Relationship between Endotoxin Content in Vaccine Preclinical Formulations and Animal Welfare: An Extensive Study on Historical Data to Set an Informed Threshold
Abstract
:1. Introduction
2. Materials and Methods
3. Results
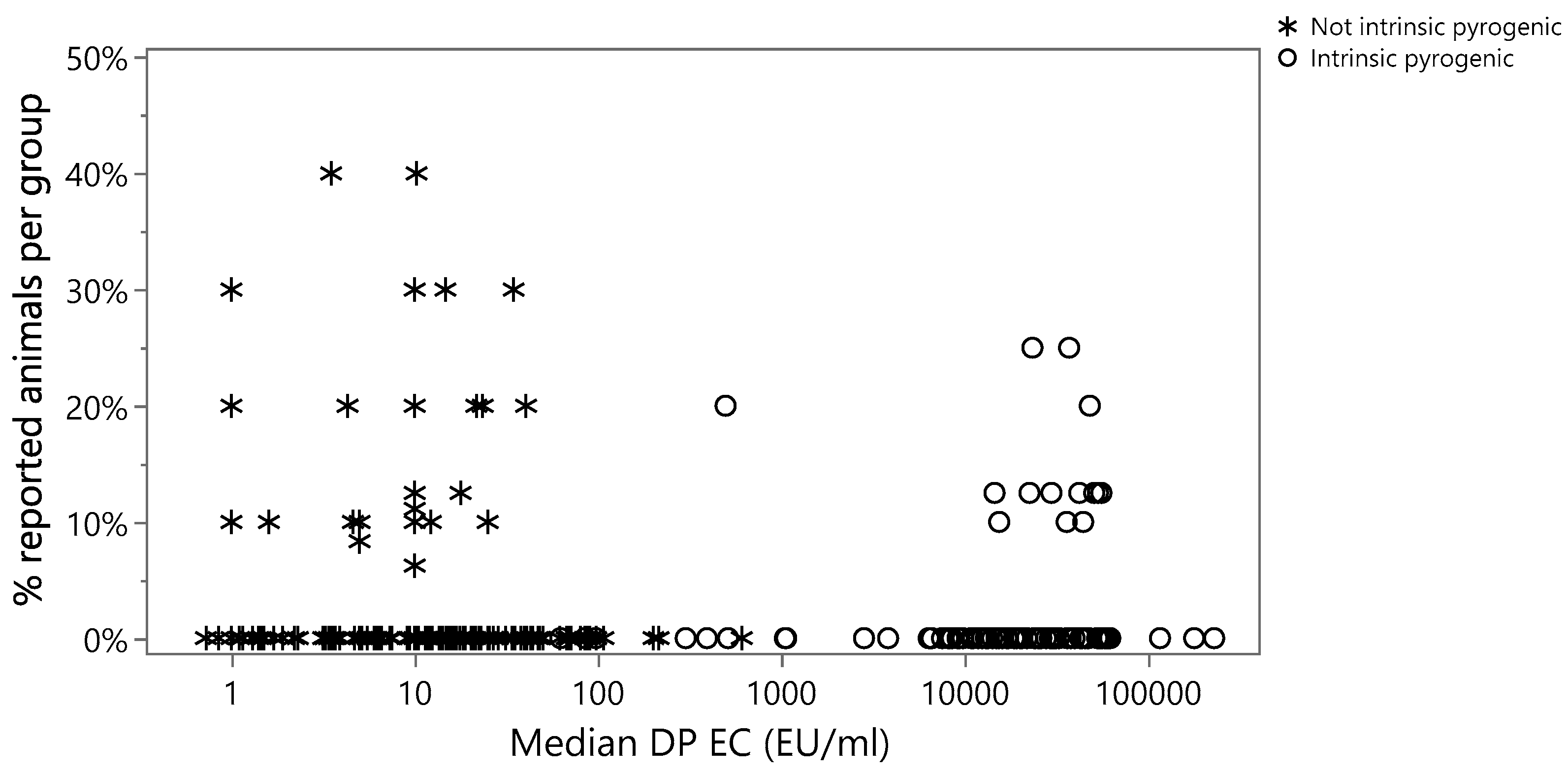
3.1. Evaluation of Potential Relationship between the EC of the DP and the Animal Welfare
3.1.1. Logistic Regression
3.1.2. Cochran–Armitage Trend Exact Test
3.1.3. Summary Results for the Evaluation of Potential Relationship between EC of the DP and the Animal Welfare
3.2. EC Threshold Definition Based on Animal Welfare
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, L.A.; Singh, M. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J. Pharm. Sci. 2011, 100, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Malyala, P.; Singh, M. Endotoxin limits in formulations for preclinical research. J. Pharm. Sci. 2008, 97, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Kiros, T.G.; Levast, B.; Auray, G.; Strom, S.; van Kessel, J.; Gerdts, V. The Importance of Animal Models in the Development of Vaccines. In Innovation in Vaccinology; Spring: Berlin/Heidelberg, Germany, 2012; pp. 251–264. [Google Scholar] [CrossRef]
- Andersen, M.L.; Winter, L.M.F. Animal models in biological and biomedical research—Experimental and ethical concerns. Acad. Bras. Ciênc. 2017, 91 (Suppl. S1), e20170238. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co. Limited: London, UK, 1960; p. 252. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Hernández-Ávalos, I.; Martínez-Burnes, J.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The importance of animal models in biomedical research: Current insights and applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, N.M.; Scher, I. Endotoxin lethality and tolerance in mice: Analysis with the B-Lymphocyte-Defective CBA/N Strain. Infect. Immun. 1979, 24, 127–131. [Google Scholar] [CrossRef]
- Glode, L.M.; Mergenhagen, S.E.; Rosenstreich, D.L. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect. Immun. 1976, 14, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.C.; Bradley, S.G. Enhanced toxicity for mice of combinations of antibiotics with Escherichia coli cells or Salmonella typhosa endotoxin. Infect. Immun. 1971, 4, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Mabley, J.G.; Horváth, E.M.; Murthy, K.G.; Zsengellér, Z.; Vaslin, A.; Benko, R.; Kollai, M.; Szabó, C. Gender differences in the endotoxin-induced inflammatory and vascular responses: Potential role of poly (ADP-ribose) polymerase activation. J. Pharmacol. Exp. Ther. 2005, 315, 812–820. [Google Scholar] [CrossRef]
- Copeland, S.; Warren, H.S.; Lowry, S.F.; Calvano, S.E.; Remick, D. Inflammation and the Host Response to Injury Investigators. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 2005, 12, 60–67. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 1991, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Committee on Use of Laboratory Animals in Biomedical and Behavioral Research; National Research Council and Institute of Medicine. Use of Laboratory Animals in Biomedical and Behavioral Research; National Academy Press: Washington, DC, USA, 1988.
- Giacomotto, J.; Segalat, L. High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, C.F. Replacement, reduction and refinement alternatives to animal use in vaccine potency measurement. Expert Rev. Vaccines 2009, 8, 313–322. [Google Scholar] [CrossRef]
- Shivatare, S.S.; Shivatare, V.S.; Wong, C.H. Glycoconjugates: Synthesis, Functional Studies, and Therapeutic Developments. Chem. Rev. 2022, 122, 15603–15671. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef]
- Micoli, F.; Alfini, R.; Giannelli, C. Methods for Assessment of OMV/GMMA Quality and Stability. Methods Mol. Biol. 2022, 2414, 227–279. [Google Scholar] [CrossRef]
- Rossi, O.; Citiulo, F.; Mancini, F. Outer membrane vesicles: Moving within the intricate labyrinth of assays that can predict risks of reactogenicity in humans. Hum. Vaccines Immunother. 2021, 17, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Mohammed, A.R.; Kirby, D.J.; McNeil, S.E.; Bramwell, V.W. Vaccine adjuvant systems: Enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008, 364, 272–280. [Google Scholar] [CrossRef]
- Tandrup Schmidt, S.; Foged, C.; Smith Korsholm, K.; Rades, T.; Christensen, D. Liposome-based adjuvants for subunit vaccines: Formulation strategies for subunit antigens and immunostimulators. Pharmaceutics 2016, 8, 7. [Google Scholar] [CrossRef]
- Shi, Y.; HogenEsch, H.; Regnier, F.E.; Hem, S.L. Detoxification of endotoxin by aluminum hydroxide adjuvant. Vaccine 2001, 19, 1747–1752. [Google Scholar] [CrossRef]
- O’Hagan, D.T. Vaccine Adjuvants: Preparation Methods and Research Protocols; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 42. [Google Scholar]
- Pulendran, B.; S Arunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Dubczak, J.; Reid, N.; Tsuchiya, M. Evaluation of limulus amebocyte lysate and recombinant endotoxin alternative assays for an assessment of endotoxin detection specificity. Eur. J. Pharm. Sci. 2021, 159, 105716. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Ding, X. Methods of Endotoxin Detection. J. Lab. Autom. 2015, 20, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Garçon, N. AS04, an aluminum salt-and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines. 2007, 6, 723–739. [Google Scholar] [CrossRef]
- Siena, E.; Schiavetti, F.; Borgogni, E.; Taccone, M.; Faenzi, E.; Brazzoli, M.; Aprea, S.; Bardelli, M.; Volpini, G.; Buricchi, F.; et al. Systems analysis of human responses to an aluminium hydroxide-adsorbed TLR7 agonist (AS37) adjuvanted vaccine reveals a dose-dependent and specific activation of the interferon-mediated antiviral response. Vaccine 2023, 41, 724–734. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis, 2nd ed.; Wiley Series in Probability and Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 165–210. [Google Scholar] [CrossRef]
- Allison, P.D. Logistic Regression Using the SAS System: Theory and Application; SAS Publishing: Cary, NC, USA, 1999. [Google Scholar]
- Cochran, W.G. Some methods for strengthening the common chi-squared tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Armitage, P. Tests for Linear Trends in Proportions and Frequencies. Biometrics 1955, 11, 375–386. [Google Scholar] [CrossRef]
- Clopper, C.J.; Pearson, E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Meeker, W.Q.; Hahn, G.J.; Escobar, L.A. Statistical Intervals: A Guide for Practitioners and Researchers, 2nd ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 86–88. [Google Scholar] [CrossRef]
- Fisher, R.A. On the interpretation of χ2 from contingency tables, and the calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]
- Agresti, A. A Survey of Exact Inference for Contingency Tables. Stat. Sci. 1992, 7, 131–153. [Google Scholar] [CrossRef]
- Danner, R.L.; Elin, R.J.; Hosseini, J.M.; Wesley, R.A.; Reilly, J.M.; Parillo, J.E. Endotoxemia in human septic shock. Chest 1991, 99, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K.; Agnihotri, S.A. Recent advances and novel strategies in pre-clinical formulation development: An overview. J. Control Release 2011, 156, 281–296. [Google Scholar] [CrossRef]
- Klein, H.J.; Bayne, K.A. Establishing a culture of care, conscience, and responsibility: Addressing the improvement of scientific discovery and animal welfare through science-based performance standards. ILAR J. 2007, 48, 3–11. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Halsey, L.G.; Bury, N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017, 220, 3007–3016. [Google Scholar] [CrossRef]


| Reported | EC ≤ 10 | 10 < EC ≤ 25.5 | 25.5 < EC | Total |
|---|---|---|---|---|
| YES | 54 | 14 | 23 | 91 |
| (1.66%) | (1.88%) | (1.88%) | ||
| NO | 3417 | 433 | 1274 | 5124 |
| (98.34%) | (98.12%) | (98.12%) | ||
| Total | 6471 | 447 | 1297 | 5215 |
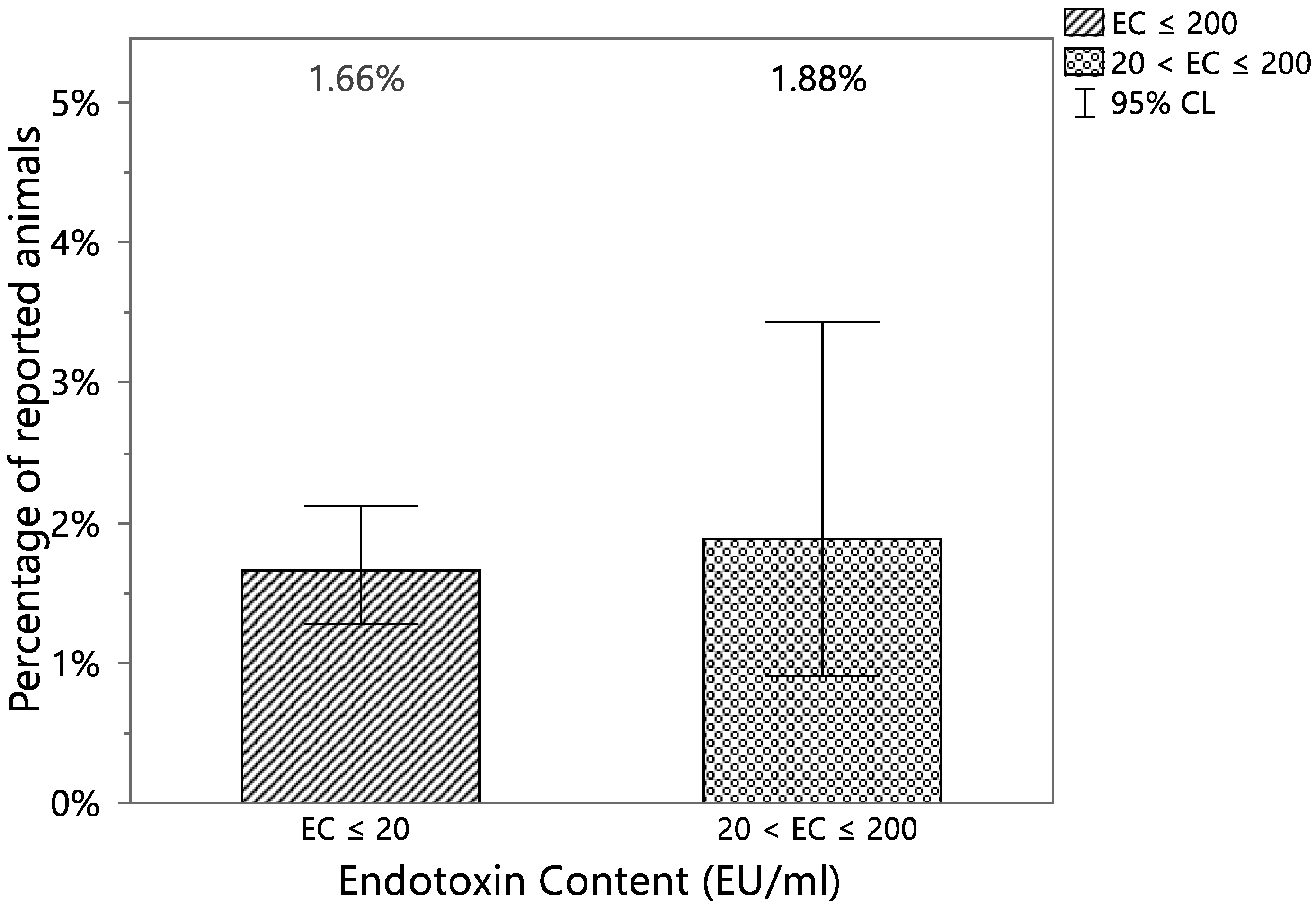
| Reported | EC ≤ 20 | 20 < EC ≤ 200 | Total |
|---|---|---|---|
| YES | 63 | 10 | 73 |
| (1.66%) | (1.88%) | ||
| NO | 3729 | 521 | 4250 |
| (98.34%) | (98.12%) | ||
| Total | 3792 | 531 | 4323 |
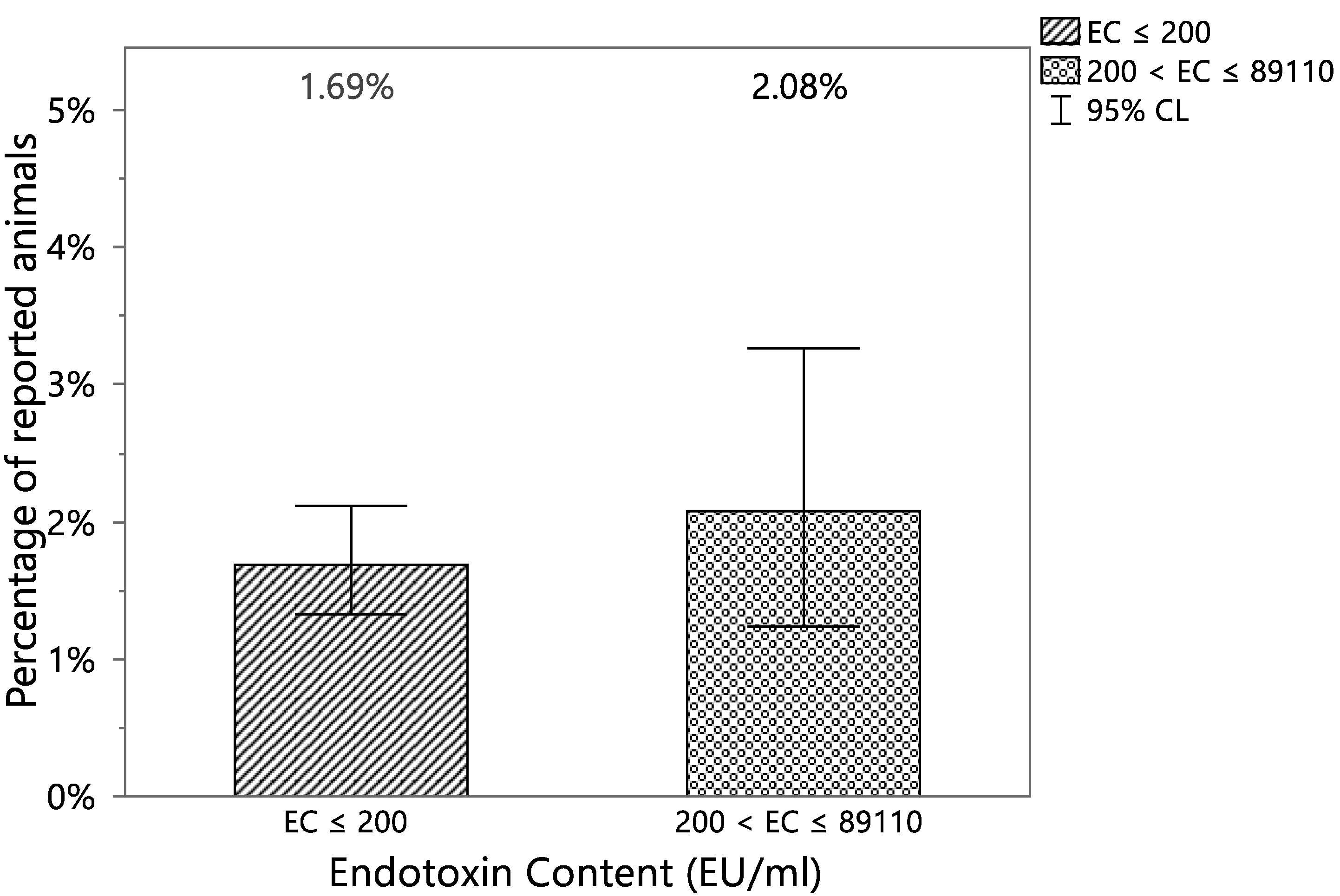
| Reported | EC ≤ 200 | 200< EC ≤ 89,110 | Total |
|---|---|---|---|
| YES | 73 | 18 | 91 |
| (1.69%) | (2.08%) | ||
| NO | 4250 | 848 | 5098 |
| (98.31%) | (97.92%) | ||
| Total | 4323 | 866 | 5189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baffetta, F.; Cecchi, R.; Guerrini, E.; Mangiavacchi, S.; Sorrentino, G.; Stranges, D. Relationship between Endotoxin Content in Vaccine Preclinical Formulations and Animal Welfare: An Extensive Study on Historical Data to Set an Informed Threshold. Vaccines 2024, 12, 815. https://doi.org/10.3390/vaccines12070815
Baffetta F, Cecchi R, Guerrini E, Mangiavacchi S, Sorrentino G, Stranges D. Relationship between Endotoxin Content in Vaccine Preclinical Formulations and Animal Welfare: An Extensive Study on Historical Data to Set an Informed Threshold. Vaccines. 2024; 12(7):815. https://doi.org/10.3390/vaccines12070815
Chicago/Turabian StyleBaffetta, Federica, Raffaella Cecchi, Eva Guerrini, Simona Mangiavacchi, Gilda Sorrentino, and Daniela Stranges. 2024. "Relationship between Endotoxin Content in Vaccine Preclinical Formulations and Animal Welfare: An Extensive Study on Historical Data to Set an Informed Threshold" Vaccines 12, no. 7: 815. https://doi.org/10.3390/vaccines12070815





