Immunometabolic Regulation of Vaccine-Induced Antibody Responses in Aging Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Vaccine
2.3. Flow Cytometry
2.4. B Cell Sorting
2.5. mRNA Extraction and Quantitative (q)PCR
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Mitostress Test
2.8. Statistical Analyses
3. Results
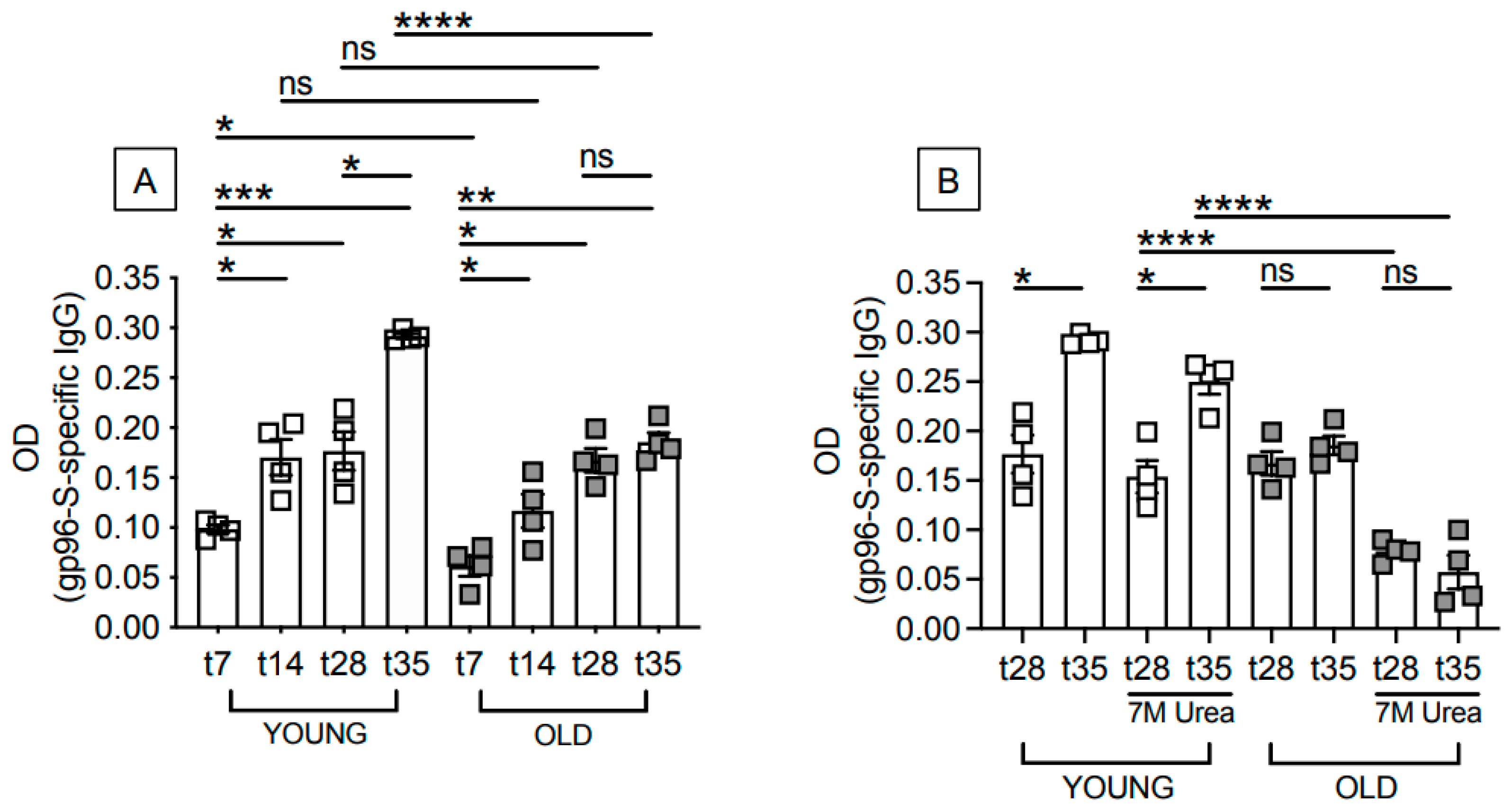
3.1. Aging Induces Defects in the Antibody Response to the gp96-Ig-S Vaccine
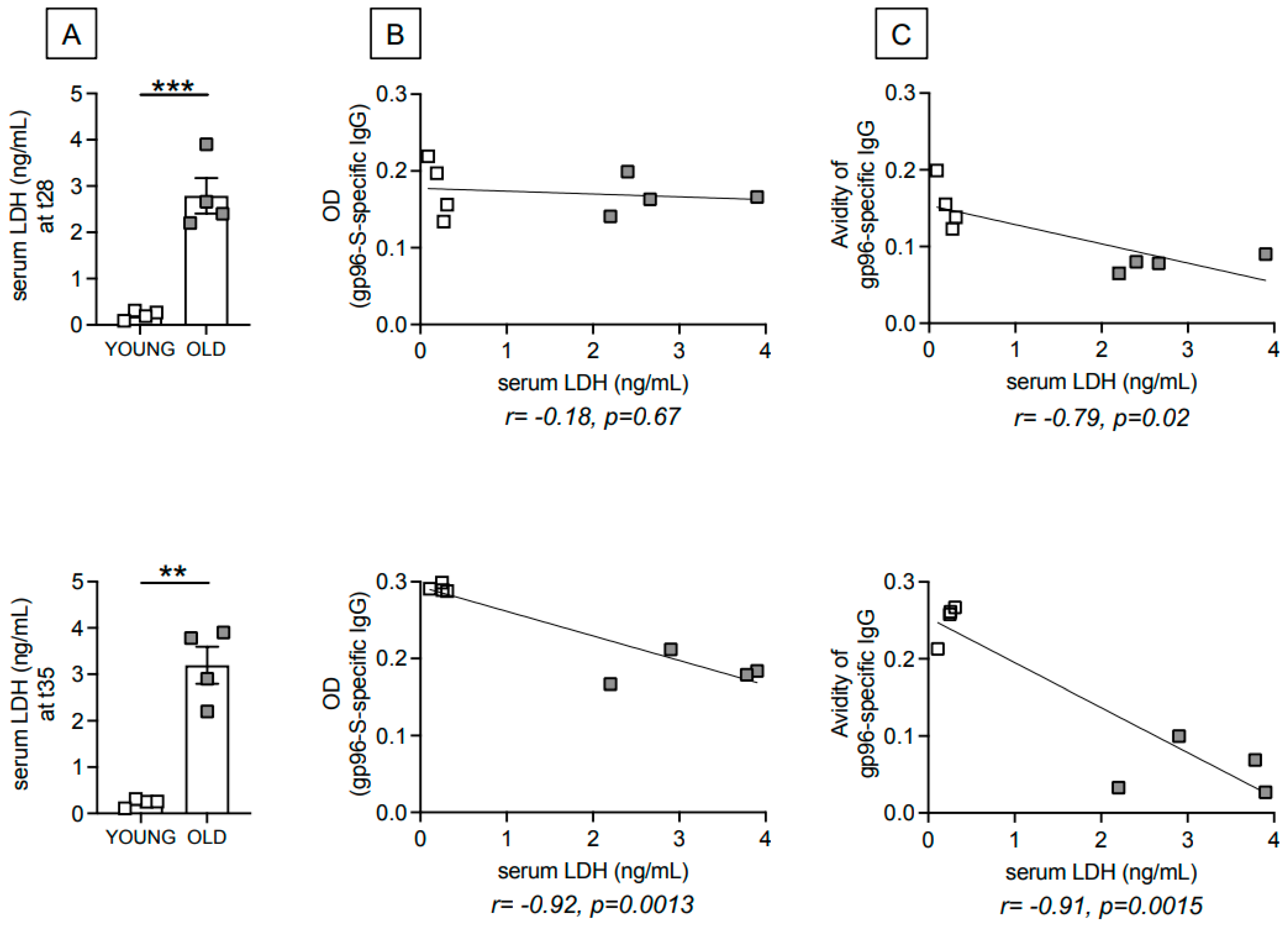
3.2. Age-Dependent Increase in Serum Levels of the Metabolic Marker Lactate Dehydrogenase (LDH) Are Negatively Associated with gp96-Ig-S Vaccine-Specific Antibodies
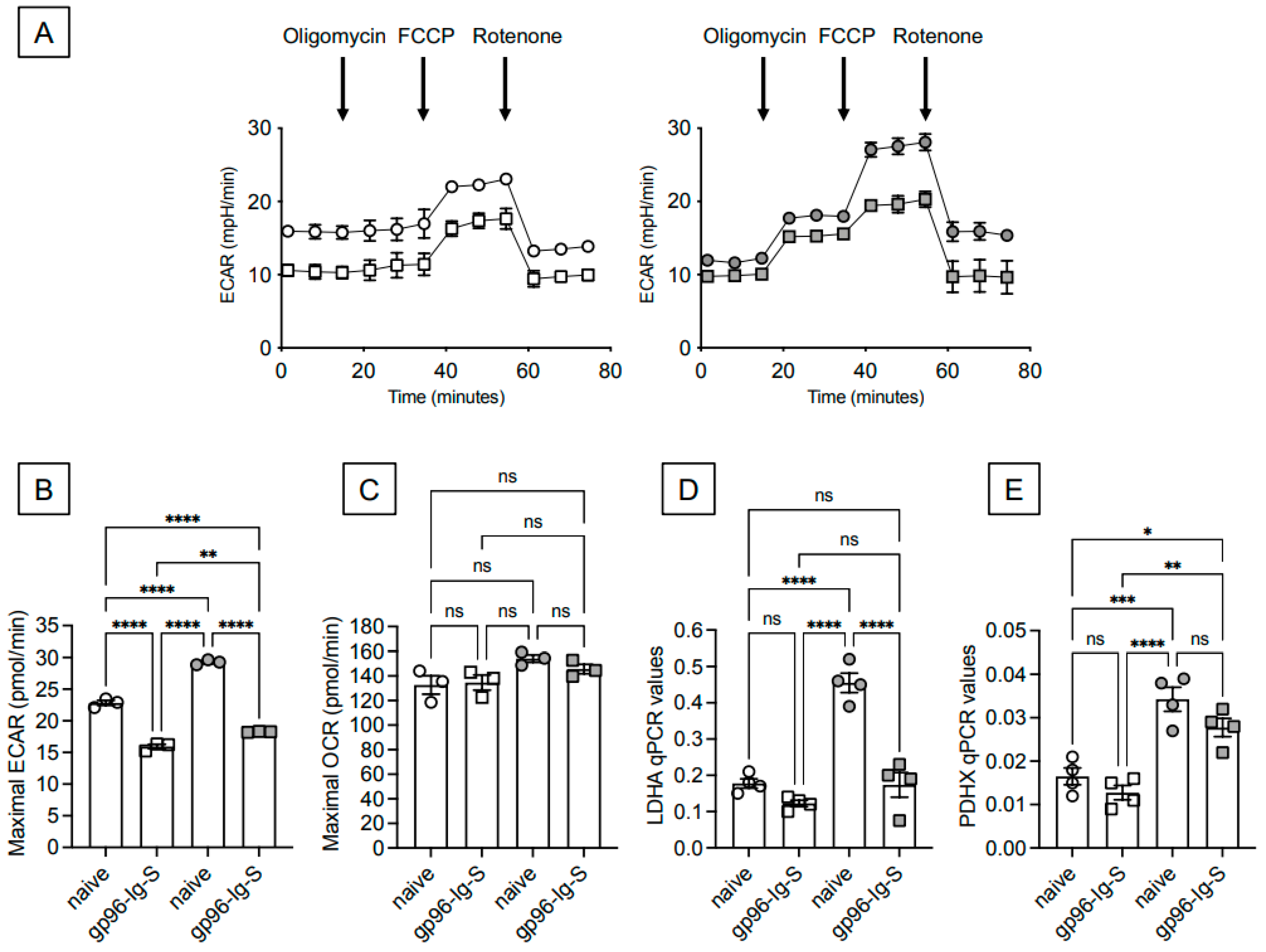
3.3. The gp96-Ig-S Vaccine Induces a Metabolic Reprogramming of B Cells from Both Young and Old Mice
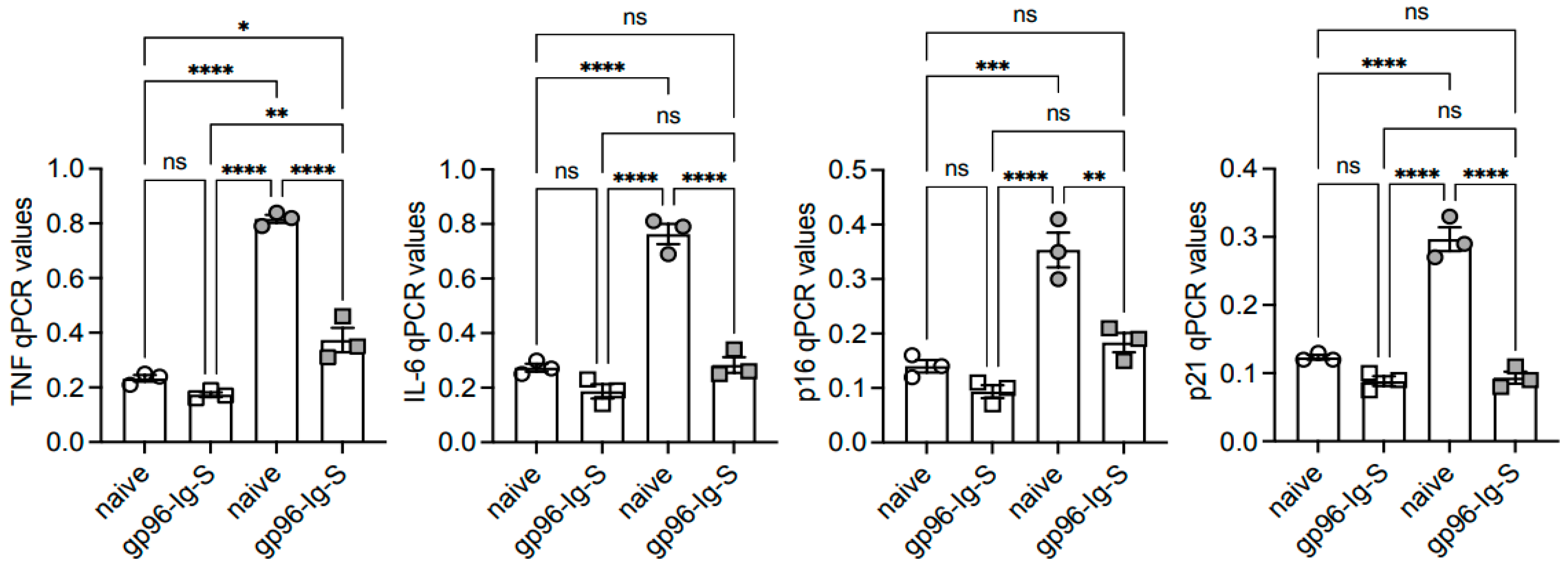
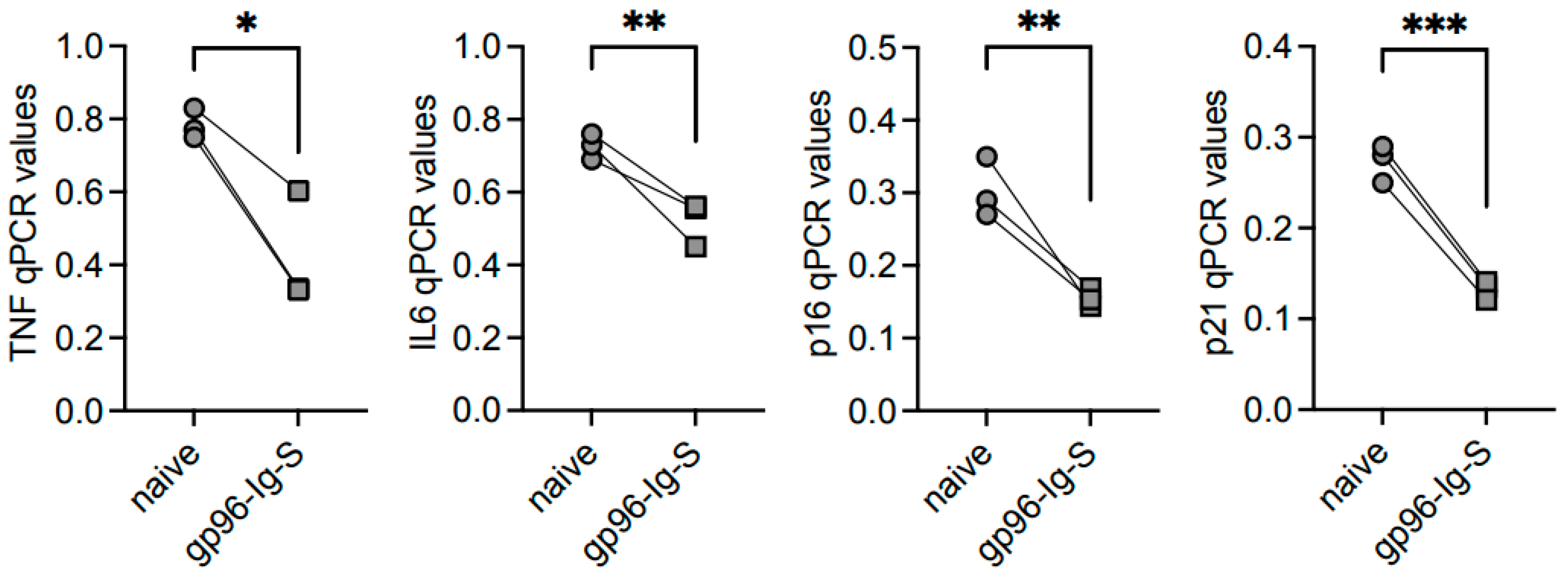
3.4. The gp96-Ig-S Vaccine Decreases RNA Expression of Inflammatory and Senescent Markers in B Cells from Old but not from Young Mice
3.5. The gp96-Ig-S Vaccine Decreases the Frequencies of Age-Associated B Cells (ABCs), Which Represent the Most Inflammatory B Cell Subsets, in Old but Not in Young Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Woudstra, T.; Thomson, A.B. Nutrient absorption and intestinal adaptation with ageing. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 1–15. [Google Scholar] [CrossRef]
- Fink, R.I.; Kolterman, O.G.; Griffin, J.; Olefsky, J.M. Mechanisms of Insulin Resistance in Aging. J. Clin. Investig. 1983, 71, 1523–1535. [Google Scholar] [CrossRef]
- Ryan, A.S. Insulin resistance with aging: Effects of diet and exercise. Sports Med. 2000, 30, 327–346. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic Shifts in Immunity and Inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef]
- Einstein, F.H.; Huffman, D.M.; Fishman, S.; Jerschow, E.; Heo, H.J.; Gil Atzmon, G.; Schechter, C.; Barzilai, N.; Muzumdar, R.H. Aging per se Increases the Susceptibility to Free Fatty Acid-Induced Insulin Resistance. J. Gerontol. Ser. A 2010, 65, 800–808. [Google Scholar] [CrossRef]
- Nicholas, D.A.; Zhang, K.; Hung, C.; Glasgow, S.; Aruni, A.W.; Unternaehrer, J.; Payne, K.J.; Langridge, W.H.R.; De Leon, M. Palmitic acid is a toll-like receptor 4 ligand that induces human dendritic cell secretion of IL-1beta. PLoS ONE 2017, 12, e0176793. [Google Scholar] [CrossRef]
- Perreault, M.; Roke, K.; Badawi, A.; Nielsen, D.E.; Abdelmagid, S.A.; El-Sohemy, A.; Ma, D.W.; Mutch, D.M. Plasma levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are positively associated, but 18:0 and 18:2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids 2014, 49, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, K.; Herbert, D.; Popkova, Y.; Lorz, A.; Schiller, J.; Gericke, M.; Kloting, N.; Bluher, M.; Franz, S.; Simon, J.C.; et al. Free fatty acids sensitize dendritic cells to amplify TH1/TH17-immune responses. Eur. J. Immunol. 2016, 46, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef]
- Zurier, R.; Rossetti, R.; Seiler, C.; Laposata, M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: Effects of unsaturated fatty acids. Prostaglandins, Leukot. Essent. Fat. Acids 1999, 60, 371–375. [Google Scholar] [CrossRef]
- Strbo, N.; Garcia-Soto, A.; Schreiber, T.H.; Podack, E.R. Secreted heat shock protein gp96-Ig: Next-generation vaccines for cancer and infectious diseases. Immunol. Res. 2013, 57, 311–325. [Google Scholar] [CrossRef]
- Fisher, E.; Padula, L.; Podack, K.; O’Neill, K.; Seavey, M.M.; Jayaraman, P.; Jasuja, R.; Strbo, N. Induction of SARS-CoV-2 Protein S-Specific CD8+ T Cells in the Lungs of gp96-Ig-S Vaccinated Mice. Front. Immunol. 2020, 11, 602254. [Google Scholar] [CrossRef]
- Padula, L.; Fisher, E.; Rivas, K.; Podack, K.; Frasca, D.; Kupritz, J.; Seavey, M.M.; Jayaraman, P.; Dixon, E.; Jasuja, R.; et al. Secreted heat shock protein gp96-Ig and OX40L-Fc combination vaccine enhances SARS-CoV-2 Spike (S) protein-specific B and T cell immune responses. Vaccine X 2022, 12, 100202. [Google Scholar] [CrossRef]
- Binder, R.J.; Srivastava, P.K. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 6128–6133. [Google Scholar] [CrossRef]
- Kinner-Bibeau, L.B.; Sedlacek, A.L.; Messmer, M.N.; Watkins, S.C.; Binder, R.J. HSPs drive dichotomous T-cell immune responses via DNA methylome remodelling in antigen presenting cells. Nat. Commun. 2017, 8, 15648. [Google Scholar] [CrossRef]
- Strbo, N.; Pahwa, S.; Kolber, M.A.; Gonzalez, L.; Fisher, E.; Podack, E.R. Cell-secreted Gp96-Ig-peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa. Mucosal. Immunol. 2010, 3, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Verma, N.; Yewdell, J.W.; Hilbert, A.K.; Castellino, F.; Lattanzi, M.; Del Giudice, G.; Rappuoli, R.; Golding, H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl. Med. 2011, 3, 85ra48. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.S. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022, 45, 2091–2123. [Google Scholar] [CrossRef]
- Lisanti, C.; Basile, D.; Parnofiello, A.; Bertoli, E.; Andreotti, V.J.; Garattini, S.K.; Bartoletti, M.; Cattaneo, M.; Di Nardo, P.; Bonotto, M.; et al. The SENECA study: Prognostic role of serum biomarkers in older patients with metastatic colorectal cancer. J. Geriatr. Oncol. 2020, 11, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Klarquist, J.; Chitrakar, A.; Pennock, N.D.; Kilgore, A.M.; Blain, T.; Zheng, C.; Danhorn, T.; Walton, K.; Jiang, L.; Sun, J.; et al. Clonal expansion of vaccine-elicited T cells is independent of aerobic glycolysis. Sci. Immunol. 2018, 3, eaas9822. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Garcia, D.; Diaz, A.; Blomberg, B.B. Hyper-metabolic B cells in the spleens of old mice make antibodies with autoimmune specificities. Immun. Ageing 2021, 18, 9. [Google Scholar] [CrossRef]
- Kawakami, S.; Johmura, Y.; Nakanishi, M. Intracellular acidification and glycolysis modulate inflammatory pathway in senescent cells. J. Biochem. 2024, 176, 97–107. [Google Scholar] [CrossRef]
- Pająk, B.; Zieliński, R.; Priebe, W. The Impact of Glycolysis and Its Inhibitors on the Immune Response to Inflammation and Autoimmunity. Molecules 2024, 29, 1298. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gomez de Las Heras, M.M.; Gabande-Rodriguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Romero, M.; Miller, K.; Gelsomini, A.; Garcia, D.; Li, K.; Suresh, D.; Frasca, D. Immunometabolic effects of lactate on humoral immunity in healthy individuals of different ages. Nat. Commun. 2024, in press.
- Cancro, M.P. Age-Associated B Cells. Annu. Rev. Immunol. 2020, 38, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, S.V.; Brahmbhatt, D.; Zomback, A.; Bassie, M.; Martinez, J.; Zhuang, J.; Schulze, J.; McLellan, J.S.; Mariani, R.; Alff, P.; et al. Engineered dityrosine-bonding of the RSV prefusion F protein imparts stability and potency advantages. Nat. Commun. 2024, 15, 2202. [Google Scholar] [CrossRef] [PubMed]
- Maciorowski, D.; Diop, C.; Bhatt, U.; Estelien, R.; Li, D.; Chauhan, R.; Vandenberghe, L.H.; Zabaleta, N. Immunogenicity of an AAV-Based COVID-19 Vaccine in Murine Models of Obesity and Aging. Viruses 2022, 14, 820. [Google Scholar] [CrossRef]
- Zhou, X.; Hopkins, J.W.; Wang, C.; Brahmakshatriya, V.; Swain, S.L.; Kuchel, G.A.; Haynes, L.; McElhaney, J.E. IL-2 and IL-6 cooperate to enhance the generation of influenza-specific CD8 T cells responding to live influenza virus in aged mice and humans. Oncotarget 2016, 7, 39171–39183. [Google Scholar] [CrossRef][Green Version]
- Dapporto, F.; Marchi, S.; Leonardi, M.; Piu, P.; Lovreglio, P.; Decaro, N.; Buonvino, N.; Stufano, A.; Lorusso, E.; Bombardieri, E.; et al. Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination. J Immunol Res. 2022, 2022, 4813199. [Google Scholar] [CrossRef]
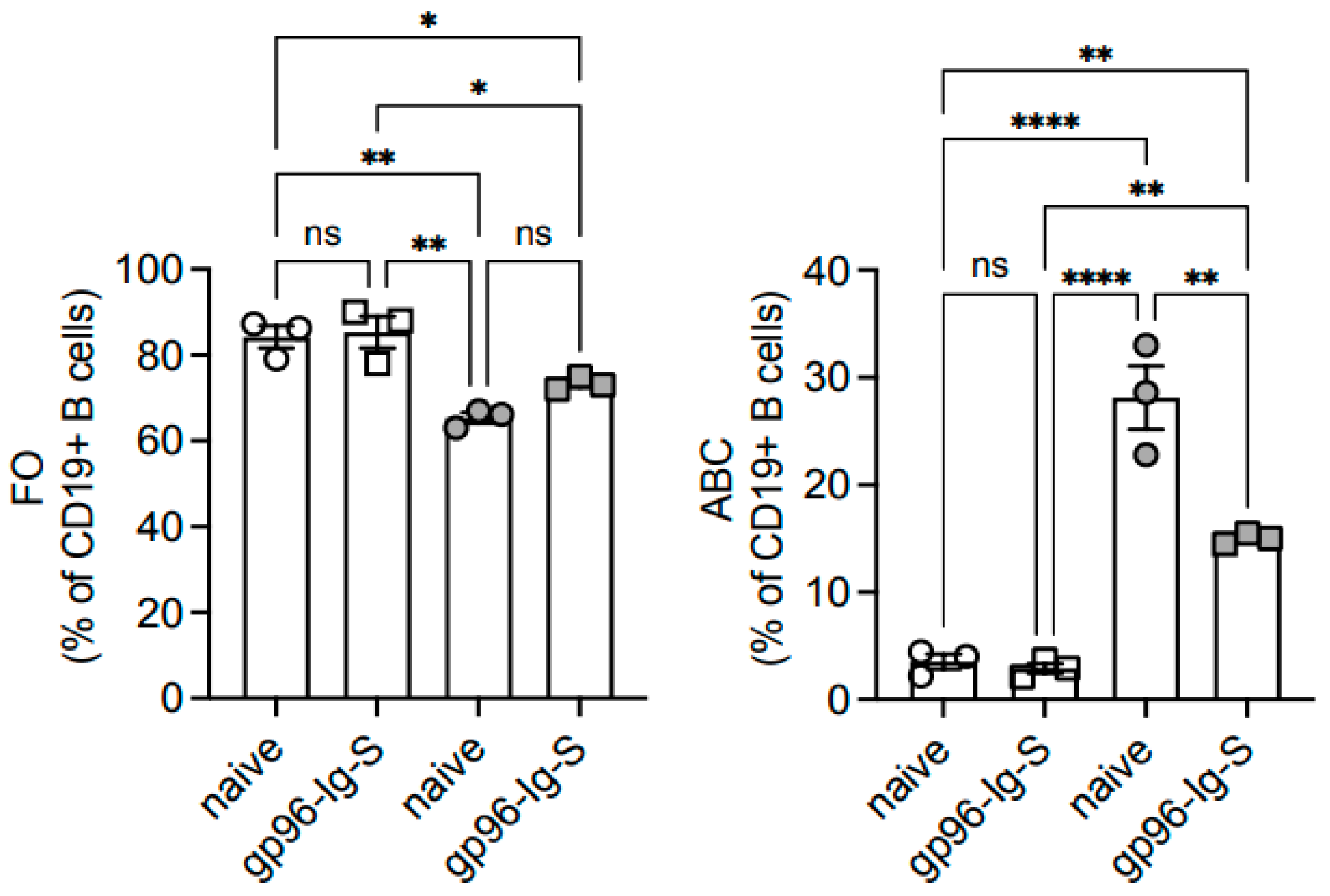
| FO | ABC | MZ | ||||
|---|---|---|---|---|---|---|
| Frequencies | Numbers | Frequencies | Numbers | Frequencies | Numbers | |
| YOUNG | 84.23 ± 2.59 * | 72.32 ± 1.01 ** | 3.55 ± 0.69 * | 3.02 ± 0.51 ** | 1.30 ± 0.70 | 1.15 ± 0.51 |
| OLD | 65.40 ± 1.22 | 54.05 ± 0.81 | 28.13 ± 2.96 | 23.23 ± 2.29 | 2.03 ± 0.38 | 1.69 ± 0.33 |
| TNF | IL6 | p16INK4 | p21CIP1/WAF1 | |||||
|---|---|---|---|---|---|---|---|---|
| FO | ABC | FO | ABC | FO | ABC | FO | ABC | |
| YOUNG | 0.004 ± 0.001 | 0.190 ± 0.012 #### | 0.004 ± 0.001 | 0.024 ± 0.005 #### | 0.022 ± 0.019 | 0.057 ± 0.007 # | 0.003 ± 0.002 | 0.017 ± 0.003 #### |
| OLD | 0.037 ± 0.004 ** | 0.783 ± 0.024 #### **** | 0.024 ± 0.00 * | 0.727 ± 0.020 #### **** | 0.057 ± 0.007 | 0.303 ± 0.024 ### ** | 0.017 ± 0.003 * | 0.273 ± 0.012 #### *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasca, D.; Romero, M.; Padula, L.; Fisher, E.; Strbo, N. Immunometabolic Regulation of Vaccine-Induced Antibody Responses in Aging Mice. Vaccines 2024, 12, 960. https://doi.org/10.3390/vaccines12090960
Frasca D, Romero M, Padula L, Fisher E, Strbo N. Immunometabolic Regulation of Vaccine-Induced Antibody Responses in Aging Mice. Vaccines. 2024; 12(9):960. https://doi.org/10.3390/vaccines12090960
Chicago/Turabian StyleFrasca, Daniela, Maria Romero, Laura Padula, Eva Fisher, and Natasa Strbo. 2024. "Immunometabolic Regulation of Vaccine-Induced Antibody Responses in Aging Mice" Vaccines 12, no. 9: 960. https://doi.org/10.3390/vaccines12090960
APA StyleFrasca, D., Romero, M., Padula, L., Fisher, E., & Strbo, N. (2024). Immunometabolic Regulation of Vaccine-Induced Antibody Responses in Aging Mice. Vaccines, 12(9), 960. https://doi.org/10.3390/vaccines12090960






