lentinan (GO-LNT) to enhance antigen uptake in macrophages, resulting in sustained long-term immune responses and increased IgG levels compared to GO/OVA. GO-LNT demonstrates potential as a safe and effective vaccine delivery system and an excellent adjuvant for eliciting long-term immune memory and boosting both cellular and humoral immunity. | [41] |
| Yang et al. | Poria cocos polysaccharide-functionalized graphene oxide nanosheet induces efficient cancer immunotherapy in mice | The abstract describes the creation of nanocomplexes named nsGO/PCP/OVA for cancer immunotherapy, using graphene oxide nanosheets as carriers for ovalbumin (OVA) and Poria cocos polysaccharides (PCPs). These nanocomplexes were successful in activating specific immune responses, inducing dendritic cell maturation, and effectively combating tumor growth, both preventatively and therapeutically. Overall, this nanovaccine platform shows great potential for enhancing anti-tumor immunity and improving cancer immunotherapy. | [42] |
| Zhao | Preparation of graphene oxide-stabilized Pickering emulsion adjuvant for Pgp3 recombinant vaccine and enhanced immunoprotection against Chlamydia Trachomatis infection | Researchers developed a graphene oxide-stabilized Pickering emulsion (GPE) to serve as a novel adjuvant for the Chlamydia trachomatis Pgp3 vaccine, demonstrating superior immune responses, including enhanced macrophage polarization and recruitment, increased cytokine production, elevated levels of specific immunoglobulins, and improved protection against Chlamydia muridarum infection compared to the vaccine with traditional adjuvants. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare-Zardini, H.; Saberian, E.; Jenča, A.; Jenča, A.; Petrášová, A.; Jenčová, J. A Narrative Review on the Promising Potential of Graphene in Vaccine Design: Evaluating the Benefits and Drawbacks of Carbon Nanoplates in Nanovaccine Production. Vaccines 2024, 12, 660. https://doi.org/10.3390/vaccines12060660
Zare-Zardini H, Saberian E, Jenča A, Jenča A, Petrášová A, Jenčová J. A Narrative Review on the Promising Potential of Graphene in Vaccine Design: Evaluating the Benefits and Drawbacks of Carbon Nanoplates in Nanovaccine Production. Vaccines. 2024; 12(6):660. https://doi.org/10.3390/vaccines12060660
Chicago/Turabian StyleZare-Zardini, Hadi, Elham Saberian, Andrej Jenča, Andrej Jenča, Adriána Petrášová, and Janka Jenčová. 2024. "A Narrative Review on the Promising Potential of Graphene in Vaccine Design: Evaluating the Benefits and Drawbacks of Carbon Nanoplates in Nanovaccine Production" Vaccines 12, no. 6: 660. https://doi.org/10.3390/vaccines12060660
APA StyleZare-Zardini, H., Saberian, E., Jenča, A., Jenča, A., Petrášová, A., & Jenčová, J. (2024). A Narrative Review on the Promising Potential of Graphene in Vaccine Design: Evaluating the Benefits and Drawbacks of Carbon Nanoplates in Nanovaccine Production. Vaccines, 12(6), 660. https://doi.org/10.3390/vaccines12060660






